Abstract
Dihydropyridine (DHP) L-type Ca2+ channel (LTCC) antagonists, such as nifedipine, have been reported to impair the extinction of conditioned fear without interfering with its acquisition. Identification of the LTCC isoforms mediating this DHP effect is an essential basis to reveal their role as potential drug targets for the treatment of specific anxiety disorders. CaV1.2 and CaV1.3 are the predominant LTCCs in the mammalian brain. However, since no isoform-selective DHP blockers are available, their individual contribution to fear memory extinction is unknown. We used a novel mouse model expressing DHP-insensitive CaV1.2 LTCCs (CaV1.2DHP−/− mice) to address this question. In line with previous studies, wild-type (WT) mice treated with systemic nifedipine displayed markedly impaired fear extinction. This DHP effect was completely abolished in CaV1.2DHP−/− mice, indicating that it is mediated by CaV1.2, but not by CaV1.3 LTCCs. Supporting this conclusion, CaV1.3-deficient mice (CaV1.3−/−) showed extinction identical to the respective WT mice. The inhibition of fear extinction was not observed after intracerebroventricular (i.c.v.) application of different doses of nifedipine, suggesting that this effect is secondary to inhibition of peripheral CaV1.2 channels. The LTCC activator BayK, which lacks neurotoxic effects in CaV1.2DHP−/− mice, did not influence the extinction time course. In summary, we demonstrate that LTCC signaling through the CaV1.2 isoform of LTCCs interferes with fear memory extinction, presumably via a peripherally mediated mechanism. Activation of other LTCC isoforms (predominantly CaV1.3) is not sufficient to accelerate extinction of conditioned fear in mice.
Reduced ability to extinguish intense fear memories is a clinically relevant problem in various anxiety disorders, e.g., post-traumatic stress disorder. Despite effective extinction-based therapies, a considerable part of the patients fail to complete therapy and/or relapse after successful completion. Therefore, drugs promoting fear extinction could represent a novel therapeutic strategy to treat anxiety disorders (Ressler et al. 2002; Richardson et al. 2004; Ledgerwood et al. 2005; Garakani et al. 2006; Hofmann et al. 2006). However, this pursuit requires a more detailed understanding of neuronal signaling events underlying fear memory extinction.
The molecular basis of fear conditioning and its extinction have been investigated, but extinction research still lags considerably behind fear acquisition research (Fendt and Fanselow 1999; Blair et al. 2001; Maren 2005; Sotres-Bayon et al. 2006; Myers and Davis 2007). The extinction of fear is thought to involve new learning of an inhibitory signal that competes with the previously learned fear memory (Pavlov 1927; Bouton 2002). Several studies have shown that the consolidation of extinction memory depends on activation of NMDA receptors and can be promoted by administration of D-cycloserine, both in animals and humans (Falls et al. 1992; Baker and Azorlosa 1996; Lee and Kim 1998; Walker et al. 2002; Ledgerwood et al. 2005; Hofmann et al. 2006). Interestingly, the acquisition of fear extinction is unaffected by systemic injection of the NMDA receptor antagonist CPP (Santini et al. 2004; Suzuki et al. 2004), but is impaired by systemic administration of the L-type voltage-gated calcium channel (LTCC) antagonists nifedipine and nimodipine (Cain et al. 2002, 2005; Suzuki et al. 2004), and therefore crucially depends on LTCCs (Santini et al. 2001). LTCCs may thus represent a particularly interesting pharmacological target to interfere also with fast (within session) extinction.
CaV1.2 and CaV1.3 are the predominant LTCCs in the mammalian brain (Sinnegger-Brauns et al. 2004; Hetzenauer et al. 2006). LTCCs can modulate neuronal function by contributing to activity-dependent gene expression (Deisseroth et al. 2003; Dolmetsch 2003; Moosmang et al. 2005; Zhang et al. 2005). Knowledge about the impact of LTCC-mediated neuronal plasticity on certain neuronal key processes is still limited, despite increasing insight into the molecular basis of synapse-to-nucleus signaling (Deisseroth et al. 2003; Dolmetsch 2003). Evidence indicates that LTCC-mediated calcium entry underlies NMDAR-independent activation of MAPK and synaptic plasticity in hippocampal neurons (Thomas and Huganir 2004; Moosmang et al. 2005). Interestingly, it was shown recently that activation of the MAPK⁄ERK signaling pathway in the basolateral amygdala (BLA) underlies the acquisition of extinction of auditory fear conditioning (Herry et al. 2006). The current lack of isoform-selective modulators of LTCCs prevents dissection of the individual LTCC subtypes contributing to fear extinction. Cognition-enhancing drugs such as nefiracetam activate LTCCs (Yoshii and Watabe 1994; Yoshii et al. 1997). Therefore, and also based on the extinction-inhibiting effect of LTCC blockers, LTCC activators, like BayK 8644 (BayK), may represent a new way of promoting extinction. However, because LTCC activators induce a toxic dystonic neurobehavioral syndrome through widespread activation of LTCCs (Jinnah et al. 1999), the reliable pharmacological testing of this hypothesis was not possible.
We have recently generated two mouse models to dissect the physiological role and pharmacotherapeutic potential of CaV1.2 and CaV1.3 LTCCs, since no isoform-selective DHP blockers are available. CaV1.2DHP−/− mice express functionally normal CaV1.2 channels at normal densities, but a point mutation in the DHP-binding pocket of the CaV1.2 α1 subunit eliminates their high sensitivity to DHP channel blockers as well as activators (Sinnegger-Brauns et al. 2004). CaV1.3−/− mice lack CaV1.3 channels (Platzer et al. 2000) and express CaV1.2 channels at normal levels (Clark et al. 2003).
Using these mice, we investigated the role of CaV1.2 and CaV1.3 LTCCs in nifedipine-induced inhibition of fear memory extinction. The BayK-induced toxic neurobehavioral syndrome is mediated by CaV1.2 channels and, hence, is absent in CaV1.2DHP−/− mice. This provided us with the additional unique possibility to test whether selective activation of CaV1.3 LTCCs by BayK leads to enhanced extinction at doses previously found to result in activation of selected brain areas in this mouse model (Sinnegger-Brauns et al. 2004).
