Abstract
Memory extinction, defined as a decrease of a conditioned response as a function of a non-reinforced conditioned stimulus presentation, has high biological and clinical relevance. Extinction is not a passive reversing or erasing of the plasticity associated with acquisition, but a novel, active learning process. Nifedipine blocks L-type voltage gated calcium channels (LVGCC) and has been shown previously to selectively interfere with the extinction, but not the acquisition, of fear memory. We studied here the effect of retrograde and anterograde shifts of nifedipine application, with respect to an extinction training, on the extinction of fear conditioning. Subcutaneous injection of 30 mg/kg nifedipine, at least up to 4 h before the extinction session, significantly impaired extinction, as did intraperitoneal injection of 15 mg/kg nifedipine, at least up to 2 h before extinction training. However, the injection of nifedipine also induced a strong and protracted stress response. The pharmacokinetics of nifedipine suggest that it was mainly this stress response that triggered the specific inhibition of extinction, not the blockade of LVGCC in the brain. Our results support recent findings that stress selectively interferes with the extinction, but not the acquisition, of fear memory. They also indicate that a pharmacological approach is not sufficient to study the role of brain LVGCC in learning and memory. Further research using specific genetically modified animals is necessary to delineate the role of LVGCC in fear memory extinction.
Pairing of a conditional stimulus (CS) with an aversive unconditional stimulus (US) results in a conditioned fear response and is a classical form of Pavlovian learning (for review, see Fanselow and Poulos 2005). In rodent models, the CS is typically an auditory cue, and the US is an electrical footshock. In addition, the context itself, which is usually the chamber in which the conditioning takes place, serves as a CS. Auditory fear conditioning, an association learned between auditory cue and footshock, depends on the functional amygdala. Contextual fear conditioning, an association between context and footshock, depends on the function of both the amygdala and hippocampus.
Learning such new associations is accomplished during the acquisition phase of fear conditioning. The molecular process of acquisition requires calcium influx through NMDA-glutamate receptor channels and is dependent on transcription and translation in the postsynaptic neurons (Milner et al. 1998). Extinction of fear conditioning is the active attenuation of an association between the CS and US and as such is different from forgetting. Extinction is induced by repeatedly presenting solely the CS without the US. In the context of fear memory extinction, analysis of the role of NMDA-receptor channels was followed by the study of L-type voltage gated calcium channels (LVGCC) in recent years (Barad 2005). Systemic application of the pharmacological LVGCC antagonists nifedipine or nimodipine inhibited the extinction, but not the acquisition, of fear conditioning in mice (Cain et al. 2002; Suzuki et al. 2004). Thus, LVGCC were proposed to play a specific role in the molecular mechanisms underlying fear extinction.
LVGCC are formed by the Cav1 family of membrane channel proteins, which comprises the Cav1.1, Cav1.2, Cav1.3, and Cav1.4 channels. Of those, Cav1.2 and Cav1.3 are the major Cav1 channels expressed in mouse brain (Striessnig et al. 2006). Cav1.3 knockout mice demonstrated an impairment in the consolidation of fear conditioning, but not of its extinction (McKinney and Murphy 2006). In addition, the NMDA-receptor-independent, but LVGCC-dependent, form of L-LTP was selectively impaired in Cav1.2 knockout mice. This deficit was accompanied by reduction of hippocampal memory formation (Moosmang et al. 2005). Thus, nifedipine-induced inhibition of fear memory extinction is more likely to be mediated by Cav1.2 (Striessnig et al. 2006).
The acquisition of fear memory requires calcium influx through NMDA-receptor channels and is dependent on transcription and translation in the postsynaptic neurons. This process requires gene expression over a period of several hours (Milner et al. 1998). Disruption of gene expression during this period by pharmacological intervention, for example, by application of a protein synthesis inhibitor (Flexner et al. 1963), interferes with memory formation. A large body of literature has investigated the “critical period” by monitoring the amnesia caused by the inhibition of protein translation. Varying the onset of protein synthesis inhibition and memory acquisition training, the critical period included the time during training and a short period afterward (Davis and Squire 1984).
Although the molecular mechanisms of fear memory extinction have attracted growing interest in recent years, the molecular pathways underlying fear memory extinction remain largely unknown. To investigate the time course of molecular processes underlying the extinction of fear memory, we studied the effect of retrograde and anterograde inhibition of LVGCC, with respect to the extinction training. Using injection of the selective dihydropyridine (DHP) LVGCC antagonist nifedipine to selectively block the fear memory extinction in mice (Cain et al. 2002), we found that the effect of nifedipine on extinction extends up to 4 h after application. Our additional findings point out the importance of the peripheral effects of the DHP antagonist nifedipine, most importantly the induction of a stress response upon its injection, in selective interference with memory extinction.
Results
Nifedipine does not affect acquisition of fear conditioning
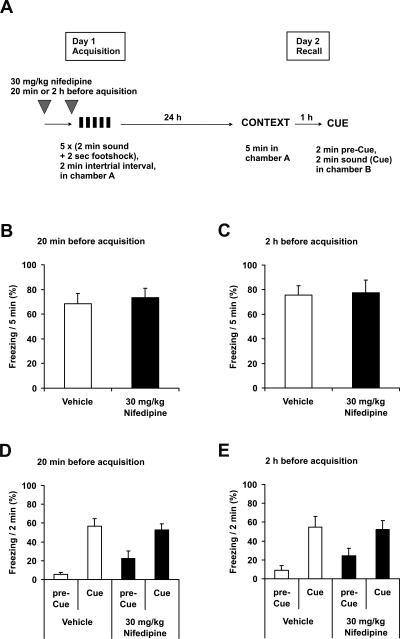
We first asked whether nifedipine injection interferes with the acquisition of fear memory. The initial study of Cain et al. (2002) had used 40–80 mg/kg of the DHP antagonist nifedipine for subcutaneous (s.c.) injection, without reporting side effects. These doses, however, led to significant mice mortality in our hands (data not shown). 30 mg/kg nifedipine s.c. was not associated with any mortality and was therefore used in the following experiments. The injection of 30 mg/kg nifedipine s.c. 20 min before conditioning did not affect the acquisition of either contextual or auditory cue fear conditioning measured 24 h after training (Fig. 1B,D). We repeated this experiment with injection of nifedipine s.c. 2 h before conditioning. With this prolonged interval, both the recall of acquisition and the freezing during acute consolidation showed similar results between nifedipine and vehicle-treated mice (Fig. 1C,E).
Figure 1.
