Abstract
The response regulator DosR is essential for promoting long-term survival of Mycobacterium tuberculosis under low oxygen conditions in a dormant state and may be responsible for latent tuberculosis in one third of the world’s population. Here we report crystal structures of full-length unphosphorylated DosR at 2.2 Å and its C-terminal DNA-binding domain at 1.7 Å resolution. The full-length DosR structure reveals several features never seen before in other response regulators. The N-terminal domain of the full-length DosR structure has an unexpected (βα)4 topology instead of the canonical (βα)5 fold observed in other response regulators. The linker region adopts a unique conformation which contains two helices forming a four-helix bundle with two helices from another subunit, resulting in dimer formation. The C-terminal domain in the full-length DosR structure displays a novel location of helix α10 which provides Gln199 to interact with the catalytic Asp54 residue of the N-terminal domain. In contrast, the structure of the DosR C-terminal domain alone displays a remarkable unstructured conformation for helix α10 residues different from the well-defined helical conformations in all other known structures, indicating considerable flexibility within the C-terminal domain. Our structures suggest a mode of DosR activation by phosphorylation via a helix rearrangement mechanism.
INTRODUCTION
With eight million new cases reported annually,1 tuberculosis remains a major global infectious disease responsible for approximately two million deaths per year worldwide.1 It is estimated that one third of the world’s population is latently infected with its causative agent, Mycobacterium tuberculosis.2 The ability to persist in infected individuals without producing any symptoms for extended periods of time makes M. tuberculosis a remarkably successful pathogen. Latent tuberculosis is a major public health concern because it acts as a reservoir of M. tuberculosis that can remain undetected for decades before reemerging as active disease. Current treatments for tuberculosis are highly effective only when the bacteria are actively growing. Tuberculosis infections require prolonged drug therapy, presumably due to the persistence of the bacteria that make them refractory to the drug treatment.
Two-component regulatory systems in bacteria are a major class of signal transduction proteins involved in adaptation to environmental changes.3 Typical two-component regulatory systems contain (i) a membrane-bound sensor kinase that plays a crucial role in sensing environmental stimuli, and (ii) a cytosolic response regulator, usually consisting of an N-terminal domain, a linker, and a C-terminal domain. This pair of proteins functions as a molecular switch that controls diverse adaptive environmental responses (for reviews see 3–5). In the presence of the appropriate stimulus, a sensor kinase catalyzes an ATP-dependent autophosphorylation of a specific His residue in the cytoplasmic domain. The signaling cascade proceeds next by a transfer of the phosphoryl group from the histidine of the sensor kinase to a conserved aspartate in the N-terminal domain of the response regulator. Phosphorylation of the response regulator switches on its C-terminal domain to perform its associated function such as DNA-binding or enzymatic activity. Multi-domain response regulators containing a C-terminal DNA-binding domain function in most cases as transcription factors by binding to promoter regions of specific genes to activate gene expression. This family of response regulators can be classified into three major subfamilies: the NarL,6 the OmpR,7,8 and the NtrC9,10 subfamilies, based on their C-terminal domain sequences.3–5 So far, unphosphorylated full-length structures have only been reported for the NarL (represented by NarL6,11 and StyR12) and OmpR (represented by DrrD13 and DrrB14) subfamilies. No full-length structures of multi-domain response regulators in the active phosphorylated state have been reported to date.
A two-component regulatory system dosS-dosR (also called devS-devR15) is among the M. tuberculosis genes induced by hypoxia or NO exposure,16,17 two conditions frequently associated with the onset and maintenance of latent tuberculosis.18,19 Sherman et al.16 proposed that dosS and dosR form a two-component signaling system involved in the adaptation of bacilli to low oxygen conditions within the host. Nearly all M. tuberculosis genes rapidly up-regulated in response to low oxygen and NO levels require DosR for their induction.17,20 The sensor kinase DosS (whose gene is upstream of the dosR gene) and an orphan sensor kinase DosT (located elsewhere) phosphorylate their conserved histidine (His395 for DosS and His392 for DosT) and subsequently transfer the phosphoryl group to Asp54 of DosR using Mg2+ as a cofactor.21–23 Phosphorylation of the catalytic Asp54 presumably leads to a conformational change which enhances the binding affinity of DosR to its cognate DNA sequence and activates transcription of regulated genes.21
By sequence homology, the N-terminal domain of DosR belongs to a larger CheY family of regulatory domains with a (βα)5 fold.24 The activation mechanism in this family of regulatory domain has been studied extensively through comparisons of available native and phosphorylation-activated regulatory domain structures of FixJ,25,26 NtrC,27,28 and Spo0A,29,30 and BeF3−-activated structures of CheY,31–33 DctD,34,35 NtrC,28,36 Spo0F,37–40 CheY2,41 ArcA,42 PhoB43 and Ntrc1.9,44 Six conserved residues have been reported to be involved in the phosphorylation activation mechanism: three aspartates (equivalent to Asp8, Asp9, and Asp54 in DosR), one Thr/Ser (Thr82), one Tyr/Phe (Tyr101), and one Lys (Lys104)45 (for residue numbers of selected proteins, see Figure 1). All these proteins share a general mechanism of “coupled rearrangement” of the Thr and Tyr residues after modification of the third Asp occurs (by a phosphoryl group or its analog BeF3−).4,36,45 Upon activation, the Thr residue changes conformation to form a hydrogen bond with an oxygen atom of a phosphoaspartate (or a fluoride atom in the BeF3−-activated proteins). This causes the Tyr residue to move from a solvent-exposed outward position to a buried inward position which induces conformational changes of nearby residues. Different response regulators have different magnitudes of conformational change38 and the outcome is different from case to case. For example, a phosphorylation-induced conformational change (i) triggers domain separation in FixJ46 and NarL,47 (ii) causes dimerization of FixJ,25,48 StyR,12,49 and PhoB,43 (iii) results in oligomerization of NtrC,50 and (iv) allows binding to different protein interaction partners with different binding affinities in the case of CheY.51–54 Therefore, while having similar global architectures, two-component response regulators act with great diversity at the functional level.
Figure 1.

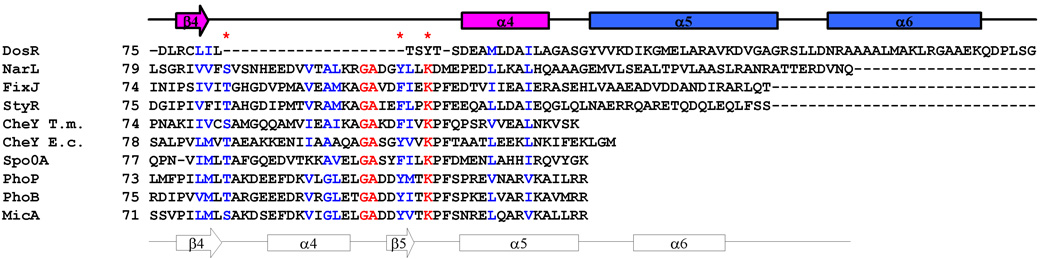
Sequence-based and structure-based sequence alignment of DosR and its homologues. The sequence alignment is color-coded red for > 80% identity and blue for > 80% homology. A schematic representation of secondary structure elements of full-length DosR is shown on top of the sequence alignment and colored magenta for the N-terminal regulatory domain, blue for the linker, and green for the C-terminal DNA binding domain. The secondary structure elements of NarL are shown on the bottom of the alignment for comparison. A * indicates conserved residues involved in phosphorylation and the coupled rearrangement mechanism for canonical regulatory domains. DosR, NarL, FixJ, and StyR are members of the NarL subfamily. T. maritima and E. coli CheY are one domain proteins. Spo0A, PhoP, PhoB, and MicA are members of the OmpR subfamily and only their N-terminal domain sequences are used for alignment. (a) Sequence-based alignment. (b) Structure-based alignment of the β4 to α6 region.