Results
Effect of BayK and nifedipine on cued and contextual fear extinction in CaV1.2DHP−/− mice
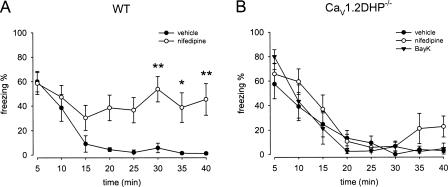
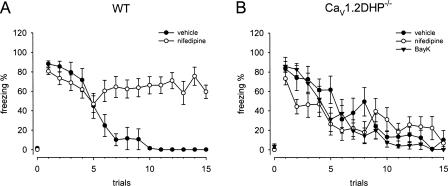
To identify individual roles of CaV1.2 and CaV1.3 LTCCs for the interference with fear extinction in mice, we studied the effects of the LTCC activator BayK and the blocker nifedipine in CaV1.2DHP−/− mice. BayK could not be tested in wild-type (WT) animals due to the neurotoxicity described above. The fear acquisition protocol involving five CS–US pairings was identical to the protocol previously used for the detailed characterization of the inhibitory DHP effects on fear memory extinction in WT mice (Cain et al. 2002). Twenty four hours after training, animals received intraperitoneal (i.p.) injections of drugs and were subjected to the extinction trial. The expression of both contextual and cued fear was indistinguishable in vehicle treated WT and CaV1.2DHP−/− mice, as indicated by an identical percentage of freezing behavior upon exposure to context A or the first 5 min of CS presentations in context B, respectively. Freezing percentages assessed during the initial 5 min of context presentation were 60% ± 8% for WT and 58% ± 11% for CaV1.2DHP−/− mice (Fig. 1). In the cued paradigm, no, or only very low levels of freezing (0%–4%, trial 0, Fig. 2) were observed during the 2-min period of habituation in context B before CS delivery. Freezing percentages assessed during the initial CS presentation were 88% ± 3% and 84% ± 11% for WT and CaV1.2DHP−/− mice, respectively (Fig. 2). Pretreatment of CaV1.2DHP−/− mice with BayK (4 mg/kg) or nifedipine (40 mg/kg) had no significant effect on fear expression in either contextual or cued paradigm (Figs. 1B, 2B).
Figure 1.
CaV1.2 LTCCs modulate contextual fear memory extinction. (A) Nifedipine (40 mg/kg) blocked extinction of contextual fear in WT mice (n = 6–7). (B) Nifedipine and BayK (4 mg/kg) had no effect on extinction in CaV1.2DHP−/− mice (n = 7). Data are presented as mean ± SEM. Statistical analysis was performed using 2-way ANOVA, *P < 0.05, **P < 0.01.
Figure 2.
CaV1.2 LTCCs modulate cued fear memory extinction. (A) Nifedipine (40 mg/kg) blocked extinction of cued fear in WT mice (n = 6–7). (B) Nifedipine and BayK (4 mg/kg) had no effect on extinction in CaV1.2DHP−/− mice (n = 8–9). Note that no, or only very low levels of freezing were observed in all groups of cued-conditioned mice upon exposure to context alone (trial 0). Data are presented as mean ± SEM. Statistical analysis was performed using 2-way ANOVA. WT mice treated with vehicle showed significantly enhanced freezing behavior compared with nifedipine-treated animals from the sixth presentation onward (6th CS, P < 0.05; 7th–15th CS, P < 0.001). Asterisks indicating significant differences were omitted for clarity.
In the extinction session of the contextual fear paradigm, freezing was assessed during 40 min of exposure to the context in the absence of US presentation. No statistically significant difference in respect to the extinction time course was observed between vehicle-treated WT and CaV1.2DHP−/− mice (for genotype: F(1,11) = 1.04, P > 0.05). More than 90% of the freezing behavior was extinguished in both groups after 25 min (Fig. 1). In excellent agreement with earlier studies (Cain et al. 2002), nifedipine resulted in a marked inhibition of the fear extinction in WT animals (Fig. 1A). This inhibition resulted in freezing levels remaining elevated at about 40% during the entire 40-min exposure to the context. Statistical analysis using 2-way ANOVA revealed a significant difference for vehicle and nifedipine treatment within the two groups (for treatment: F(1,11) = 39.51, P < 0.001). Post-hoc Bonferroni’s test showed that this was particularly due to differences in freezing behavior during the last 15 min of the 40 min of the extinction trial (Fig. 1A). In contrast, neither nifedipine nor BayK (4 mg/kg) modified extinction compared with vehicle treatment in CaV1.2DHP−/− mice (Fig. 1B). Both drug treatment groups displayed slight but statistically insignificant increases in initial freezing response. The decline in freezing over time was comparable to that in vehicle-treated controls. Although statistical analysis using 2-way ANOVA showed overall effects within the treatment groups (F(2,18) = 3.60, P < 0.05), post-hoc Bonferroni’s test revealed no significantly different freezing percentages at any time point for nifedipine and BayK compared with vehicle treatment, respectively. In addition, we did not observe treatment × time interactions within the groups (F(14,126) = 0.86, P = 0.61) (Fig. 1B).
In the extinction session of the cued fear paradigm, CS-induced freezing continuously declined in vehicle-treated mice following repeated nonreinforced CS presentations. Starting from around 80%, levels of 45% were reached after five and 1% after 10 CS presentations. Again, no difference in fear extinction between vehicle-treated WT and CaV1.2DHP−/− mice was observed (Fig. 2). Nifedipine-treated WT mice appeared to start to extinguish fear similarly; however, they failed to display a decline in extinction below 60% during the entire CS presentations (50% after five, 65% after 10, and 60% after 15 presentations). This difference was significant (for treatment: F(1,11) = 141.10, P < 0.001 and for treatment × time interaction: F(14,154) = 8.03, P < 0.001). In particular, from the sixth presentation onward, WT mice treated with nifedipine showed significantly increased freezing levels compared with vehicle controls (Fig. 2A).
Similar to the contextual fear extinction experiments, the nifedipine effect was completely absent in CaV1.2DHP−/− mice during extinction of cued fear. Again, BayK treatment did not affect the extinction time course of cued fear in CaV1.2DHP−/− mice. CaV1.2DHP−/− mice treated with either of the drugs or vehicle displayed a similar decline of initial freezing levels following repeated exposure to the CS (for treatment: F(2,22) = 2.97, P > 0.05 and for treatment × time interaction: F(28,308) = 1.45, P > 0.05) (Fig. 2B).