Nifedipine does not affect acquisition of fear conditioning. (A) Schematic overview of the protocol. (B–E) Recall of fear conditioning on day 2 of the protocol. (B,D) 30 mg/kg nifedipine s.c. (n = 8) or vehicle (n = 8) were injected 20 min before conditioning. (C,E) 30 mg/kg nifedipine s.c. (n = 8) or vehicle (n = 8) were injected 2 h before conditioning. (B,C) Freezing 24 h after conditioning in context A. (D,E) Freezing 1 h later in context B (pre-Cue) and with acoustic cue (Cue). (Black symbols) Injection with nifedipine, (white symbols) injection with vehicle. Data are expressed as mean and SEM.
Nifedipine blocks extinction of fear conditioning
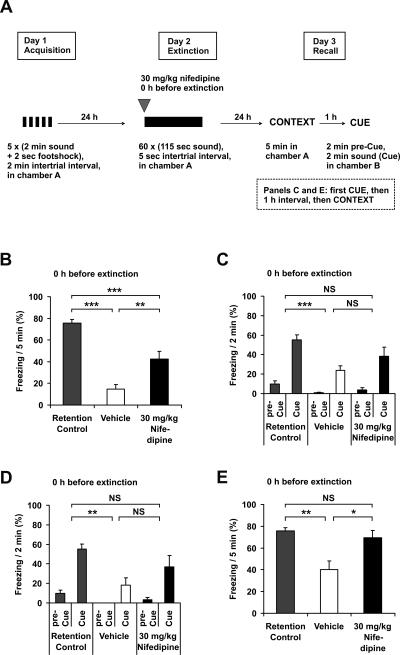
We next investigated the effect of nifedipine on extinction of fear memory. Vehicle-treated animals showed a dramatic reduction of conditioned fear after being exposed to the 2 h of extinction training, compared with retention control animals (Fig. 2B,D). The injection of 30 mg/kg nifedipine s.c. immediately before the extinction training led to a significantly reduced suppression of contextually conditioned fear. This finding supports the conclusion of the previous study by Cain et al. (2002). The extinction of auditory cue conditioned fear was also reduced, albeit to a lesser extent. Since we tested all experimental animals first for contextual and then for auditory cue conditioning, with an interval of 1 h between both sessions, we analyzed whether the order of the recall sessions had an influence on memory recall. When auditory fear conditioning was tested first (Fig. 2C), we observed a nonsignificant tendency of the reduction of extinction, similar to the situation when auditory fear conditioning was tested 1 h after the recall of contextual conditioning (Fig. 2D). When contextual conditioning was tested after the recall of auditory fear conditioning (Fig. 2E), the injection of 30 mg/kg nifedipine s.c. led to a significantly lower reduction of contextually conditioned fear, similar to the situation when contextual conditioning was tested first (Fig. 2B). However, the extinction of contextual conditioning, for both nifedipine and vehicle-treated groups, appeared less pronounced when recall of contextual conditioning was performed after recall of auditory cue conditioning (Fig. 2, cf. E and B). By contrast, there was no difference in the extent of auditory fear conditioning with respect to the order of context and cue recall (Fig. 2C,D). Thus, in all further experiments we analyzed context recall first, and then cue recall.
Figure 2.
Nifedipine blocks extinction of fear conditioning. (A) Schematic overview of the protocol. (B–E) Recall of fear conditioning on day 3 of the protocol. (B) Freezing 48 h after conditioning and 24 h after extinction in context A, with antecedent injection of 30 mg/ kg nifedipine s.c. (n = 8) or vehicle (n = 8) immediately before extinction. Retention control animals (n = 8) were in their home cages on day 2. (C) Freezing 48 h after conditioning and 24 h after extinction in context B (pre-Cue) and with acoustic cue (Cue), with antecedent injection of 30 mg/kg nifedipine s.c. (n = 8) or vehicle (n = 8) immediately before extinction. Retention control animals (n = 8) were in their home cages on day 2. (D) Freezing 1 h after (B) in context B (pre-Cue) and with acoustic cue (Cue) (n = 8, all groups). (E) Freezing 1 h after C in context A (n = 8, all groups). (Black symbols) Injection with nifedipine, (white symbols) injection with vehicle, (gray symbols) retention control. Data are expressed as mean and SEM. *P < 0.05, **P < 0.01, ***P < 0.001, (NS) not significant. (B) One-way ANOVA (F(2,21) = 32.6; P < 0.001) followed by Tukey post-hoc tests: retention control vs. vehicle P < 0.001; retention control vs. nifedipine P < 0.001; nifedipine vs. vehicle P = 0.004. (C) Two-way repeated measures ANOVA (F(2,21) = 9.8; P < 0.001) followed by Tukey post-hoc tests: retention control vs. vehicle P < 0.001; retention control vs. nifedipine P = 0.081; nifedipine vs. vehicle P = 0.105. (D) Two-way repeated measures ANOVA (F(2,21) = 8.4; P = 0.002) followed by Tukey post-hoc tests: retention control vs. vehicle P = 0.002; retention control vs. nifedipine P = 0.071; nifedipine vs. vehicle P = 0.214. (E) One-way ANOVA (F(2,21) = 8.8; P < 0.002) followed by Tukey post-hoc tests: retention control vs. vehicle P = 0.002; retention control vs. nifedipine P = 0.772; nifedipine vs. vehicle P = 0.010.
Nifedipine applied subcutaneously inhibits extinction of fear conditioning up to 4 h after injection
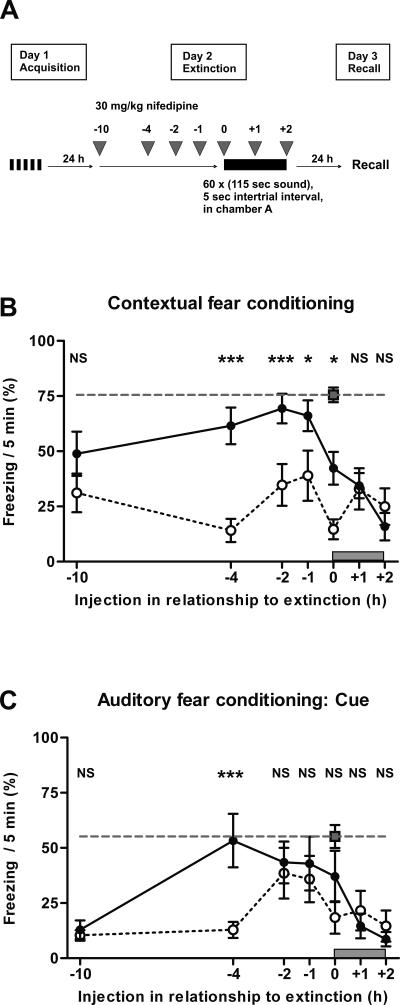
In the previous experiments, nifedipine was applied either immediately before (Fig. 2) or, as in the initial study (Cain et al. 2002), 20 min before extinction training. We now asked whether moving the time point of nifedipine injection backward or forward with respect to extinction training would change the extent of extinction of fear memory. We injected mice with 30 mg/kg nifedipine s.c. or vehicle at different time points in an interval from 10 h before up to the end of the 2-h extinction session. Injection of nifedipine during or at the end of the extinction session had no effect on extinction of both contextual and auditory cue learning, similar to vehicle (Fig. 3B,C). By contrast, when nifedipine was injected 0, 1, 2, or 4 h before the extinction session, the extinction of contextual fear conditioning was significantly impaired (Fig. 3B). For auditory fear conditioning, extinction was significantly impaired if nifedipine was injected 4 h before the extinction session (Fig. 3C). For contextual conditioning, there was still a nonsignificant tendency toward impaired extinction if nifedipine was injected even 10 h before the extinction session (Fig. 3B).