Our previous crystal structures of the DosR C-terminal domain (DosRC) and of its complex with DNA identify DosR as a member of the NarL subfamily of response regulators on the basis of the structural topology of the C-terminal domain.55 A DosRC dimer is the functional unit for DNA binding where residues in the C-terminal helix α10 form a dimerization interface.55 Two such DNA-binding dimers assemble as a tetramer via an interface involving helices α7 and α8 of the C-terminal domain.55 However, due to the absence of the N-terminal regulatory domain, a molecular mechanism for phosphorylation activation of DosR remained unclear. Here we report a crystal structure of full-length unphosphorylated DosR at 2.2 Å resolution. Our structure reveals a novel topology for both the N-terminal domain and the linker region, and a unique conformation for the C-terminal domain. In addition, we present a 1.7 Å resolution structure of the C-terminal domain fragment (DosRC) with yet another conformation of the residues forming helix α10 which is different from our previous structures of DosRC 55 and, to the best of our knowledge, all related structures.6,11,12,56–62 Our structures reported here provide insight on how DosR conformational changes might be induced by phosphorylation through a helix rearrangement mechanism.
RESULTS
Overall structure of full-length DosR
The structure of full-length DosR was solved by the SeMet MAD technique and refined using native data to 2.2 Å resolution (Table 1). The asymmetric unit contains two DosR molecules forming a dimer related by a non-crystallographic two-fold axis. The electron density is well defined for the first 210 residues with C-terminal tail residues 211–217 being disordered (Figure 1). The N-terminal domain of DosR (formed by residues 1 to 97) is connected to the C-terminal domain (residues 150–210) by a two-helix linker (Figure 2). Quite remarkably, the structure of each of these three elements is different from other members of the NarL subfamily to which DosR belongs.
Table 1.
Data collection and refinement statistics
| SeMet derivative DosR | Native DosR | DosRC-II | ||
|---|---|---|---|---|
| Data collection | ||||
| Space group | P212121 | P21 | P212121 | |
| Wavelength (Å) | 0.9791 | 0.9641 | ||
| (peak) | (remote) | |||
| Unit cell dimension | 53.68, 95.81, | 53.68, 95.83, | 48.11, 98.08, | 39.64, 47.40, |
| a, b, c (Å) | 97.77 | 97.70 | 53.71, β=93.81 | 66.44 |
| Resolution rangea (Å) | 50–2.88 | 50–4.1 | 50–2.2 | 50–1.7 |
| (2.98–2.88) | (4.25–4.10) | (2.28–2.20) | (1.76–1.70) | |
| Total reflections | 69,241 | 25,716 | 100,603 | 90,800 |
| Unique reflections | 11,508 | 4,334 | 22,915 | 14,128 |
| I/σ(I)a | 8.9 (3.5) | 10.1 (6.3) | 22.1 (3.1) | 19.3 (2.2) |
| Completenessa (%) | 95.6 (95.2) | 99.5 (99.8) | 90.5 (64.3) | 98.5 (92.2) |
| Rsyma (%) | 19.8 (33.9) | 18.2 (29.8) | 7.4 (39.7) | 9.1 (59.0) |
| MAD phasing | ||||
| Resolution range (Å) | 50.0–2.88 | |||
| No. methionine residues | 16 | |||
| No. heavy-atom sites found | 16 | |||
| FOMb | 0.28 | |||
| Solvent flattening FOMb | 0.59 | |||
| TLS Refinement | ||||
| Resolution range (Å) | 50–2.2 | 38.6–1.7 | ||
| Reflections used (working/free) | 21,670/1,172 | 13,384/704 | ||
| Rwork (%) | 18.6 | 18.8 | ||
| Rfree (%) | 24.9 | 20.6 | ||
| Average residual B-factor of all atoms (Å2) | 38.5 | 14.3 | ||
| No. protein molecules per asymmetric unit | 2 | 2 | ||
| R.m.s. deviations from ideal geometry | ||||
| Bond length (Å) | 0.010 | 0.012 | ||
| Bond angles (deg.) | 1.27 | 1.33 | ||
| Ramachandran plot | ||||
| Most favored (%) | 93.8 | 98.9 | ||
| Additional allowed (%) | 6.2 | 1.1 | ||
| Generously allowed (%) | 0 | 0 | ||
| Disallowed (%) | 0 | 0 | ||
Values in parentheses are for the highest-resolution shell.
According to RESOLVE.72
Figure 2.

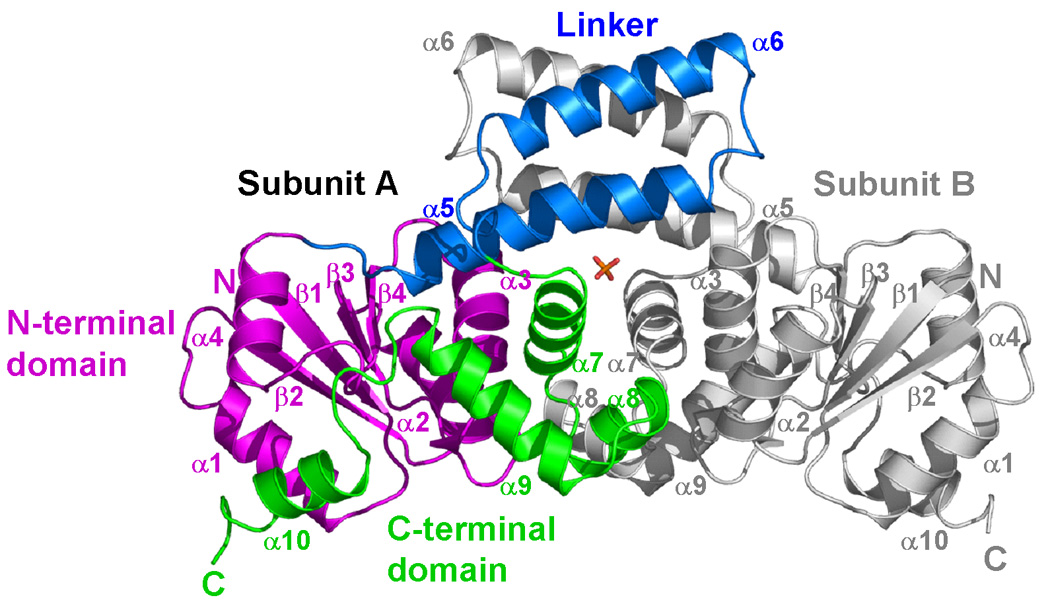
Crystal structure of full-length DosR. (a) The DosR monomer. The structure is shown in magenta for the N-terminal domain, blue for the linker, and green for the C-terminal DNA binding domain. (b) The DosR dimer. Subunit A is shown as above. Subunit B is shown in gray. A sulfate molecule (shown as a stick model) is found in the dimer interface between the two DosR molecules.