Locomotor activity in the open field and in the conditioning chamber
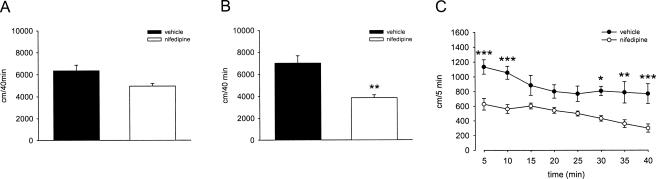
To test for potential effects of nifedipine treatment on locomotor activity, we performed an open field test. WT mice pretreated i.p. with nifedipine (40 mg/kg) 50 min prior to placement in the open field tended to show reduced locomotor activity compared with vehicle-treated mice. The total distance traveled during 40 min was 4953 ± 230 and 6346 ± 519 cm/40 min, respectively (n = 7 each, P = 0.064). This difference failed to reach statistical significance (Fig. 3A). However, when this experiment was performed with naive mice in the conditioning chamber, nifedipine treatment induced a statistically significant decrease in locomotor activity (Fig. 3B,C).
Figure 3.
Nifedipine influences spontaneous locomotor activity in the conditioning chamber. (A) Total distance traveled in an open field during a 40-min session. WT mice were injected 50 min before the session with vehicle (n = 7) or 40 mg/kg of nifedipine (n = 7). (B) Total distance traveled in the conditioning chamber during a 40-min session. WT mice were injected 50 min before the session with vehicle (n = 10) or 40 mg/kg of nifedipine (n = 9). Data are presented as mean ± SEM. Statistical analysis was performed using a Mann Whitney U-test. **P < 0.01, WT nifedipine vs. vehicle. (C) Distance traveled in the conditioning chamber broken down to 5-min time bins. WT mice were injected 50 min before the session with vehicle (n = 9) or 40 mg/kg of nifedipine (n = 10). Data are presented as mean ± SEM. Statistical analysis was performed using 2-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001, WT nifedipine vs. vehicle.
We have shown previously that BayK at the dose administered has no effect on spontaneous locomotor activity and motor coordination in CaV1.2DHP−/− mice (Sinnegger-Brauns et al. 2004). We did not evaluate effects of nifedipine on motor coordination and locomotion in CaV1.2DHP−/− mice, since no behavioral effect following nifedipine treatment was observed in our fear conditioning experiments.
Fear memory extinction in CaV1.3−/− mice
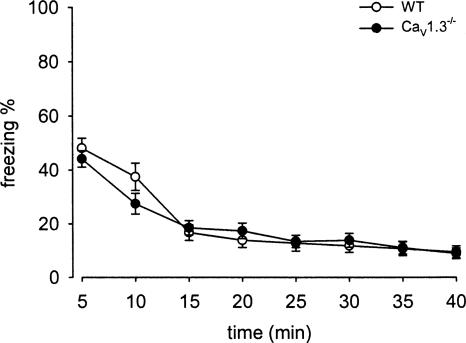
Using our CaV1.3−/− mouse model, McKinney and Murphy (2006) investigated fear extinction using a very mild fear conditioning protocol. They applied one footshock (2-sec 0.50 mA) during a short acquisition session of 3.5 min. Based on the protocol of Cain and coworkers (Cain et al. 2002), we used the stronger fear conditioning paradigm (five footshocks of 0.7 mA) utilized also in the above described experiments. Due to the deaf phenotype of CaV1.3−/− mice, only the contextual fear paradigm was used. Expression of contextual fear assessed 24 h after acquisition was indistinguishable between WT and CaV1.3−/− mice, as indicated by an identical percentage of freezing behavior during the initial 5 min of context presentation. Freezing percentages were 48% ± 4% for WT and 44% ± 3% for CaV1.3−/− mice (Fig. 4). WT and CaV1.3−/− mice showed a similar decline of freezing in the extinction session (for genotype: F(1,21) = 0.27, P > 0.05). Already, after 15 min in the context, both strains expressed less than half of the initial freezing, 17% ± 3% and 18% ± 3% for WT and CaV1.3−/− mice, respectively (Fig. 4). A very low percentage of freezing was observed until the end of the 40-min extinction session.
Figure 4.
CaV1.3 LTCCs are not involved in fear memory extinction. CaV1.3−/− (n = 12) as the WT animals (n = 11) showed a similar, normal extinction of contextual fear memory. Data are presented as mean ± SEM. No significant differences were found (2-way ANOVA).
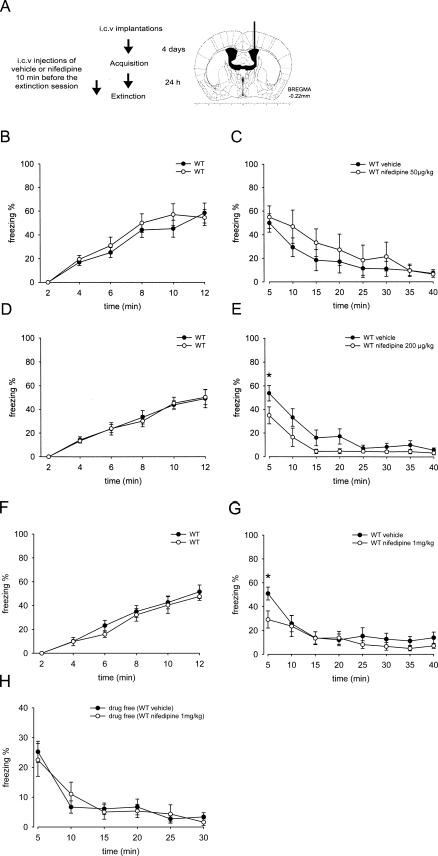
Effect of intracerebroventricular (i.c.v.) nifedipine administration on extinction of learned fear
CaV1.2 channels are widely expressed outside the CNS, especially in the cardiovascular system. In order to address the important question to which extent inhibition of CaV1.2 channels outside the CNS is responsible for the nifedipine effect, we injected different doses of nifedipine directly into the CNS of WT mice through i.c.v. application (Fig. 5A). Doses were adjusted to reach CNS concentrations within a range similar to i.p. injections based on previously determined pharmacokinetic parameters (Larkin et al. 1992).
Figure 5.