Figure 3.
Nifedipine applied subcutaneously inhibits extinction of fear conditioning up to 4 h after injection. (A) Schematic overview of the protocol. (B,C) Recall of fear conditioning on day 3 of the protocol. (B) Freezing in context A 48 h after acquisition of fear and 24 h after extinction training, with injection of 30 mg/kg nifedipine s.c. (n = 8, for each time of injection) or vehicle (n = 8, for each time of injection) before, during, or after extinction at the times indicated. Retention control animals (n = 8) were in their home cages on day 2. (C) Freezing in context B and acoustic cue 1 h later (n = 8, for all groups). (Open circles) s.c. injection with vehicle, (black circles) injection with 30 mg/kg nifedipine, (gray dashed line) retention control that was determined in parallel with the animals injected immediately before extinction training. The gray block above the X-axis represents the 2 h of extinction training. Data are expressed as mean and SEM. *P < 0.05, ***P < 0.001, (NS) not significant. The statistical information in the figure represents the P-value of nifedipine vs. vehicle for the time point indicated. (B) Two-way ANOVA (F(2,147) = 89.2; P < 0.001) followed by Tukey post-hoc tests: Injection 10 h before extinction session (injection −10 h); retention control (RC) vs. vehicle (V) P < 0.001; retention control (RC) vs. nifedipine (N) P = 0.015; nifedipine (N) vs. vehicle (V) P = 0.152. Injection −4 h; RC vs. V P < 0.001; RC vs. N P = 0.308; N vs. V P < 0.001. Injection −2 h; RC vs. V P < 0.001; RC vs. N P = 0.794; N vs. V P < 0.001. Injection −1 h; RC vs. V P < 0.001; RC vs. N P = 0.582; N vs. V P = 0.013. Injection 0 h; RC vs. V P < 0.001; RC vs. N P = 0.002; N vs. V P = 0.011. Injection 1 h; RC vs. V P < 0.001; RC vs. N P < 0.001; N vs. V P = 0.988. Injection 2 h; RC vs. V P < 0.001; RC vs. N P < 0.001; N vs. V P = 0.988. (C) Two-way ANOVA (F(2,147) = 39.4; P < 0.001) followed by Tukey post-hoc tests: Injection −10 h; RC vs. V P < 0.001; RC vs. N P < 0.001; N vs. V P = 0.968. Injection −4 h; RC vs. V P < 0.001; RC vs. N P = 0.984; N vs. V P < 0.001. Injection −2 h; RC vs. V P = 0.037; RC vs. N P = 0.513; N vs. V P = 363. Injection −1 h; RC vs. V P = 0.166; RC vs. N P = 0.483; N vs. V P = 0.787. Injection 0 h; RC vs. V P = 0.002; RC vs. N P = 0.207; N vs. V P = 0.188. Injection 1 h; RC vs. V P < 0.001; RC vs. N P = 0.005; N vs. V P = 0.971. Injection 2 h; RC vs. V P < 0.001; RC vs. N P < 0.001; N vs. V P = 0.842.
Nifedipine inhibits extinction in a dose-dependent manner
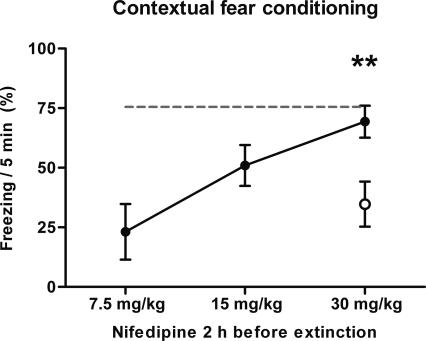
The s.c. injection of 30 mg/kg nifedipine 2 h before the extinction session resulted in significantly reduced extinction of contextual conditioning, as shown before (Fig. 3B). Doses of 7.5 mg/kg and 15 mg/kg nifedipine s.c. produced a less pronounced reduction of extinction, suggesting a dose-dependent correlation (Fig. 4).
Figure 4.
Nifedipine inhibits extinction in a dose-dependent manner. Freezing in context A 48 h after acquisition of fear and 24 h after extinction training, with antecedent injection of nifedipine or vehicle 2 h before extinction. (Open circle) s.c. injection with vehicle; (black circles) injection with 7.5 mg/kg, 15 mg/kg, or 30 mg/kg nifedipine (n = 8, for all groups); (gray dashed line) retention control that was determined in the previous experiment (Fig. 3). Data are expressed as mean and SEM. **P < 0.01. The statistical information in the figure represents the P-value of nifedipine vs. vehicle for the time point indicated. One-way ANOVA (F(2,21) = 9.9; P < 0.001) followed by Tukey post-hoc tests: retention control vs. vehicle P < 0.001; retention control vs. nifedipine P = 0.806; nifedipine vs. vehicle P = 0.006.