The N-terminal regulatory domain
The N-terminal domain of DosR contains an α/β fold with a (βα)4 arrangement and is topologically different from the canonical regulatory domain fold with a (βα)5 arrangement.24 The canonical secondary structure elements α4 and β5 appear to be absent in the DosR structure (Figure 2). Surprisingly, the DosR helix α4 is positioned where the canonical helix α5 is located in structures of other members of the subfamily (as discussed below).
The catalytic Asp54 residue, involved in the phosphorylation reaction, is located in the β3-α3 loop surrounded by the conserved Asp8 and Asp9 residues from the β1-α1 loop from the same subunit (Figure 3). The Oδ1 atom of Asp54 makes a hydrogen bond to a water molecule. This water molecule also forms two hydrogen bonds with the Oδ1 and Oδ2 atoms of Asp9. Interestingly, the Oδ2 atom of Asp54 makes two hydrogen bonds to the Oε1 and Nε2 atoms of Gln199 located in the beginning of the C-terminal domain α10 helix. This unique feature will also be discussed further below.
Figure 3.
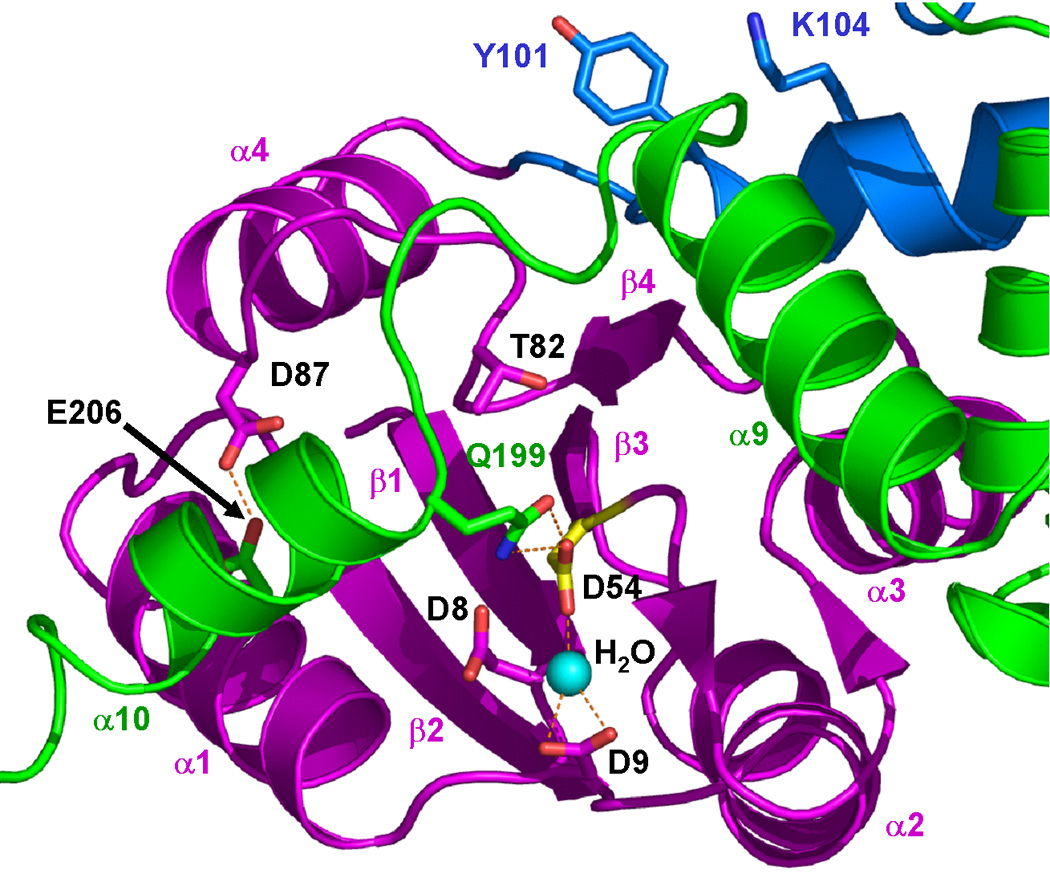
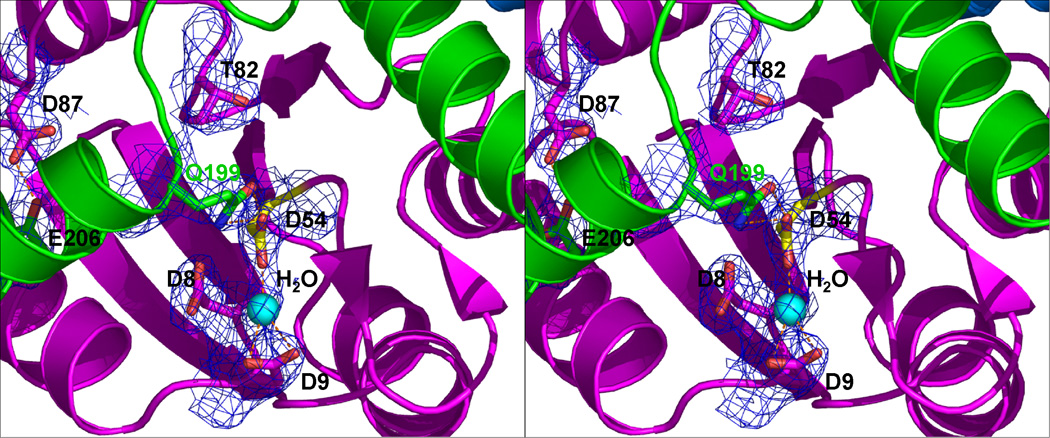
The DosR phosphorylation site. (a) The side chains of D8, D9, D54 (highlighted in yellow), T82 and D87 in the N-terminal domain (magenta), Y101 and K104 in the linker (blue), Q199 and E206 in the C-terminal domain (green) are shown in stick model. Y101 is ~12 Å away from T82. The D54-Q199 and D87-E206 hydrogen bonds contribute to contacts between helix α10 and the N-terminal domain. The latter hydrogen bonds require protonation of at lease one of the carboxylates. (b) Stereo view of the phosphorylation site. The σA-weighted 2Fobs-Fcalc electron density map of the phosphorylation site D54, contoured at the 1σ level is shown including neighboring residues.