Inhibition of fear memory extinction is not centrally mediated. (A) Experimental design. (B,C) Percentage of freezing during contextual fear acquisition and extinction sessions. WT mice were injected i.c.v. 10 min before the extinction session with vehicle (n = 7) or 50 μg/kg of nifedipine (n = 7). (D,E) Percentage of freezing during contextual fear acquisition and extinction sessions. WT mice were injected i.c.v. 10 min before the extinction session with vehicle (n = 10) or 200 μg/kg of nifedipine (n = 9). (F,G) % of freezing during contextual fear acquisition and extinction sessions. WT mice were injected i.c.v. 10 min before the extinction session with vehicle (n = 10) or 1 mg/kg of nifedipine (n = 8). (H) Percent of freezing during a long-term extinction session. Vehicle- and nifedipine- (1 mg/kg) treated groups when tested 24 h later in drug-free conditions. Data are presented as mean ± SEM. Statistical analysis was performed using a 2-way ANOVA. *P < 0.05, WT nifedipine vs. vehicle.
Figure 5, B, D and F illustrates that fear acquisition prior to injection was similar in the two groups, later assigned to vehicle and the drug group. Nifedipine dose-dependently inhibited the expression of contextual fear assessed by freezing in the initial 5 min of context presentation, but did not prevent fear extinction. Expression of fear reached ∼50% and was indistinguishable between mice treated i.c.v. with vehicle or 50 μg/kg of nifedipine (Fig. 5C). However, nifedipine, 200 μg/kg and 1 mg/kg, significantly reduced freezing to 36% and 29%, respectively (Fig. 5E–G). Although statistical analysis using a 2-way ANOVA revealed a highly significant difference between vehicle and the 200 μg/kg nifedipine group (for treatment: F(1,17) = 15.28, P < 0.001), post-hoc Bonferroni’s test showed only a significant difference in the percentage of freezing during the first 5 min of the extinction session between the two treatment groups (Fig. 5E). A similar result (reduced freezing during the first 5 min) was observed with 1 mg/kg nifedipine (2-way ANOVA analysis, for treatment: F(1,16) = 5.07, P < 0.05) (Fig. 5G).
WT mice injected i.c.v. with nifedipine (200 μg/kg) or vehicle 10 min prior to placement in the open field showed no significant differences in locomotor activity (data not shown). The total distance traveled during the first 5 min was 1175 ± 162 and 1382 ± 191 cm, respectively (2-way ANOVA analysis, F(1,22) = 1.049, P = 0.3114, n = 12 each). The effect on fear expression was clearly drug dependent, because the previously vehicle- or nifedipine- (1 mg/kg) treated groups showed comparable freezing when tested 24 h later under drug-free conditions (Fig. 5H).
Discussion
In the present study we used a unique mouse model to unequivocally identify CaV1.2 as the LTCC isoform mediating nifedipine-induced impairment of extinction of both contextual as well as cued conditioned fear in mice. Evidence is provided showing that this effect is initiated by a peripheral action of nifedipine. We also show that activation of other LTCC isoforms (predominantly CaV1.3) is not sufficient to accelerate extinction of conditioned fear in mice.
In addition to the reported importance of LTCC for consolidation and for the formation of long-term memory of conditioned fear (Bauer et al. 2002; McKinney and Murphy 2006), there is evidence that LTCCs may represent potential targets to modulate fear extinction. For example, LTCC-dependent long-term potentiation (LTP) has been described in synapses between thalamic afferents and neurons of the lateral amygdala in which LTP was blocked by nifedipine (Weisskopf et al. 1999). As these synapses have been implicated in fear conditioning (Rogan and LeDoux 1995), the inhibition of extinction by LTCC antagonists may, at least in part, be due to blockade of LTCC activity in these synapses. This hypothesis is further supported by another study showing that LTCC blockers such as nimodipine reduce the amplitude of excitatory postsynaptic currents (EPSC) of lateral amygdala neurons in animals subjected to fear conditioning, but not in naive controls (Shinnick-Gallagher et al. 2003), thus suggesting that these synapses are sensitized by conditioned fear. Using two different DHPs, nifedipine and nimodipine, effects of LTCC blockers on the extinction of fear memory in mice has first been discovered by Cain et al. (2002), demonstrating that these drugs selectively impair extinction, while acquisition or expression of conditioned fear was not affected. Part of these findings have been confirmed in another study (Suzuki et al. 2004), showing impairment of within-session extinction by nimodipine. Interestingly, it has been shown that nifedipine-induced impairment of extinction is not evident when extinction training occurs immediately after acquisition (Cain et al. 2005), indicating a difference in the mechanism of short- and long-interval extinction (Myers and Davis 2007). We therefore used the initially described 24-h interval, and indeed confirmed the extinction-impairing effect of nifedipine in both contextual and cued fear conditioning in WT mice.
Based on these findings showing that blocking of LTCCs impairs extinction, calcium entry through LTCCs may be an important induction mechanism to initiate plasticity underlying extinction mechanisms. We therefore asked the question whether (therapeutically meaningful) facilitation of extinction can be accomplished by activation of LTCC, using e.g., BayK. However, it is not possible to test BayK effects in WT mice, because LTCC activators induce a toxic dystonic syndrome through widespread activation of CaV1.2 (Sinnegger-Brauns et al. 2004). The doses of BayK used in the present study do not cause neurotoxicity in CaV1.2DHP−/− mice (Sinnegger-Brauns et al. 2004) but still stimulate CaV1.3 channels in CaV1.2DHP−/− brains, leading to the activation of selected brain areas (Sinnegger-Brauns et al. 2004; Hetzenauer et al. 2006). In CaV1.2DHP−/− mice, BayK failed to affect freezing behavior at the beginning and during the extinction trial, indicating that activation of CaV1.3 channels is not sufficient to accelerate extinction of conditioned fear. Although CaV1.2 and CaV1.3 channels have been reported to show a broad and largely overlapping expression pattern in the mammalian CNS (Hell et al. 1993; Tanaka et al. 1995; Ludwig et al. 1997), and are known to be present on both excitatory and inhibitory neurons (Landfield 1996), there is only scarce information available concerning the precise expression patterns of the different subtypes of LTCCs in the mPFC, amygdala, and hippocampus. Current models for fear extinction propose interactions between these brain areas (Sanders et al. 2003; Sotres-Bayon et al. 2004, 2006; Maren 2005; Corcoran and Quirk 2007; Myers and Davis 2007). Using c-Fos mapping, we observed previously (Hetzenauer et al. 2006) that BayK-induced activation in the mPFC, the amygdala, and the hippocampus is predominantly mediated via CaV1.2. The failure of BayK to enhance c-Fos expression in these areas in CaV1.2DHP−/− mice may be in accordance with the failure of CaV1.3 activation to modify behavioral measures of both cued and contextual fear extinction (Herry and Mons 2004; Santini et al. 2004). These findings are further supported by a report showing, in particular, CaV1.2 in abundance within the BLA (Pinard et al. 2005) and the demonstration of an up-regulation of CaV1.2 but not CaV1.3 following fear conditioning in the amygdala (Shinnick-Gallagher et al. 2003).