Acute effects of nifedipine on acquisition and extinction
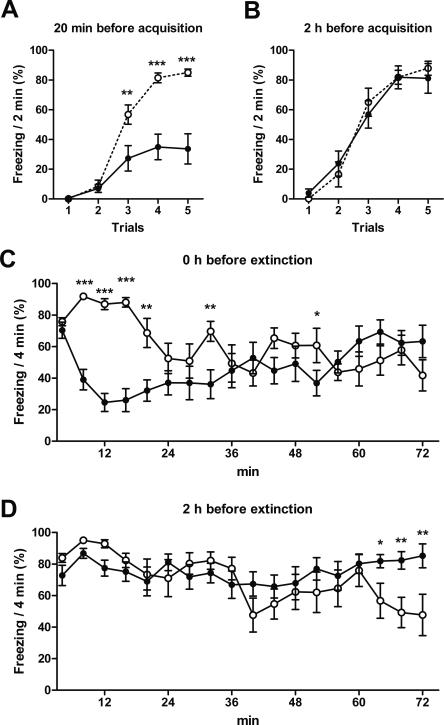
The previous data have shown only the effects of nifedipine on the recall of memory at least 24 h after fear memory conditioning or its extinction. We next analyzed the acute effects of 30 mg/kg nifedipine s.c. during these experiments (Figs. 1, 3). During the first 30 min after injection, nifedipine caused a strong suppression of freezing (Fig. 5A,C). Similar to the recall of acquisition (Fig. 1), acute acquisition was not affected by injection of 30 mg/kg nifedipine 2 h before the extinction session (Fig. 5B). And, similar to the recall of extinction (Fig. 3), acute extinction was impaired after nifedipine injection (Fig. 5D).
Figure 5.
Acute effects of nifedipine on acquisition and extinction. The panels show the acute acquisition and acute extinction measured during the previous experiments (Figs. 1, 3). (A,B) Acute acquisition 20 min and 2 h after s.c. injection of 30 mg/kg nifedipine or vehicle (Fig. 1). One trial has 2-min length; the intertrial interval is 2 min. (C,D) Acute extinction immediately and 2 h after s.c. injection of 30 mg/kg nifedipine or vehicle (Fig. 3). (Black circles) Injection with nifedipine, (open circles) injection with vehicle. Data are expressed as mean and SEM. *P < 0.05, **P < 0.01, ***P < 0.001. For reasons of clarity, nonsignificant Tukey post-hoc tests are unlabeled in this figure. The data of all significant Tukey post-hoc tests are described in the following. (A) Two-way repeated measures ANOVA (F(1,56) = 16.7; P < 0.001) followed by Tukey post-hoc tests: trial 3 P = 0.001; trial 4 P < 0.001; trial 5 P < 0.001. (B) Two-way repeated measures ANOVA (F(1,56) = 0.01; P = 0.919) followed by Tukey post-hoc tests. (C) Two-way repeated measures ANOVA (treatment: F(1,238) = 5.0; P < 0.041; interaction of treatment and time point: F(17,238) = 6.9; P < 0.001) followed by Tukey post-hoc tests: 4–8 min P < 0.001; 8–12 min P < 0.001; 12–16 min P < 0.001; 16–20 min P = 0.003; 28–32 min P = 0.005; 48–52 min P = 0.045. (D) Two-way repeated measures ANOVA (treatment: F(1,238) = 0.625; P = 0.443; interaction of treatment and time point: F(17,238) = 2.69; P < 0.001) followed by Tukey post-hoc tests: 60–64 min P = 0.033; 64–68 min P = 0.005; 68–72 min P = 0.002.
Pharmacokinetics of nifedipine after subcutaneous and intraperitoneal injection
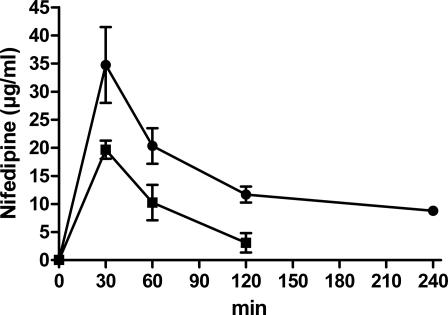
As the previous experiments have demonstrated a protracted effect of nifedipine on extinction (Fig. 3), we asked whether this effect was caused by prolonged pharmacokinetics of the substance or by other secondary mechanisms. The concentration of nifedipine in serum can be determined by high-performance liquid chromatography (HPLC). Because of easy permeability of nifedipine through the blood–brain barrier, the pharmacokinetics of nifedipine in the blood and brain are very similar (Larkin et al. 1992). We injected mice with 30 mg/kg nifedipine by s.c. application and measured the nifedipine concentration in serum at the times indicated (Fig. 6, round symbols). Nifedipine concentration in serum reaches its peak within the first 30 min after injection. Despite the described half-life of nifedipine of 7.5 min in serum (Uchida et al. 1997), the actual serum concentration upon s.c injection decreases slowly.
Figure 6.
Pharmacokinetics of nifedipine after s.c. and i.p. injection. 30 mg/kg nifedipine was injected s.c. or 15 mg/kg nifedipine i.p.. Mice were sacrificed at the times indicated (n = 3, for all groups), and blood was collected. The graph shows the concentration of nifedipine that was determined from 200 μL of serum. (Black circles) Injection with 30 mg/kg nifedipine s.c., (black squares) injection with 15 mg/kg nifedipine i.p. Data are expressed as mean and SEM.
In a previous study, injection of 6 mg/kg nifedipine by intraperitoneal (i.p.) application in mice resulted in a peak of the concentration ∼10 min after injection. Thirty minutes after i.p. injection, the concentration had dramatically decreased in a logarithmic fashion, and 120 min after injection, it was almost undetectable (Larkin et al. 1992). The kinetics of the lipophilic substance 3,4-benzpyrene in mouse blood after s.c. injection in an oily solution was about 10 times slower than after i.p. injection (Berenblum and Schoental 1942). Our injection vehicle (10% cremophor–90% PBS) contained an oily solution and was injected into the subcutaneous fat. We therefore asked whether the protracted effect of nifedipine on extinction upon s.c injection could be due to such a depot effect.
Injection of 30 mg/kg nifedipine i.p. resulted in significant mice mortality (data not shown). Thus, we injected 15 mg/kg nifedipine i.p. and measured the serum concentration by HPLC (Fig. 6, square symbols). Similar to s.c. injection, nifedipine concentration reaches its peak within 30 min. This concentration, however, decreases faster compared with s.c. injection and was almost at detection limits 120 min after i.p. injection.