The linker region
The linker formed by residues 98 to 149, between the N- and the C-terminal domains contains helices α5 (residues 101 to 121) and α6 (residues 127 to 143). These two helices form a four-helix bundle dimerization interface with their counterparts from another subunit of the dimer (Figure 2). According to a B-factor plot (not shown), helix α6 has the highest average B-factor in the structure (78 Å2 for residues 125–144 vs. 44 Å2 for the remainder of protein), suggesting flexibility in this part of the molecule. The conserved Tyr101 and Lys104 residues of DosR are located at the beginning of helix α5 and their side chains point into the solvent. This arrangement is entirely different from what is seen in the structures of other NarL subfamily members.6,11,12
The C-terminal DNA-binding domain
In the full-length DosR structure, the C-terminal domain folds back from the linker region to interact with the N-terminal domain of the same subunit (Figure 2). This domain has an unusual conformation where helix α10, which starts at Gln199, moves away from the core formed by helices α7 to α9 of the C-terminal domain to pack against helix α1 of the N-terminal domain (Figure 2). The Oε1 and Nε2 atoms of Gln199 from helix α10 form hydrogen bonds to the Oδ2 atom of Asp54 of the N-terminal domain (Figure 3), shielding Asp54 and surrounding residues from solvent and stabilizing the protein in the inactive closed conformation.
Domain Interfaces within a DosR Subunit
The interactions between the N-terminal domain, the linker region, and the C-terminal domain of DosR are extensive. The largest interdomain interface occurs between the N-terminal domain plus the linker region and the C-terminal domain, burying ~2,500 Å2 solvent accessible surface. This interface has a gap volume index (gap volume per interface solvent accessible surface) of 1.4 Å which is a low value indicating an excellent complementarity of the interaction.63,64 The N-terminal domain alone and the C-terminal domain bury 1,900 Å2 solvent accessible surface, with the same gap volume index. Interestingly, most of the interface between the N- and the C-terminal domains is due to contacts between the N-terminal domain and the α10 helix region (residues 194–210) which bury 1,400 Å2 solvent accessible interface with a very small gap volume index of 0.8 Å, indicating a very tight interaction given that the average gap volume index is 2.5 ± 1.0 Å for heterocomplexes.64 The major contacts between the N-terminal domain and the α10 helix region are hydrogen bonds between Asp54 Oδ2 and Gln199 Oε1 and Nε2 atoms; Leu81 carbonyl oxygen and Arg197 Nε and Nη2 atoms; Thr82 Oγ1 and Met194 carbonyl oxygen; Thr82 carbonyl oxygen and Arg196 main chain N; Asp87 Oδ1 and Arg197 Nη1; and Asp87 Oδ2 and Glu206 Oε2 (Figure 3). The rest of the C-terminal domain (helices α7 to α9) and the N-terminal domain bury only 500 Å2 solvent accessible interface with a gap volume index of 4.9 Å, indicating minimal interactions.
Inspection of all hydrogen bonds present in all interdomain interfaces within the subunit reveals that only the hydrogen bonds between Asp54 Oδ2 and Gln199 Oε1, and between Asp87 Oδ2 and Glu206 Oε2 atoms are made possible by protonation of side chain carboxylate groups at pH 3.0 of our crystallization mother liquor. Only a small Gln199 side chain rotation is required, and possible, to maintain the Gln199-Asp54 contact via the Nε2 of Gln199 at neutral pH. Similarly, the loss of a proton in the Asp87 to Glu206 hydrogen bond in our current structure might be followed by small rotations about the Cβ-Cγ bond of Asp87 and the Cβ-Cγ and Cγ-Cδ bonds of Glu206 so that these residues are not repulsed by the negative charges on the side chain carboxylate groups at neutral pH. Therefore, it is likely that at neutral pH, the α10 helix can also adopt the conformation observed in our structure. Because no contact involving protonated carboxylate groups occurs elsewhere within the same subunit, we believe that our structure may be physiologically relevant and crystallization conditions at low pH only helped stabilize the dynamically flexible helix α10 into a well-defined conformation which assists crystal growth.
Dimer Interface of Full-length DosR
The two subunits in the asymmetric unit form a dimer related by a non-crystallographic two-fold axis (Figure 2b). The dimerization interface buries 2,900 Å2 of solvent accessible surface of which 33% is polar and its gap volume index is 2.4 Å (compared to an average of 2.2 ± 0.9 Å for homodimers).64 The two-helix linker (helices α5 and α6) from both subunits form a four-helix bundle and bury 1,800 Å2 of the solvent accessible surface, i.e. 62% of the total dimerization interface. The second part of the dimerization interface comes from interactions between the N-terminal domain helices α2 and α3 of one subunit and the C-terminal domain helices α7 and α8 of the other subunit. A sulfate molecule is found buried in the dimer interface mediating hydrogen bonds between its oxygen atoms and side chains of Arg113 and of a protonated Asp117, and mediating longer-range electrostatic interactions with the guanidinium group of Arg67 at a distance of ~3.6 Å.
Structure of the DosR C-terminal domain in crystal form II
The DosRC structure in a new crystal form II (DosRC-II), different from our crystal from I (DosRC-I) reported previously,55 was obtained at pH 6.5, and solved by molecular replacement and refined to 1.7 Å resolution (Table 1). Interestingly, molecular replacement using all four helices of the DosRC-I structure was not successful, but using only helices α7 to α9 gave a clear solution. In the DosRC-II crystals, two DosRC molecules form a dimer in the asymmetric unit (Figure 4a). The dimerization interface buries 1,300 Å2 solvent accessible surface with a gap volume index of 2.7 Å. In this structure, helices α7, α8, and α9 are arranged in the same manner as observed previously in the structures of the DosRC-I and DosRC-DNA complexes55 (Figure 4b) and in the full-length structure reported here (Figure 2). Most surprisingly, however, helix α10 is not observed in this structure with residues Arg196 to Gln199 adopting a coiled coil structure and residues beyond Gln199 being disordered. The latter probably explains the differences in success with three and four helix molecular replacement probes.
Figure 4.