These data suggest that CaV1.3 LTCCs do not play a prominent role for fear extinction-related behavior. This suggestion is further supported by the finding that CaV1.3−/− mice displayed normal extinction of contextual conditioned fear using the same fear conditioning paradigm. We cannot exclude the possibility that such effects can be unmasked under other experimental conditions (e.g., different fear conditioning protocols). However, normal short- and long-term extinction was also demonstrated in the same animal model by using a milder, one pair-shock fear conditioning paradigm (McKinney and Murphy 2006). Interestingly, it was demonstrated in this study that CaV1.3 channels are involved in the consolidation of contextual fear conditioning.
Taken together, the findings suggest that CaV1.2 is the LTCC subtype mediating DHP effects on fear extinction. To proof this hypothesis, we investigated the extinction-impairing effect of nifedipine in the CaV1.2DHP-insensitive mouse model. This effect of nifedipine in WT animals was completely lost in CaV1.2DHP−/− mice in both contextual and cued conditioning protocols. This finding unequivocally demonstrates that indeed CaV1.2, and not CaV1.3, LTCCs or other potentially DHP-sensitive targets (such as, for example, nicotinic acetylcholine receptors; Wheeler et al. 2006) mediate extinction impairment by nifedipine.
Since CaV1.2 LTCCs are abundantly expressed outside of the central nervous system (Catterall et al. 2005) and also mediate the vasodilating effects of dihydropyridine calcium channel blockers (as shown in CaV1.2DHP−/− mice; Sinnegger-Brauns et al. 2004), our aim was to clarify by i.c.v. injection whether the nifedipine-induced impairment of extinction is centrally mediated. Nifedipine can easily cross the blood brain barrier due to its high lipophilicity (Larkin et al. 1992). We investigated the effect of nifedipine at concentrations calculated to be at the lower and higher end of the expected brain concentrations reached after i.p. injection (see Materials and Methods). We started with the lowest i.c.v. dose previously reported to cause CNS-mediated pharmacological effects in rodents (withdrawal of sympathetic tone; Laurent et al. 1989) to minimize potential peripheral effects of the drug. As no effect on extinction was noted at this concentration, we increased the dose up to 1 mg/kg to reach nifedipine brain concentrations that should be within the range of those reached after i.p. injections (see Materials and Methods) at the beginning of the experiment (Larkin et al. 1992). Proper cannula placement was confirmed both histologically and functionally, by applying BayK (70 μg/μL) at the end of the experiment, resulting in dystonic behavior within 1-2 min after injection.
While the lowest dose of nifedipine had no effect at all, the two higher doses significantly reduced freezing in the first 5-min block of the extinction trial, indicating an effect of nifedipine on fear expression. Since this effect was absent in the drug-free extinction retention test, it represents an acute drug effect. This provides further evidence that nifedipine indeed reached brain area(s) relevant for the processing of fear/anxiety under the experimental conditions used. Although we have not further characterized this drug effect, it may reflect anxiolytic properties previously described for LTCC blockers (Soubrie 1989; Matsumoto et al. 1994; El Ganouni et al. 1998). Despite this attenuation of fear expression, none of the i.c.v. doses inhibited extinction of fear memory as observed after i.p. administration. The finding that the extinction-impairing effect of nifedipine could not be reproduced by central application strongly suggests that this effect is secondary to a peripheral action of the drug on CaV1.2 channels (see also Waltereit et al. 2007).
Since LTCC blockers including nifedipine elicit vasodilatation and subsequent hypotension in rodents (Kubo et al. 1981; Laurent et al. 1987; Barrett et al. 1988), there is the possibility that these cardiovascular effects contribute to the observed extinction-impairing effect. This interaction may involve, e.g., secondary effects on locomotion. Indeed, other antihypertensive drugs, e.g., moxonidine or clonidine, also have been shown to attenuate general locomotion (Von Voigtlander et al. 1978; Zhu et al. 2003). Cain et al. (2002) have demonstrated that nifedipine in the dose used here (40 mg/kg) does not influence locomotor activity in the open field under their experimental conditions. Since there might be differences between the animals used, we also tested this in our mice. In line with the results of Cain and colleagues, we found a trend to reduced locomotion in nifedipine-treated mice, when the observation period (40 min) was averaged. However, when a detailed time course analysis was performed, we noted a clear, statistically significant depressive effect of nifedipine on general locomotion.
Although this attenuating effect can confound measures of freezing, it seems that the effect on locomotion cannot be the only explanation for the extinction-inhibiting effect of nifedipine. Freezing (symptoms, see Materials and Methods) can be dissociated from reduced locomotion/resting (Tang et al. 2001) if evaluated manually by an observer, as was the case in the present experiments. Indeed, looking at the time course of the nifedipine effects on locomotion and contextual freezing, it is clear that the effect on freezing is much more pronounced, which is particularly evident from min 15 to 40 (Figs. 1A, 3C). Furthermore, while the central application of nifedipine reduced freezing in the first 5 min, this treatment had no effect on locomotor activity, supporting a dissociation between freezing and locomotion. Finally, the pre-CS values in the cued version (Fig. 2) show that freezing was not induced by nifedipine injection, making it unlikely that an unspecific depressive effect on locomotion accounts for the extinction-inhibiting effect of nifedipine. The exact peripheral mechanism finally leading to interference with the extinction of conditioned fear remains to be clarified.