Intraperitoneal injection of nifedipine blocks extinction of fear conditioning, similar to application by s.c. injection
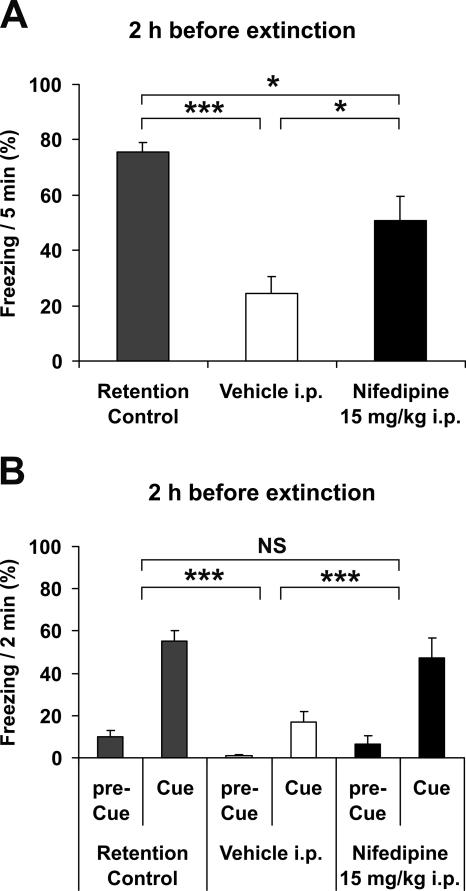
We next examined whether 15 mg/kg nifedipine injected i.p. 2 h before the extinction session will still affect extinction. Under these conditions, nifedipine in serum was almost undetectable at the moment of extinction training, as demonstrated previously (Fig. 6). However, injection of 15 mg/kg nifedipine i.p. 2 h before the extinction session still significantly blocked the extinction of both contextual conditioning (Fig. 7A) and auditory fear conditioning (Fig. 7B).
Figure 7.
Intraperitoneal injection of nifedipine blocks extinction of fear conditioning, similar to application by s.c. injection. (A) Freezing in context A 48 h after acquisition of fear and 24 h after extinction training, with antecedent injection of 15 mg/kg nifedipine i.p. (n = 8) or vehicle (n = 8) 2 h before extinction training. Retention control animals (n = 8) were in their home cages on day 2. (B) Freezing 1 h later in context B (pre-Cue) and with acoustic cue (Cue) (n = 8, for all groups). (Black bars) Injection with nifedipine, (open bars) injection with vehicle, (gray bars) retention control. Data are expressed as mean and SEM. *P < 0.05, ***P < 0.001, (NS) not significant. (A) One-way ANOVA (F(2,21) = 16.4; P < 0.001) followed by Tukey post-hoc tests: retention control vs. vehicle P < 0.001; retention control vs. nifedipine P = 0.030; nifedipine vs. vehicle P = 0.020. (B) Two-way repeated measures ANOVA (F(2,21) = 9.4; P = 0.001) followed by Tukey post-hoc tests: retention control vs. vehicle P < 0.001; retention control vs. nifedipine P = 0.029; nifedipine vs. vehicle P < 0.001.
Locomotor activity is reduced after nifedipine injection
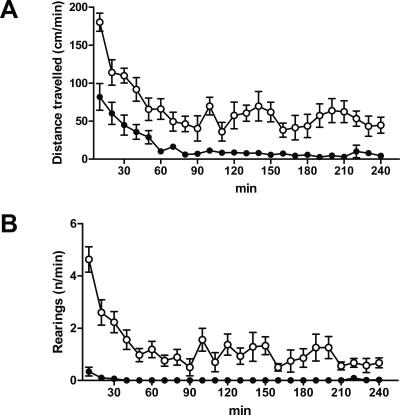
Nifedipine relaxes the smooth muscles of small arteries and thus reduces the arterial blood pressure. It is therefore used as an antihypertensive drug in humans (Motro et al. 2001). An overdose of nifedipine in humans results in severe arterial hypotonia and bradycardia and can cause death (DeWitt and Waksman 2004). Even though the pharmacokinetics and drug effects of nifedipine in humans and mice are not directly comparable, a dose of 30 mg/kg in one injection as used in our experiments is substantially higher than the maximal therapeutic dose in humans, which rarely exceeds 1 mg/kg per day. Hypotonia causes reduced locomotor activity. We used two paradigms to indirectly test the hypotensive effect of 30 mg/kg nifedipine after s.c. application in mice. The previous study had observed reduced locomotion after 16 mg/kg s.c. nimodipine injection, but not after 40 mg/kg s.c. nifedipine (Cain et al. 2002). We injected mice with 30 mg/kg nifedipine s.c. and measured the locomotor activity in the open field. Whereas after vehicle injection the animals showed locomotor activity during the whole 240 min of observation, nifedipine-treated animals traveled a continuously reduced distance during the same period. (Fig. 8A). Rearings to the ceiling, which are part of normal mouse exploratory behavior, are an even more sensitive indicator of low blood pressure. Whereas vehicle-treated animals demonstrated continuous rearing activity over the whole observed period, nifedipine-injected mice did not perform any rearings between 40 and 210 min after application of the drug (Fig. 8B).
Figure 8.
Locomotor activity is reduced after nifedipine injection. 30 mg/kg nifedipine (n = 8) or vehicle (n = 8) were injected by s.c. application. Five minutes later, the animals were analyzed for 240 min in an open field. (A) Distance traveled, (B) rearings. (Open circles) s.c. injection with vehicle, (black circles) injection with 30 mg/kg nifedipine. No animal died during or within 24 h after the experiment. Data are expressed as mean and SEM. (A) Two-way repeated measures ANOVA (F(1,299) = 50.3; P < 0.001). (B) Two-way repeated measures ANOVA (F(1,299) = 190.4; P < 0.001).
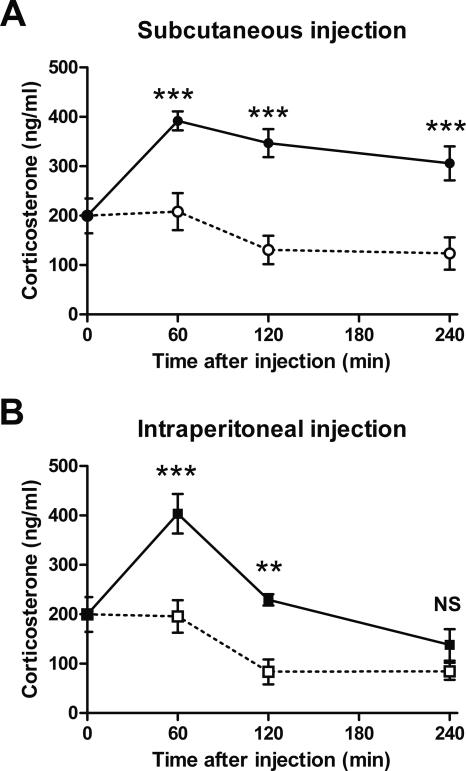
Nifedipine induces up-regulation of corticosterone
Injection of 30 mg/kg nifedipine s.c. results in significant reduction of locomotor activity and rearing, which is presumably caused by the peripheral, antihypertensive effects of the drug (Fig. 8). The combination of severe arterial hypotonia and bradycardia, as is the effect of an overdose of nifedipine, causes cardiovascular shock and, ultimately, death (DeWitt and Waksman 2004). Thus, injection of 30 mg/kg nifedipine s.c. may be a strong stressor for a mouse, despite its anxiolytic effects (Mogilnicka et al. 1988; Sinnegger-Brauns et al. 2004). Corticosterone is released in rodents from the adrenal glands during the stress response (Selye 1950). To investigate the induction of stress, we injected mice, during morning times, with 30 mg/kg nifedipine s.c., 15 mg/kg nifedipine i.p., and vehicle and measured the corticosterone response in serum at the times indicated (Fig. 9). From 60 to 240 min after injection of 30 mg/kg nifedipine s.c., the corticosterone response was strongly elevated in comparison to s.c. vehicle injection (Fig. 9A). From 60 to 120 min after injection of 15 mg/kg nifedipine i.p., the corticosterone response was similarly significantly elevated in comparison to injection of vehicle i.p. (Fig. 9B). Thus, nifedipine injections as applied here to inhibit the extinction of fear memory are associated with a strong stress response.