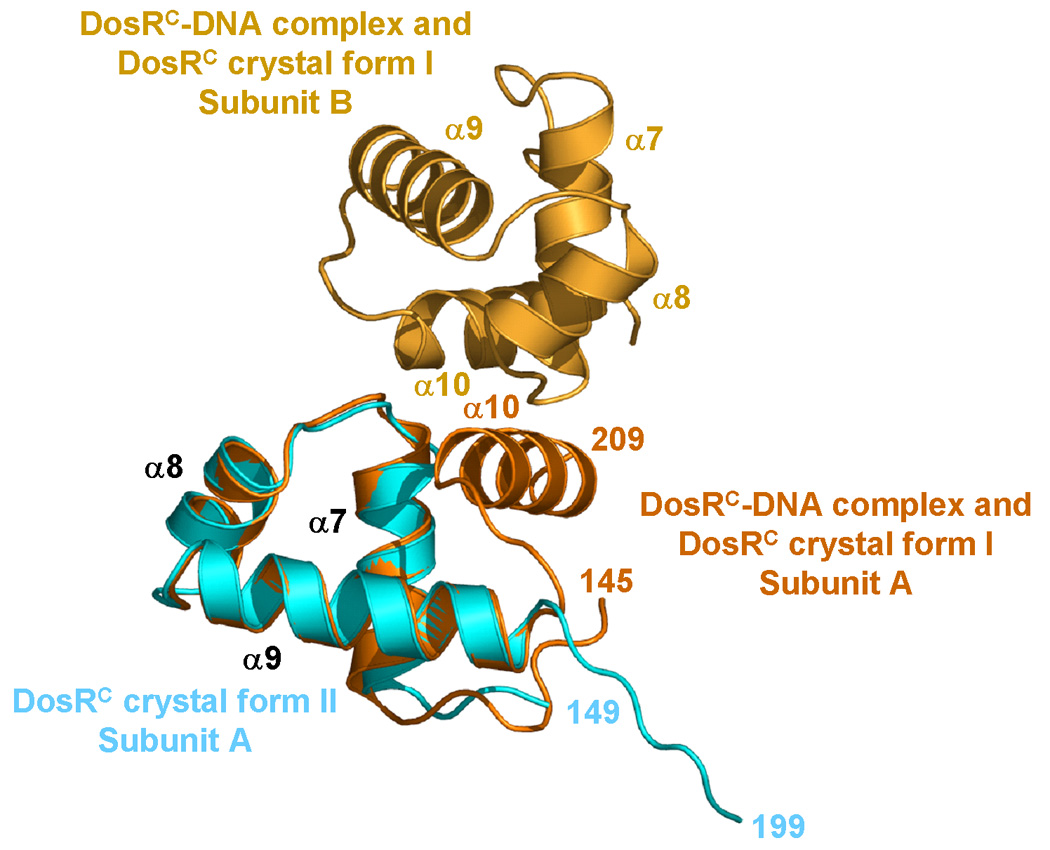
The crystal structures of the DosR C-terminal domain. (a) The DosRC dimmer structure from crystal form II (DosRC-II). Subunit A is shown in cyan and subunit B in blue. The α10 helix is "melted" into a coiled coil, and the C-terminal tail after Gln199 is disordered. (b) Superposition of helices α7 to α9 of DosRC-II subunit A (cyan) onto subunit A of the DosRC dimer from the DosRC-DNA complex crystal structure (subunit A shown in orange and subunit B in gold) reported previously.55
Comparison of the DosR C-terminal domain structures
The full-length DosR and the new DosRC-II structures presented here reveal two novel features with helix α10 separated from the rest of the C-terminal domain in both cases. In our previous structures of DosRC-I55 and the DosRC-DNA complex,55 helix α10 is formed by residues Arg196 to Arg209 and packs in the canonical conformation against the hydrophobic core of the rest of the domain (α7–α9 helices). In the two new structures described here, residues Leu150 to Leu192, comprising helices α7, α8, and α9, superimpose well in all four available structures (DosRC-I, DosRC-DNA, DosRC-II, and full-length DosR) with mutual r.m.s. deviations of less than 1 Å (Figure 5). However, helix α10 behaves totally different. In the current full-length structure, helix α10 starts at Gln199 (instead of Arg196 in the previous structures) and rotates about 113° from the canonical conformation to a new position enabling the helix to interact with the N-terminal domain. In the new DosRC-II structure, residues Arg196 to Gln199 become a coiled coil structure and residues after Gln199 are disordered. It is possible that residues Gln199 to Arg209 may still adopt a helical conformation in the DosRC-II crystal but due to high flexibility, this region is invisible in the electron density map. The orientation of this coiled coil structure is accompanied by a 180° kink at Gly193 compared to the DosRC-I and the DNA complex structures (Figure 5). The three different conformations of the residues forming helix α10 in different crystal structures suggest major flexibility within the C-terminal domain. This flexibility is most likely of great importance for DosR function as discussed below.
Figure 5.

Superpositions of the DosR C-terminal domain in three different conformations. The C-terminal domain from the full-length DosR is shown in green, the DosRC-DNA complex55 (which is the same as DosRC in crystal form I, not shown) in orange, and the DosRC crystal form II in cyan. The structures are superimposed with r.m.s. deviations less than 1 Å for helices α7–α9. Helix α10 evidently adopts three entirely different positions or conformations. Only the conformation observed of the DosRC-DNA complex and of DosRC in crystal form I (orange) is able to bind DNA.55
Comparison with homologous structures
DosR is a member of the NarL subfamily by sequence homology of the C-terminal domain (Figure 1). Two other members of this subfamily with known full-length structures are NarL6,11 and StyR,12 both in the unphosphorylated state.
The N-terminal domain
Full length DosR adopts an unusual (βα)4 fold with the canonical helix α4 and strand β5 absent in the N-terminal domain compared to the (βα)5 fold of homologous structures. Superposition of the DosR N-terminal domain to its closest sequence homologue NarL11 yields an r.m.s. deviation of 1.7 Å for 82 superimposable Cα atoms, and 1.2 Å for the superposition of 77 Cα atoms with StyR (Figure 6). Their equivalent secondary structure elements comprise β1 to β3 plus α5 in NarL and StyR and β1 to β3 plus α4 in DosR. Most unexpectedly, helix α4 of DosR is shifted and positioned in a place where the canonical helix α5 is located in NarL and StyR. In another surprise, a sequence segment Tyr101 to Gly121 of DosR, corresponding to the β5-α5 of the canonical regulatory domain becomes helix α5 of the linker region.
Figure 6.

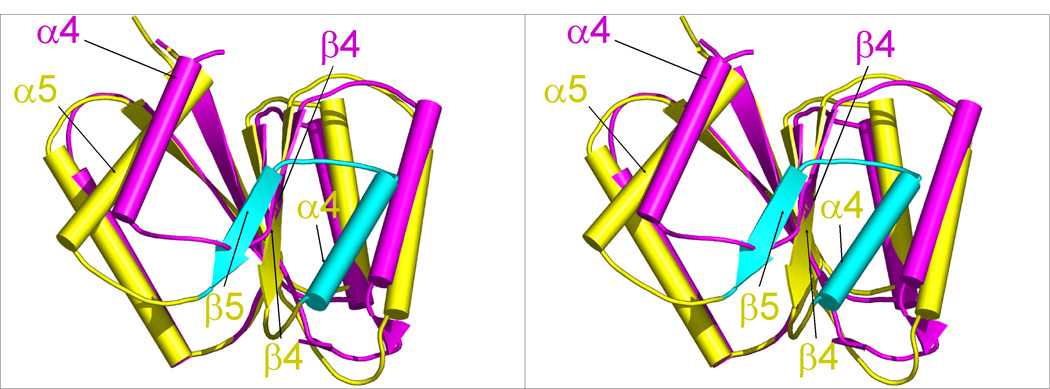
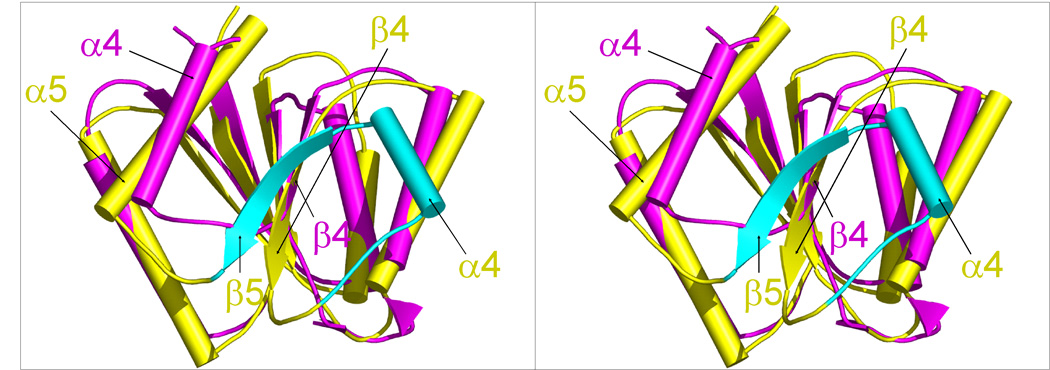
Comparison of DosR with other NarL subfamily members. (a) Structures of full-length unphosphorylated DosR, NarL, and StyR monomers arranged in the same orientation of their N-terminal domains. Domains are color-coded with magenta for the N-terminal domain, blue for the linker, and green for the C-terminal domain. (b and c) N-terminal domain superpositions of full length DosR (magenta) onto the NarL (b) and StyR (c) structures (yellow). Loops are smoothened for clarity. DosR lacks the canonical β4 and α4 segments of NarL or StyR (shown in cyan) and its α4 is situated roughly at the position of the canonical α5.