In conclusion, our data indicate that CaV1.3 LTCCs do not play an important role in extinction of contextual and cued conditioned fear, since fear extinction was normal in CaV1.3 knockouts, the inhibition of extinction by nifedipine observed in WT mice was absent in CaV1.2DHP−/− mice, and BayK did not promote extinction compared with vehicle treatment in these mice. Thus, selective activation of CaV1.3 does not appear to be a useful strategy regarding the design of potential extinction-enhancing drugs. Moreover, we provide unequivocal evidence that the DHP-induced inhibition of conditioned fear extinction occurs through the inhibition of CaV1.2 LTCCs. However, this is most likely caused by an indirect effect initiated through the inhibition of CaV1.2 channels outside the CNS. This implies that neither CaV1.3 nor CaV1.2 LTCCs in the CNS are involved in the acquisition of conditioned fear extinction learning. However, we must emphasize that much higher concentrations of DHP LTCC blockers are required to inhibit LTCCs in neurons than, e.g., in arterial smooth muscle (Welling et al. 1993; Helton et al. 2005). Perhaps experiments biochemically down-regulating CaV1.2 channels (e.g., using siRNA or appropriate knockout mouse models) in the brain will provide a final answer regarding the role of CaV1.2 channels for fear memory extinction.
Materials and Methods
Animals
Experiments comparing mutant with WT mice were performed using male CaV1.2DHP−/−, CaV1.3−/−, and C57BL/6N WT mice obtained from homozygous breedings. CaV1.2DHP−/− animals were backcrossed for at least 10 generations (Sinnegger-Brauns et al. 2004) and CaV1.3−/− animals for at least five generations (Platzer et al. 2000) into a C57BL/6N genetic background. C57BL/6N WT mice used for the i.c.v. implantations were purchased from Charles River. All of the animals were 3-5 mo old, weighing 20-30 g at the time of experiments, and were accommodated in our animal facility for at least 3 wk before experiments. Procedures were approved by the national Ethical Committee on Animal Care and Use (Bundesministerium für Wissenschaft und Verkehr, Kommission für Tierversuchsangelegenheiten, Austria) and are in compliance with international laws and policies.
Fear conditioning
Contexts
Two contexts, A and B, were used for all fear conditioning experiments. A fear conditioning chamber (26 × 30 × 32 cm; Coulbourn Instruments) served as context A and a standard empty mouse cage (20.7 × 26.7 × 14 cm, Euro standard Type II) served as context B. In order to maximally reduce the contribution of context to cued fear conditioning, tactile, visual, and olfactory cues were different in context B compared with context A. Thus, context A was equipped with a metal grid, the illumination was bright light (300 lux), and the context was cleaned with water after each use. In contrast, context B had a smooth surface, illumination was a dim red light (∼10-15 lux), and the context was wiped out with ethanol after each session. Individual video cameras were mounted above each context and connected to a standard video recorder for later scoring of freezing behavior by an experienced investigator who was blinded to the treatment classification of the mice. Freezing, defined as the absence of all nonrespiratory movements (Fanselow 1980), was characterized according to Tang et al. (2001) by various symptoms: (1) horizontal positioning of the head, (2) rigid hunched position of the body, (3) stiffening/lifting of the tail. Auditory stimuli for the cued fear conditioning task were delivered via a speaker (Coulbourn Instruments) mounted ∼20 cm above the contexts. Unconditioned stimuli (US) were delivered via an interface connected to the metal grid of context A.
Contextual fear conditioning
Both the acquisition and extinction of contextual fear were performed in context A. For acquisition, mice were placed in the context and delivered five unsignaled mild foot shocks (US: 0.7 mA, 2 sec). Two-minute stimulus-free periods preceded, separated, and followed the US presentations. After 24 h of memory consolidation, mice were placed in the same context for 40 min of contextual extinction testing.
Cued fear conditioning
For acquisition, mice were placed in context A. Fear acquisition was elicited by presenting audible cues (CS: white noise, 80 dB, 2 min) that coterminated with the US. Two-minute stimulus-free periods preceded, separated, and followed the pairings. Extinction was carried out in context B after 24 h of memory consolidation. Mice were habituated to the context for 2 min and were subsequently presented 15 CS presentations with an intertrial interval of 5 sec.
Locomotor activity
Naive mice were placed into context A and their spontaneous locomotor activity was tracked for 40 min by a video camera positioned above the chamber and subsequently analyzed using the TSE VideoMot2 system (TSE Technical & Scientific Equipment GmbH).
Open field
Spontaneous locomotor activity was monitored by using the Tru Scan system (Coulbourn Instruments), consisting of a plastic box (41 × 41 × 41 cm) equipped with photobeam sensor rings linked to a PC. Illumination at floor level was 150 lux. The area of the open field was divided into a 28 × 28 cm central zone and the surrounding border zone. Previously conditioned mice were individually placed into the periphery of the open field, and the overall distance traveled by the mice was quantified during an interval of 40 min.
Intracerebroventricular (i.c.v.) implantations
WT mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). A 25-gauge stainless-steel guide cannula, 8.0 mm in length, was implanted using a stereotaxic apparatus (TSE Systems) into the right lateral ventricle, at coordinates 0.20 mm posterior, 1.00 mm lateral to bregma, and 1.80 mm ventral to the skull surface (Franklin and Paxinos 1997). The guide cannula was fixed to the skull with two stainless steel screws (1 mm diameter, Paul Korth) and dental cement (Dentalon plus). A stylus (0.25 mm diameter, Science Products) was inserted into the guide cannula to maintain cannula patency. Mice were single housed post-surgery to prevent cage mate interference with the cannula and left 4 d to recover.
A stainless steel injector (31 gauge; 8.5 mm long) was designed to extend 0.5 mm beyond the end of the guide cannula into the lateral ventricle. One end of the injector was attached to polyethylene tubing (1.09 mm diameter, Portex), which, in turn, was connected to a 5.0-μL Hamilton syringe.
Nifedipine was administrated at three different doses; 50, 200, and 1000 μg/kg 10 min before extinction trials or prior to the placement into an open field. Nifedipine and vehicle were delivered in a volume of 1 μL over a 30-sec period with the injection cannula remaining in position for an additional 30 sec to ensure a correct dispersion into the ventricle.
The exact injection sites were verified histologically in brain sections using cresyl violet dye to facilitate localizations, and data were analyzed only from those animals that had received injections in the correct target sites.