Figure 9.
Nifedipine induces up-regulation of corticosterone. 30 mg/kg nifedipine s.c., vehicle s.c., 15 mg/kg nifedipine i.p., and vehicle i.p. (n = 6, for all groups) were applied at 9 a.m. by the indicated type of injection. Mice were sacrificed at the times indicated, and full blood was collected. The graph shows the concentration of corticosterone that was determined from 200 μL of serum. (Open symbols) Injection with vehicle, (black symbols) injection with nifedipine, (circles) s.c. injection, (squares) i.p. injection. (A) Corticosterone response after subcutaneous injection of 30 mg/kg nifedipine. (B) Corticosterone response after intraperitoneal injection of 15 mg/kg nifedipine. Data are expressed as mean and SEM. **P < 0.01, ***P < 0.001, (NS) not significant. (A) Two-way ANOVA (F(1,30) = 59.9; P < 0.001) followed by Tukey post-hoc tests: 60 min after injection P < 0.001; 120 min after injection P < 0.001; 240 min after injection P < 0.001. (B) Two-way ANOVA (F(1,30) = 32.6; P < 0.001) followed by Tukey post-hoc tests: 60 min after injection P < 0.001; 120 min after injection P = 0.002; 240 min after injection P = 0.215.
Discussion
We found that anterograde application of 30 mg/kg nifedipine s.c. at least up to 4 h before an extinction session selectively inhibits extinction of fear memory. Since the removal of nifedipine from serum after i.p. application was faster in comparison to s.c. injection, we tested fear memory extinction after i.p injection of nifedipine. Two h after injection of 15 mg/kg nifedipine i.p., nifedipine was almost washed out from the serum, but extinction of fear conditioning was still significantly inhibited. Application of 30 mg/kg nifedipine strongly inhibited locomotor activity and rearings of the animals, suggesting substantial cardiovascular effects. Finally, 30 mg/kg nifedipine s.c. induced up-regulation of serum corticosterone for at least 4 h; 15 mg/kg nifedipine i.p. induced up-regulation of corticosterone for at least 2 h.
Our findings confirm the result of the previous study (Cain et al. 2002), that s.c. injection of nifedipine selectively inhibits the extinction of fear conditioning, whereas the acquisition of fear memory is undisturbed (Figs. 1, 2). These findings also correspond with a third study (Suzuki et al. 2004) that reproduced the findings of Cain et al. (2002), demonstrating that another LVGCC DHP antagonist, nimodipine, inhibits extinction of fear conditioning. The use of different substrains of C57BL/6 mice may explain the differences in mortality after injection of nifedipine injection and the extent of auditory cue conditioning between the study by Cain et al. (2002) and our findings. As was also shown in the previous study, we observed here an acute suppression of freezing by nifedipine during acquisition training (Fig. 5). The reason for this acute suppression of freezing could be anxiolytic effects of nifedipine (Mogilnicka et al. 1988, Sinnegger-Brauns et al. 2004). However, the effect observed by us was stronger than in the previous study and was also seen during extinction training. In addition, the effect of nifedipine on acute extinction was less pronounced in our experiments. An explanation for these differences could be, in addition to strain distinctions, that we used for our study an automatic and computer-based system for movement analysis, whereas the previous study made use of traditional and investigator-based assessment of videotapes.
The half-life of nifedipine in mouse blood is 7.5 min (Uchida et al. 1997), and the kinetics of nifedipine in serum and brain are very similar (Larkin et al. 1992), due to easy passage through the blood–brain barrier. The clearance of nifedipine after i.p. injection was faster than after s.c. injection (Fig. 6). The kinetics of 3,4-benzpyrene in mouse blood after s.c. injection of an oily solution was about 10 times longer than after i.p. injection (Berenblum and Schoental 1942). Therefore, it may be possible that the prolonged action of nifedipine after s.c. injection was caused by extended action of the higher dose combined with an accumulation of the drug in the subcutaneous fat. Regardless of the mechanism underlying the pharmacokinetics, 240 min after 30 mg/kg nifedipine s.c., the serum concentration was less than one-third of the initial serum concentration, and 120 min after 15 mg/kg nifedipine i.p., the serum concentration was almost undetectable (Fig. 6). Under these conditions, the extinction of fear memory was still significantly blocked, over a period of 2 h of extinction training that started at these time points (Figs. 3, 4, 7). It is thus implausible that the inhibition of fear memory extinction can exclusively be explained by an effect of nifedipine on LVGCC in the brain.
LVGCC are not restricted to the brain; they are also found in heart, arteries, skeletal muscle, and neuroendocrine cells. In fact, the most important application of nifedipine is its use as an antihypertensive medication in human medicine. We therefore asked whether the observed reduction of extinction may have been caused by nifedipine acting on LVGCC outside of the brain. We did not measure the blood pressure directly, but the severe effects of 30 mg/kg nifedipine s.c. on locomotor activity and especially on rearings (Fig. 8) render such a hypotensive effect very likely. Substantial arterial hypotonia is a strong biological stressor. Irrespective of the detailed understanding of the mechanism of nifedipine action on locomotion, the corticosterone response, which is an indicator of the stress reaction (Selye 1950), was significantly elevated 240 min after 30 mg/kg nifedipine s.c. and 120 min after 15 mg/kg nifedipine i.p. (Fig. 9).
Can a strong stress reaction be the actual reason for why the acquisition of fear conditioning was undisturbed, but the extinction of fear memory was inhibited after injection of the LVGCC antagonist? There is evidence from both human and animal model studies that acute or chronic stress inhibits the extinction of fear memories, which is seen as a major psychopathological mechanism in post-traumatic stress disorder (PTSD) (Akirav and Maroun 2007). For example, the extinction of an autonomic response to newly learned auditory tones was slower in crime victims with PTSD (Rothbaum et al. 2001). In rats, stress did not affect the acquisition of conditioned fear, but increased freezing was observed on the second day of extinction training (Miracle et al. 2006). Finally, brief uncontrollable stress impaired the extinction, but not the acquisition, of fear memory in mice (Izquierdo et al. 2006).