When considering conserved residues involved in phosphorylation activation, it appears that Asp8 and Asp9 in the β1-α1 loop and Asp54 in the β3-α3 loop are within 1 Å from equivalent residues in other structures. However, the key Thr82 residue located at the end of strand β4 is shifted 3–4 Å away compared to the position of the equivalent Thr residue in other structures as the β4-α4 loop has to connect to helix α4 in its new position (Figure 6b and 6c). Tyr101, conserved in the primary sequence alignment (Figure 1a) and known to be involved in the activation mechanism, is positioned in the linker region with its Cα atom 12 Å away from Cα of Thr82. The Cα of Tyr101 is shifted by 5–6 Å compared to the equivalent Tyr106 in NarL and Phe102 in StyR. A conserved Lys104, which makes a salt bridge with a phosphoryl oxygen of phosphoaspartate or BeF3− analog in activated response regulator structures, is located in the α5 linker helix in our structure 21 Å away from the position of the equivalent Lys in NarL and StyR. This has implications for the activation mechanism as will be discussed below.
The linker region
The interdomain linker of the full-length DosR structure shows no resemblance to those of NarL and StyR.6,11,12 Quite surprisingly, DosR uses the amino acid sequence for the canonical β5-α5 secondary structure elements to form a “new” helix α5 (Figure 1 and 2) which clearly is part of the linker connecting the N-terminal and the C-terminal domains. Together with helix α6, the two-helix linker forms a four-helix bundle with the other subunit in the dimer (Figure 2). In contrast, in the unphosphorylated NarL structure, helix α6 is a linker allowing both domains to make contacts in a compact “closed” conformation (Figure 6a).6,11 In the case of StyR, the α5 helix adopts its canonical position but residues 108 to 141 form a long helical extension of helix α5 which separates the C-terminal domain from the N-terminal domain by 48 Å (blue helical segment in Figure 6a).12 (Although specific residues involved in activation adopt an “active-like” conformation, the reported structure of StyR is likely to be an inactive monomer from an oligomerization point of view.12) In the case of DosR, however, the linker positions the C-terminal domain near the phosphorylation site to make extensive contacts with the N-terminal domain. Moreover, the DosR linker provides a dimerization interface for the unphosphorylated protein in contrast to both StyR and NarL whose structures are both monomeric (Figure 2 and Figure 6a).6,11,12
The C-terminal domain
Except for helix α10, the C-terminal domain in the full-length DosR and the DosRC-II structures are similar to the canonical conformation observed before in all related structures.6,11,12,55–62 In contrast, the entire C-terminal domains of the full-length NarL and StyR structures are both in the canonical conformation (Figure 6a).6,11,12 In all three full-length proteins, the C-terminal domain does not form the α10 interface dimer as seen in the previous structure of DosRC-I and its DNA complex55 and the NarLC-DNA complex.59
DISCUSSION
How phosphorylation activates response regulators is one of the central questions in two-component regulatory system research. Response regulators are believed to be in equilibrium between inactive and active conformations, with phosphorylation acting as a molecular switch to shift this equilibrium towards the active form.45,65–67 In all activated structures of the N-terminal regulatory domain, phosphorylation (or BeF3− activation) of the critical Asp residue causes a movement of a conserved Lys in the β5-α5 loop to form a salt bridge to the phosphoryl group. Phosphorylation also causes a conformational change of a conserved Thr residue in strand β4 to form a hydrogen bond with a phosphoryl oxygen atom. This allows the sidechain of a conserved Tyr residue in β5 to reorient from a solvent-exposed outward position to fill a pocket vacated by the Thr residue in a buried inward position with concomitant conformational changes of surrounding residues.45
The puzzle remaining after our structure determination is that, in DosR, Tyr101 and Lys104 (i) deviate considerably from the positions seen in other structures; (ii) the sidechains in their new positions are essentially totally solvent exposed, while (iii) these residues are conserved nevertheless. The main reason for all these differences is a major rearrangement of secondary structure element such that our full length DosR structure lacks the canonical α4 and β5 secondary structure elements in the N-terminal domain in which the conserved Tyr101 and Lys104 are located. These residues are now found in helix α5 of the linker region 15–20 Å away from the catalytic Asp54.
The DosR activation mechanism may involve a phosphorylation-dependent equilibrium switching between the inactive conformation seen in our full-length structure and the active canonical conformation where the C-terminal domain forms a dimer at the α10 interface.55 Our full-length DosR structure indicates that in the inactive conformation, the C-terminal domain is bound to the N-terminal domain where Gln199 of helix α10 may makes contacts with and buries Asp54 residues from accessibility to solvent. Interestingly, a similar interaction has been observed for Spo0F where the catalytic Asp54 residue is blocked by a salt bridge interaction of an Arg side chain from a neighboring molecule.68 The extensive interactions between the N-terminal domain and helix α10 in the DosR structure could be assisted by hydrogen bonds from protonated carboxylate groups at low crystallization pH. It is likely that at neutral pH, helix α10 is dynamically in equilibrium between different conformations, of which at least two are seen in our structures reported here. This would allow Asp54 to be solvent exposed part of the time and to become phosphorylated in the presence of sensor His kinases or acetyl phosphate.