Drugs
We used nifedipine (40 mg/kg) for investigating fear conditioning because it has been shown not to affect locomotor activity (Cain et al. 2002), nor does it act as a potent adenosine uptake inhibitor as described for nimodipine (Striessnig et al. 1985). For intraperitoneal injections, nifedipine (Sigma-Aldrich) was suspended in saline containing 2% Tween 80. Nifedipine partly dissolved upon ultrasonication (at least 30 min) and care was taken to thoroughly mix the drug before injection. BayK (Sigma Aldrich) was dissolved in an aqueous solution consisting of 0.8% ethanol and 1% Tween 80 in saline. All drugs and vehicle were administered in a volume of 10 μL/g body weight. Time of drug applications prior to behavioral tests were chosen according to previous studies. Nifedipine was administered 50 min before the extinction trials, since this protocol has been reported to elicit marked extinction-inhibiting effects (Cain et al. 2002). BayK was administered 20 min before testing, when it exerts pronounced behavioral effects (e.g., in the forced swim test) (Sinnegger-Brauns et al. 2004). For i.c.v. injections, nifedipine was dissolved in dimethyl sulfoxide (DMSO).
Approximate brain concentrations were calculated on the basis of the pharmacokinetic parameters of nifedipine in mice reported by Larkin et al. (1992). Assuming equilibrium of nifedipine within 10 min (i.e., before start of extinction) and a brain distribution volume of 0.5 g (approximate weight of adult C57BL/6 mouse brain; www.mbl.org), injection of 50, 200, and 1000 μg/kg (∼4 μg/mouse), should result in about 1, 4, and 20 μg/g concentrations at the beginning of the experiment. This should cover a concentration range around the concentrations (3-5 μg/g) obtained 50 min after i.p. injections (i.e., at the start of extinction trials). Brain concentrations were extrapolated from the concentration time courses obtained after 6 and 60 mg/kg i.p. injections reported by Larkin et al. (1992). The elimination half-life from brain is 14.7 min and 11.2 min from blood (Larkin et al. 1992). Faster elimination should therefore not account for a lack of nifedipine effects after i.c.v. application.
Statistical analysis
Percentage of freezing scores were calculated for each mouse, and data represent mean ± SEM for groups of mice during 5-min time periods for contextual extinction and during presentation of each CS for cued extinction. Statistical analysis of fear conditioning experiments was performed using 2-way ANOVA with repeated measurements followed by post-hoc Bonferroni’s test where appropriate. Statistical analysis of locomotor activity was performed by using a 2-way ANOVA followed by post-hoc Bonferroni’s test, or a Mann-Whitney U-test. P-levels < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by the Austrian Science Fund (P-17159, NFN-S-102, W11) and the University of Innsbruck.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.886208
References
- Baker J.D., Azorlosa J.L. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav. Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Barrett R.J., Wright K.F., Taylor D.R., Proakis A.G. Cardiovascular and renal actions of calcium channel blocker chemical subgroups: A search for renal specificity. J. Pharm. Pharmacol. 1988;40:408–414. doi: 10.1111/j.2042-7158.1988.tb06305.x. [DOI] [PubMed] [Google Scholar]
- Bauer E.P., Schafe G.E., LeDoux J.E. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J. Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H.T., Schafe G.E., Bauer E.P., Rodrigues S.M., LeDoux J.E. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear conditioning. Learn. Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Cain C.K., Blouin A.M., Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J. Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C.K., Godsil B.P., Jami S., Barad M. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learn. Mem. 2005;12:277–284. doi: 10.1101/lm.88805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A., Perez-Reyes E., Snutch T.P., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Clark N.C., Nagano N., Kuenzi F.M., Jarolimek W., Huber I., Walter D., Wietzorrek G., Boyce S., Kullmann D.M., Striessnig J., et al. Neurological phenotype and synaptic function in mice lacking the CaV1.3 alpha subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience. 2003;120:435–442. doi: 10.1016/s0306-4522(03)00329-4. [DOI] [PubMed] [Google Scholar]
- Corcoran K.A., Quirk G.J. Recalling safety: Cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Deisseroth K., Mermelstein P.G., Xia H., Tsien R.W. Signaling from synapse to nucleus: The logic behind the mechanisms. Curr. Opin. Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R.2003Excitation-transcription coupling: Signaling by ion channels to the nucleus Sci. STKE 2003PE4 . 10.1126/stke.2003.166.pe4 [DOI] [PubMed] [Google Scholar]
- El Ganouni S., Tazi A., Hakkou F. Potential serotonergic interactions with the anxiolytic-like effects of calcium channel antagonists. Pharmacol. Biochem. Behav. 1998;60:365–369. doi: 10.1016/s0091-3057(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Falls W.A., Miserendino M.J., Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M.S. Conditioned and unconditional components of post-shock freezing. Pavlov. J. Biol. Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fendt M., Fanselow M.S. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Franklin B.J., Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Garakani A., Mathew S.J., Charney D.S. Neurobiology of anxiety disorders and implications for treatment. Mt. Sinai J. Med. 2006;73:941–949. [PubMed] [Google Scholar]
- Hell J.W., Westenbroek R.E., Warner C., Ahlijanian M.K., Prystay W., Gilbert M.M., Snutch T.P., Catterall W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J. Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton T.D., Xu W., Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J. Neurosci. 2005;25:10247–10251. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C., Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur. J. Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Herry C., Trifilieff P., Micheau J., Luthi A., Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur. J. Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hetzenauer A., Sinnegger-Brauns M.J., Striessnig J., Singewald N. Brain activation pattern induced by stimulation of L-type Ca2+-channels: Contribution of CaV1.3 and CaV1.2 isoforms. Neuroscience. 2006;139:1005–1015. doi: 10.1016/j.neuroscience.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Pollack M.H., Otto M.W. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah H.A., Yitta S., Drew T., Kim B.S., Visser J.E., Rothstein J.D. Calcium channel activation and self-biting in mice. Proc. Natl. Acad. Sci. 1999;96:15228–15232. doi: 10.1073/pnas.96.26.15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Fujie K., Yamashita M., Misu Y. Antihypertensive effects of nifedipine on conscious normotensive and hypertensive rats. J. Pharmacobiodyn. 1981;4:294–300. doi: 10.1248/bpb1978.4.294. [DOI] [PubMed] [Google Scholar]