The role of LVGCC in learning and memory has also been studied in mice with genetic ablation of Cav1.2 and Cav1.3. The study by Moosmang et al. (2005) has analyzed spatial memory in mice with an inactivation of Cav1.2 in the hippocampus and neocortex. They found impaired performance in the Morris water maze, a task that requires function of the hippocampus. McKinney and Murphy (2006) studied fear conditioning including extinction in conventional Cav1.3 knockout mice. They reported impairment of the consolidation of fear conditioning, but not of the extinction of the fear memory trace. However, this study does not distinguish the different biological roles of LVGCC inside and outside of the brain.
In summary, we show here that the stress response may significantly contribute to the specific inhibition of extinction by nifedipine. It is possible that a specific effect of nifedipine by blockade of brain LVGCC on extinction was still present in our experiments, but was superimposed by the stress effect. Our data support the growing evidence that stress specifically inhibits the extinction of fear. In addition, our data indicate an important limitation of the pharmacological approach in the study of the role of brain LVGCC on memory extinction. Further research, notably using conditional and tissue-specific manipulations of LVGCC in animal models, is necessary to delineate the role of LVGCC on fear memory extinction and other brain functions.
Materials and Methods
Animals
Naïve 12- to 18-wk-old C57BL/6 male mice (Charles River) were housed in single cages, maintained on a reversed 12-h light/dark schedule, and were allowed access to food and water ad libitum. All testing was conducted during the dark phase.
Preparation of nifedipine
Solutions were always freshly prepared before injection. The LVSCC antagonist nifedipine (Sigma) was sonicated into 100% Cremophor EL (Fluka) at 80 mg/mL. PBS was added to prepare the final injection vehicle (10% Cremophor–90% PBS). Mice were injected s.c. (subcutaneously) or i.p. (intraperitoneally) as indicated.
Conditioning system
All testing took place in a sound- and light-protected isolation cubicle (Habitest H10-24TA, Coulbourn Instruments). The 0.7-mA scrambled footshock was delivered from a precision animal shocker (H13-15, Coulbourn Instruments). The sound stimulus was a 5000-Hz, 80- to 85-dB sinus tone, and was generated with a conventional sound card, amplified with a HiFi amplifier (PR530A, Pyramid) and delivered via speakers in the chamber walls of two different chambers (contexts A and B). Context A had two transparent walls, stainless steel grid floors, and was 17 cm × 18 cm × 32 cm in size (H10-11M-TC, Coulbourn Instruments). Context B had four transparent walls, stainless steel grid floors, and was 30 cm × 25 cm × 25 cm in size (Med Associates). The hardware was operated from a personal computer equipped with FreezeFrame software (Actimetrics Software) and an IMAQ-A6822 interface card (National Instruments). The movements of the tested animal were recorded with a digital video camera mounted at the ceiling of the cubicle and analyzed for the percentage of freezing using FreezeView software (Actimetrics Software). In all experiments, the virtual threshold for freezing was set to 10.
Fear conditioning
The protocol was designed according to a previously published study (Cain et al. 2002). The following passages summarize the experimental procedure in the order of the figures. For schematic descriptions, see also in Figures 1A, 2A and 3A.
Influence of nifedipine s.c. on acquisition of fear conditioning (Fig. 1)
Day 1 (acquisition): 30 mg/kg nifedipine or vehicle were applied by s.c. injection. Two hours or 20 min later, mice were placed in context A. Conditional fear was induced by presenting five pairings of 2-min sound stimulus which co-terminated with 2-sec foot shocks. Two minutes of stimulus-free periods preceded, separated, and followed the pairings. Day 2 (recall): 24 h later, mice were placed in context A to measure context conditioning. Freezing was analyzed for 5 min. The mice were then set back into their home cages for 60 min. For measurement of auditory conditioning, mice were placed in context B. After 2 min of acclimatization, the sound stimulus was presented for 2 min. Freezing was recorded during both periods (pre-cue, cue).
Influence of nifedipine s.c. on extinction of fear conditioning, and influence of the order of context and cue recording during recall (Fig. 2)
Day 1 (acquisition): Mice were placed in context A. Conditional fear was induced by presenting five pairings of 2-min sound stimulus which co-terminated with 2-sec foot shocks. Two minutes of stimulus-free periods preceded, separated, and followed the pairings. Day 2 (extinction): 24 h later, 30 mg/kg nifedipine or vehicle were applied by s.c. injection. Two hours later, mice were again placed in context A. Extinction was induced by 60 presentations of 115-sec sound stimulus with 5-sec intertrial intervals. Mice that did not undergo extinction training (retention control) did not receive any treatment on the second day of the protocol and stayed in their home cages. Day 3 (recall): Version 1. 24 h later, mice were placed in context A to measure context conditioning. Freezing was analyzed for 5 min. The mice were then set back into their home cages for 60 min. For measurement of auditory conditioning, mice were placed in context B. After 2 min of acclimatization, the sound stimulus was presented for 2 min. Freezing was recorded during both periods. Version 2. 24 h later, mice were placed in context B to measure auditory conditioning. After 2 min of acclimatization, the sound stimulus was presented for 2 min. Freezing was recorded during both periods. The mice were then set back into their home cages for 60 min. For measurement of context conditioning, mice were placed in context A. Freezing was analyzed for 5 min.
Influence of various time points of subcutaneous nifedipine injection on extinction of fear conditioning (Fig. 3)
Day 1 (acquisition): Mice were placed in context A. Conditional fear was induced by presenting five pairings of 2-min sound stimulus which co-terminated with 2-sec foot shocks. Two minutes of stimulus-free periods preceded, separated, and followed the pairings. Day 2 (extinction): 24 h later, 30 mg/kg nifedipine or vehicle were applied by s.c. injection. 10, 4, 2, or 1 h later or immediately after injection, mice were again placed in context A. Extinction was induced by 60 presentations of 115-sec sound stimulus with 5-sec intertrial intervals. In one experiment, mice were taken out of context A, and 30 mg/kg nifedipine or vehicle were applied by s.c. injection in the middle of the training. The mice were then set back into context A. In another experiment, 30 mg/kg nifedipine or vehicle was applied by s.c. injection immediately after the extinction training. Nonextinguished mice (retention control) did not receive any treatment on the second day of the protocol and stayed in their home cages. Day 3 (recall): 24 h later, mice were placed in context A to measure context conditioning. Freezing was analyzed for 5 min. The mice were then set back into their home cages for 60 min. For measurement of auditory conditioning, mice were placed in context B. After 2 min of acclimatization, and the sound stimulus was presented for 2 min. Freezing was recorded during both periods.