A proposed helix rearrangement activation mechanism
On the basis of the structures reported here and previously,55 we propose the arrangement of helices α4, α5, and α10 from the conformations seen in our structure to new conformations in a phosphorylated active state to occur via a helix rearrangement mechanism (Figure 7). The unphosphorylated DosR dimer in an inactive/closed conformation (1 in Figure 7) as seen in the crystal structure of full-length DosR, is in equilibrium with the monomer (2) in solution. At neutral pH, helix α10 adopts multiple conformations including one with no contacts with the N-terminal domain (3), allowing Asp54 to be solvent exposed part of the time. Phosphorylation of Asp54 is likely to promote a cascade of events which may be as follows. First, the new phosphoryl group will prevent helix α10 from returning to bind the N-terminal domain due to steric hindrance between the phosphoaspartate and Gln199 sidechains. Secondly, phosphorylation presumably induces a conformational change in the N-terminal domain such that interactions with the C-terminal domain (as seen in our structure) become unfavorable. This change includes a rearrangement of the (βα)4 domain and of the α5-α6 linker seen in our structure to become the canonical (βα)5 fold. In this case, helix α4 moves to resume its canonical position and helix α5 becomes the canonical β5-α5 unit. Such a conformational change will cause a collision between helix α4 and the C-terminal domain. Thirdly, the conserved Thr82 sidechain may abandon its interactions with the C-terminal domain (i.e. with residues Met194 and Arg196) in order to reorient and form a hydrogen bond with phosphoaspartate. Alternatively, the adoption of the canonical (βα)5 fold could potentially take place before phosphorylation of Asp54 occurs. In agreement with the fact that the interdomain interface in our structure occurs extensively only through the helix α10 region, while the rest of the interface is minimal, a separation of the two domains after phosphorylation of the N-terminal domain is likely to occur (4). This allows the C-terminal domain to arrange helix α10 to its canonical position, leading to an active/open conformation (5) and form a dimer with the α10 helix interface (6), resulting in a DosR dimer-DNA complex in the presence of cognate DNA (7).
Figure 7.

A helix rearrangement activation mechanism of DosR by phosphorylation. The N-terminal regulatory domain is shown in magenta, the linker in blue, and the C-terminal DNA-binding domain in green. Helices are numbered. The critical Asp54 is depicted as a red star with a yellow core. Unphosphorylated DosR dimer in an inactive/closed conformation (1) as seen in the crystal structure of full-length DosR and is in a concentration-dependent equilibrium with monomer (2) in solution. At neutral pH, helix α10 adopts multiple conformations including one with no contacts with the N-terminal domain (3), allowing Asp54 to be solvent exposed. One of these conformations might be the "canonical" conformation, which facilitates phosphorylation of Asp54 which in its turn may contribute to shifting the equilibrium towards that of canonical conformation. The linker helix α5 becomes now the canonical β5 and α5 secondary structure elements (4), resulting in an active/open conformation with the two domains separated by linker α6 (5). This allows the C-terminal domain to arrange helix α10 to its canonical position and interact with α10 from a second subunit to form the α10 helix interface, resulting in a DosR dimer (6). This dimer recognizes cognate DNA yielding DosR dimer-DNA complex (7).
In the absence of a phosphorylated DosR structure, this scenario remains to be validated. Nevertheless, once the C-terminal domain is released from the N-terminal domain and the α10 helix is reorganized to the canonical conformation, DosR becomes active and binds to DNA21 as a dimer as observed in the structures of the DosRC-DNA complex.55
MATERIAL AND METHODS
Protein expression and purification
The E. coli expression system for full-length DosR was constructed as described previously.21 The selenomethionine-substituted (SeMet) DosR with a C-terminal His-tag was expressed in M9 minimal media supplemented with selenomethionine. The growth temperature was reduced from 37°C to 30°C when the OD600 reached 0.6 and the culture was induced with 1 mM IPTG for 4 hours. The cells were harvested by centrifugation at 5,000 rpm for 20 min at 4 °C and stored at −80 °C. Cell pellets containing SeMet DosR were resuspended in a buffer containing 20 mM TrisHCl buffer pH 8.0, 500 mM NaCl, and 10% glycerol, supplemented with 1 mM PMSF, Complete EDTA-free protease inhibitor cocktail (Roche), and benzonase nuclease, and lysed by sonication. To the lysate, protamine sulfate was added and subsequently incubated on ice for 30 minutes. The mixture was centrifuged at 20,000×g for 30 minutes and the supernatant was clarified by filtration and loaded onto a Ni-NTA affinity chromatography column. The non-specifically bound proteins were eluted with 30 mM imidazole in 20 mM Tris-HCl and 500 mM NaCl, 10% glycerol. Subsequently, SeMet DosR was eluted with 300 mM imidazole in the same buffer. Fractions containing SeMet DosR were concentrated and subsequently purified using a Superdex75 HR10/30 size-exclusion column (Amersham Biosciences) equilibrated in a buffer containing 20 mM Tris-HCl pH 7.5, 200 mM NaCl, 10% glycerol, 1 mM EDTA, and 1 mM TCEP (buffer A). SeMet DosR from peak fractions was diluted to 3 mg/ml in the same buffer for crystallization. Native DosR was expressed in LB media and purified using the same procedure as the SeMet protein.
Crystallization
SeMet DosR crystals were grown at 4° C by sitting drop vapor diffusion by adding 2 µl of 10% glycerol, 1 µl of 10 mM MgCl2, and 1 µl of precipitant containing 0.5 M Li2SO4 and 0.1 M citric acid pH 3.0 to 2 µl of protein (3 mg/ml) to minimize showers of microcrystals. Thin plate crystals appeared after 1 week and reached their maximum size after about 1 month. For acetyl phosphate soaking experiments, crystals were transferred from the mother liquor to a working solution containing 25 mM lithium potassium acetyl phosphate and 5 MgCl2 in 1:1:1 ratio by volume of buffer A:50% glycerol:precipitant and were soaked in this solution at 4°C for 1 hour. Crystals were subsequently transferred to a cryoprotectant solution containing 25 mM acetyl phosphate and 5 mM MgCl2 in 1:1:1 ratio of buffer A:75% glycerol:precipitant and flash frozen under liquid nitrogen.
Native DosR was crystallized at 4° C by sitting drop vapor diffusion by adding 2 µl of 10% glycerol, 1 µl of 10 mM MnCl2, and 1 µl of precipitant solution containing 0.5 M lithium sulfate and 0.1 M citric acid pH 3.0 to 2 µl of protein solution. Crystals were soaked for 10 seconds in a solution containing 5 mM MnCl2 in 1:1:1 ratio by volume of buffer A:50% glycerol precipitant followed by a cryoprotectant solution containing 5 mM MnCl2 in 1:1:1 ratio by volume of buffer A:75% glycerol precipitant, and subsequently flash frozen under liquid nitrogen.
DosRC was expressed natively and purified as described previously for SeMet DosRC.55 The protein was crystallized with 24% w/v PEG 5000mme, 0.2 M ammonium sulfate, 10% glycerol and 0.1 M MES buffer pH 6.5. Crystals were transferred to a cryoprotectant solution containing the same concentration of precipitant plus additional 15% ethylene glycol and subsequently flash frozen under liquid nitrogen.