- Landfield P.W. Aging-related increase in hippocampal calcium channels. Life Sci. 1996;59:399–404. doi: 10.1016/0024-3205(96)00318-9. [DOI] [PubMed] [Google Scholar]
- Larkin J.G., Thompson G.G., Scobie G., Forrest G., Drennan J.E., Brodie M.J. Dihydropyridine calcium antagonists in mice: Blood and brain pharmacokinetics and efficacy against pentylenetetrazol seizures. Epilepsia. 1992;33:760–769. doi: 10.1111/j.1528-1157.1992.tb02358.x. [DOI] [PubMed] [Google Scholar]
- Laurent S., Girerd X., Tsoukaris-Kupfer D., Legrand M., Huchet-Brisac A.M., Schmitt H. Opposite central cardiovascular effects of nifedipine and BAY k 8644 in anesthetized rats. Hypertension. 1987;9:132–138. doi: 10.1161/01.hyp.9.2.132. [DOI] [PubMed] [Google Scholar]
- Laurent S., Brisac A.M., Champeroux P., Lacolley P., Huguet F., Legrand M., Lucet B., Tsoucaris D., Briand V., Schmitt H. Central cardiovascular effects of dihydropyridines in spontaneously hypertensive rats. Fundam. Clin. Pharmacol. 1989;3:47s–56s. doi: 10.1111/j.1472-8206.1989.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L., Richardson R., Cranney J. D-cycloserine facilitates extinction of learned fear: Effects on reacquisition and generalized extinction. Biol. Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lee H., Kim J.J. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J. Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Flockerzi V., Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Kataoka Y., Watanabe Y., Miyazaki A., Taniyama K. Antianxiety actions of Ca2+ channel antagonists with Vogel-type conflict test in rats. Eur. J. Pharmacol. 1994;264:107–110. doi: 10.1016/0014-2999(94)90645-9. [DOI] [PubMed] [Google Scholar]
- McKinney B.C., Murphy G.G. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn. Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Muller J., Stiess M., Marais E., Schulla V., Lacinova L., et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K.M., Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Pavlov I.G. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford University Press; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard C.R., Mascagni F., McDonald A.J. Neuronal localization of Cav1.2 L-type calcium channels in the rat basolateral amygdala. Brain Res. 2005;1064:52–55. doi: 10.1016/j.brainres.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Ressler K.J., Paschall G., Zhou X.L., Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J. Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R., Ledgerwood L., Cranney J. Facilitation of fear extinction by D-cycloserine: Theoretical and clinical implications. Learn. Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Rogan M.T., LeDoux J.E. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Sanders M.J., Wiltgen B.J., Fanselow M.S. The place of the hippocampus in fear conditioning. Eur. J. Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Santini E., Muller R.U., Quirk G.J. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J. Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E., Ge H., Ren K., de Pena Ortiz S., Quirk G.J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick-Gallagher P., McKernan M.G., Xie J., Zinebi F. L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann. N. Y. Acad. Sci. 2003;985:135–149. doi: 10.1111/j.1749-6632.2003.tb07078.x. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns M.J., Hetzenauer A., Huber I.G., Renstrom E., Wietzorrek G., Berjukov S., Cavalli M., Walter D., Koschak A., Waldschutz R., et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J. Clin. Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F., Bush D.E., LeDoux J.E. Emotional perseveration: An update on prefrontal-amygdala interactions in fear extinction. Learn. Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F., Cain C.K., LeDoux J.E. Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Soubrie P. Neuropsychological profiles of calcium antagonists. Fundam. Clin. Pharmacol. 1989;3:71s–78s. doi: 10.1111/j.1472-8206.1989.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Zernig G., Glossmann H. Human red-blood-cell Ca2+-antagonist binding sites. Evidence for an unusual receptor coupled to the nucleoside transporter. Eur. J. Biochem. 1985;150:67–77. doi: 10.1111/j.1432-1033.1985.tb08989.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Josselyn S.A., Frankland P.W., Masushige S., Silva A.J., Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka O., Sakagami H., Kondo H. Localization of mRNAs of voltage-dependent Ca2+-channels: Four subtypes of α1- and β-subunits in developing and mature rat brain. Brain Res. Mol. Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- Tang J., Wotjak C.T., Wagner S., Williams G., Schachner M., Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res. 2001;919:232–241. doi: 10.1016/s0006-8993(01)03020-7. [DOI] [PubMed] [Google Scholar]
- Thomas G.M., Huganir R.L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander P.F., Triezenberg H.J., Losey E.G. Interactions between clonidine and antidepressant drugs: A method for identifying antidepressant-like agents. Neuropharmacology. 1978;17:375–381. doi: 10.1016/0028-3908(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Ressler K.J., Lu K.T., Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R., Mannhardt S., Bartels K., Nescholta S., Maser-Gluth C., Bartsch D. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf M.G., Bauer E.P., LeDoux J.E. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J. Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling A., Kwan Y.W., Bosse E., Flockerzi V., Hofmann F., Kass R.S. Subunit-dependent modulation of recombinant L-type calcium channels. Molecular basis for dihydropyridine tissue selectivity. Circ. Res. 1993;73:974–980. doi: 10.1161/01.res.73.5.974. [DOI] [PubMed] [Google Scholar]
- Wheeler D.G., Barrett C.F., Tsien R.W. L-type calcium channel ligands block nicotine-induced signaling to CREB by inhibiting nicotinic receptors. Neuropharmacology. 2006;51:27–36. doi: 10.1016/j.neuropharm.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Yoshii M., Watabe S. Enhancement of neuronal calcium channel currents by the nootropic agent, nefiracetam (DM-9384), in NG108-15 cells. Brain Res. 1994;642:123–131. doi: 10.1016/0006-8993(94)90913-x. [DOI] [PubMed] [Google Scholar]
- Yoshii M., Watabe S., Sakurai T., Shiotani T. Cellular mechanisms underlying cognition-enhancing actions of nefiracetam (DM-9384) Behav. Brain Res. 1997;83:185–188. doi: 10.1016/s0166-4328(97)86066-4. [DOI] [PubMed] [Google Scholar]
- Zhang H., Maximov A., Fu Y., Xu F., Tang T.S., Tkatch T., Surmeier D.J., Bezprozvanny I. Association of CaV1.3 L-type calcium channels with Shank. J. Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Paul I.A., Stec D.E., Peeler D.F., Piletz J.E. Non-adrenergic exploratory behavior induced by moxonidine at mildly hypotensive doses. Brain Res. 2003;964:9–20. doi: 10.1016/s0006-8993(02)03754-x. [DOI] [PubMed] [Google Scholar]