Dose dependence of nifedipine s.c. on extinction of fear conditioning (Fig. 4)
The protocol was similar to the protocol described for Figure 2, with two exceptions: Doses of 7.5, 15, and 30 mg/kg nifedipine or vehicle were used (day 2), and only version 1 was used during recall (day 3).
Influence of nifedipine i.p. on extinction of fear conditioning (Fig. 7)
The protocol was similar to the protocol described for Figure 2, with two exceptions: Instead of 30 mg/kg nifedipine by s.c. application, 15 mg/kg nifedipine or vehicle were applied by i.p. injection (day 2), and only version 1 was used during recall (day 3).
Nifedipine pharmacokinetics (Fig. 6)
Mice were sacrificed by decapitation, and blood was collected in a 50-mL tube. At least 200 μL of serum was obtained after centrifugation and was stored at −20°C. The high-performance liquid chromatography (HPLC) for nifedipine was performed at a commercial clinical laboratory (Dr. Eberhardt und Partner, Dortmund, Germany). All procedures were performed under sodium light.
Locomotor activity (Fig. 8)
Eight equal chambers (48 cm × 48 cm × 40 cm in size) had dark floors and transparent walls. The chambers were surrounded at all sidewalls, at 1 cm and 6 cm above the floor, with a photobeam sensor ring, which detected movements on the floor level and rearings (TruScan, Coulbourn Instruments). Locomotor activity was monitored for 4 h, and signals were analyzed using TruScan software on a personal computer.
Corticosterone response (Fig. 9)
Mice were sacrificed by decapitation, and blood was collected in a 50-mL tube. At least 200 μL of serum was obtained and was stored at −20°C. Corticosterone was measured by a specific in–house radioimmunoassay (RIA) established at the Steroid Laboratory of the Department of Pharmacology as previously described (Bielohuby et al. 2007). Before RIA, a recovery-corrected extraction was performed. Intra-assay and inter-assay coefficients of variance were <10% and <15%, respectively.
Statistical analysis and presentation of data
Statistical analysis was performed with SigmaStat software (Systat Software) as indicated in the figure legends. Differences were considered statistically significant if P < 0.05. Graphical artwork was conducted with Excel (Microsoft Corporation), Prism (GraphPad Software), and CorelDraw software (Corel Corporation).
Acknowledgments
We thank Hans-Joachim Krause and Heinz Bussemas for excellent technical support, Dr. Michael Deuschle and Dr. Jörg Striessnig for valuable discussions, and Dr. Miriam Schneider for statistical advice. This research was supported by a Deutsche Forschungsgemeinschaft (DFG) collaborative research grant (SFB 636), EU IP Integrated technologies for in-vivo molecular imaging (LSHG-CT-2003-503259), and EU Marie Curie Research Training Networks CavNET (MRTN-CT-2006-035367) to D.B.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.808608.
References
- Akirav I., Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: Basic insight to clinical promise. Curr. Opin. Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Schoental R. The rate of disappearance of 3:4-benzpyrene from the mouse after subcutaneous and intraperitoneal injection. Biochem. J. 1942;36:92–97. doi: 10.1042/bj0360092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielohuby M., Herbach N., Wanke R., Maser-Gluth C., Beuschlein F., Wolf E., Hoeflich A. Growth analysis of the mouse adrenal gland from weaning to adulthood: Time- and gender-dependent alterations of cell size and number in the cortical compartment. Am. J. Physiol. Endocrinol. Metab. 2007;293:E139–E146. doi: 10.1152/ajpendo.00705.2006. [DOI] [PubMed] [Google Scholar]
- Cain C.K., Blouin A.M., Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J. Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H.P., Squire L.R. Protein synthesis and memory: A review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- DeWitt C.R., Waksman J.C. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol. Rev. 2004;23:223–238. doi: 10.2165/00139709-200423040-00003. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Poulos A.M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Flexner J.B., Flexner L.B., Stellar E. Memory in mice as affected by intracerebral puromycin. Science. 1963;260:57–59. doi: 10.1126/science.141.3575.57. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Wellman C.L., Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J.G., Thompson G.G., Scobie G., Forrest G., Drennan J.E., Brodie M.J. Dihydropyridine calcium antagonists in mice: Blood and brain pharmacokinetics and efficacy against pentylenetetrazol seizures. Epilepsia. 1992;33:760–769. doi: 10.1111/j.1528-1157.1992.tb02358.x. [DOI] [PubMed] [Google Scholar]
- McKinney B.C., Murphy G.G. The L-type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn. Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B., Squire L.R., Kandel E.R. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Miracle A.D., Brace M.F., Huyck K.D., Singler S.A., Wellman C.L. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learn. Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mogilnicka E., Czyrak A., Maj J. BAY K 8644 enhances immobility in the mouse behavioral despair test, an effect blocked by nifedipine. Eur. J. Pharmacol. 1988;151:307–311. doi: 10.1016/0014-2999(88)90813-8. [DOI] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motro M., Shemesh J., Grossman E. Coronary benefits of calcium antagonist therapy for patients with hypertension. Curr. Opin. Cardiol. 2001;16:349–355. doi: 10.1097/00001573-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Rothbaum B.O., Kozak M.J., Foa E.B., Whitaker D.J. Posttraumatic stress disorder in rape victims: Autonomic habituation to auditory stimuli. J. Trauma. Stress. 2001;14:283–293. doi: 10.1023/A:1011160800958. [DOI] [PubMed] [Google Scholar]
- Selye H. The physiology and pathology of exposure to stress. ACTA Medical Publishers; Montreal: 1950. [Google Scholar]
- Sinnegger-Brauns M.J., Hetzenauer A., Huber I.G., Renström E., Wietzorrek G., Berjukov S., Cavalli M., Walter D., Koschak A., Waldschütz R., et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J. Clin. Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Koschak A., Sinnegger-Brauns M.J., Hetzenauer A., Nguyen N.K., Busquet P., Pelster G., Singewald N. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem. Soc. Trans. 2006;34:903–909. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Josselyn S.A., Frankland P.W., Masushige S., Silva A.J., Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Yamada S., Nagai K., Deguchi Y., Kimura R. Brain pharmacokinetics and in vivo receptor binding of 1,4-dihydropyridine calcium channel antagonists. Life Sci. 1997;61:2083–2090. doi: 10.1016/s0024-3205(97)00881-3. [DOI] [PubMed] [Google Scholar]