Data Collection and Structure Determination
DosR crystals often diffracted anisotropically and had very high mosaicity (2°–2.5°) unsuitable for data collection. Hundreds of crystals had to be screened for diffraction quality. Data sets were eventually collected from a native crystal to 2.2 Å resolution, and from an acetyl phosphate-soaked SeMet crystal. The SeMet data sets were collected at the Se peak and high-energy remote wavelengths at the Advanced Photon Source (APS) and processed separately (due to random crystal orientations on a goniometer) with HKL200069 to 2.9 Å for the peak wavelength data and 4.1 Å for the remote wavelength data. The peak wavelength was used for finding Se sites and phasing. The systematic absence of the data suggested space group P212121. The Matthews’ coefficient is 2.6 Da/Å3 for 2 molecules in the asymmetric unit (with an anticipated 16 SeMet per asymmetric unit). While the self-rotation function revealed no non-origin peak, the self-Patterson function contains one strong peak at ν = 0.5, suggesting a non-crystallographic translation along the b axis which may contribute to the systematic absence of 0 k 0 reflections. Hence, space group P21212 was also tested. The programs SHELX70 and BnP (SnB)71 were used to locate heavy atoms. Heavy atom sites were refined by the programs SOLVE/RESOLVE72 or SHARP.73,74 All programs were able to find probable solutions for both P212121 and P21212 space groups. However, heavy atom sites in space group P212121 show clearly a drop of peak height after 16 sites were found and maintained high site occupancy after refinement, while in P21212 the peak heights decreased gradually without a clear cut. The program RESOLVE72 was able to find the NCS operators from these sites only in space group P212121 which incorporated a translation of 0.5 along ν in agreement with the self-Patterson. Anomalous Fouriers using phases from RESOLVE confirmed the presence of all 16 sites. However, the map at this point was not interpretable. Molecular replacement with known structures of the DosR C-terminal domain55 did not yield a solution either by using Fobs or experimental phased map. Molecular replacements using homologous protein structures of the N-terminal domain did not yield any solutions either.
The remote wavelength data set was then used to improve phasing power. The program SHELX70 was used to search for 16 Se sites which were found and which appeared to be identical to those found previously using only the peak data. By subsequently using (i) the program SHARP73,74 to refine Se sites and calculate phases;. (ii) RESOLVE72 for density modification with NCS averaging; (iii) the RESOLVE_BUILD script for iterative model building and refinement, RESOLVE75 was able to build 296 residues of which 80 residues had side chains assigned (out of the expected 450 residues in the asymmetric unit) with Rwork/Rfree = 43.9/52.1%.
This initial model was used as a search model for molecular replacement to the native data set collected at Stanford Synchrotron Radiation Laboratory (SSRL) and processed with HKL200069 in space group P21. The program MOLREP76 yielded a clear solution with a 44.1% R-factor and 60% correlation coefficient. At this point, the native data was used for chain tracing. Cycles of RESOLVE_BUILD75 and manual building using program COOT77 and restrained refinement using the program REFMAC78 were carried out. The refinement of the native DosR structure was completed with TLS refinement79,80 using REFMAC78 after subsequent cycles of manual building and water addition. Data collection and refinement statistics are provided in Table 1. Subsequently, the native DosR model was used for molecular replacement for the MAD data set to verify the accuracy of chain trace using anomalous Fourier peaks of methionine residues in the MAD electron density map and to see if Asp54 was phosphorylated. After partial refinement, the SeMet DosR structure turned out to be almost identical to the native structure but, unfortunately, no electron density for a phosphorylated aspartate was observed.
A data set for the native DosRC crystal form II was collected at the Advance Light Source (ALS). The data set was processed with HKL200069 in space group P212121, while the SeMet DosRC crystal form I, crystallized under similar conditions, belongs to space group P1.55 The structure could not be solved by molecular replacement using the complete C-terminal domain of crystal form I55 as search model. A new search model using only helices α7 to α9 was used and two solutions were found using the program MOLREP,76 resulting in subunits A and B per asymmetric unit. The σA-weighted Fobs-Fcalc electron density map after several cycles of rigid body and restrained refinements using REFMAC78 revealed extra electron density for the C-terminal portion of the molecule in a conformation different from what had been observed previously.55 Automatic model building using RESOLVE_BUILD script75 was carried out followed by manual building and water adding using the program COOT.77 Subsequently REFMAC78 was used for TLS refinement,79,80 yielding statistics as summarized in Table 1.
Protein Data Bank accession codes
The coordinates and structure factors of DosR and DosRC have been deposited in the RCSB Protein Data Bank with accession codes 3C3W and 3C57, respectively.
ACKNOWLEDGEMENTS
We thank Konstantin Korotkov and Adrian Rice for providing the full-length DosR and DosRC constructs, respectively. We gratefully acknowledge the use of the Advanced Photon Source (APS) beamline 19-BM (SBC-CAT) at Argonne National Laboratory, the Advanced Light Source (ALS) beamline 8.2.2 (HHMI) at Lawrence Berkeley National Laboratory, the Stanford Synchrotron Radiation Laboratory (SSRL) at Stanford Linear Accelerator Center, and their staffs for technical assistance. Use of the APS, ALS, and SSRL are supported by the U.S. Department of Energy. This work was supported by the Howard Hughes Medical Institute (W.G.J.H.) and by the National Institute of Health grants CA65656 to W.G.J.H. and AI47744 to D.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Parthania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001;26:369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 4.Robinson VL, Buckler DR, Stock AM. A tale of two components: a novel kinase and a regulatory switch. Nat. Struct. Biol. 2000;7:626–633. doi: 10.1038/77915. [DOI] [PubMed] [Google Scholar]
- 5.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 6.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus RP, Dickerson RE. Structure of the Escherichia coli response regulator NarL. Biochemistry. 1996;35:11053–11061. doi: 10.1021/bi960919o. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Hackert E, Stock AM. The DNA-binding domain of OmpR: crystal structures of a winged helix transcription factor. Structure. 1997;5:109–124. doi: 10.1016/s0969-2126(97)00170-6. [DOI] [PubMed] [Google Scholar]
- 8.Mizuno T, Tanaka I. Structure of the DNA-binding domain of the OmpR family of response regulators. Mol. Microbiol. 1997;24:665–667. doi: 10.1046/j.1365-2958.1997.3571723.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee SY, De La Torre A, Yan D, Kustu S, Nixon BT, Wemmer DE. Regulation of the transcriptional activator NtrC1: structural studies of the regulatory and AAA+ ATPase domains. Genes Dev. 2003;17:2552–2563. doi: 10.1101/gad.1125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelton JG, Kustu S, Wemmer DE. Solution structure of the DNA-binding domain of NtrC with three alanine substitutions. J. Mol. Biol. 1999;292:1095–1110. doi: 10.1006/jmbi.1999.3140. [DOI] [PubMed] [Google Scholar]
- 11.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Cascio D, Gunsalus RP, Dickerson RE. NarL dimerization? Suggestive evidence from a new crystal form. Biochemistry. 1998;37:3665–3676. doi: 10.1021/bi972365a. [DOI] [PubMed] [Google Scholar]
- 12.Milani M, Leoni L, Rampioni G, Zennaro E, Ascenzi P, Bolognesi M. An active-like structure in the unphosphorylated StyR response regulator suggests a phosphorylation- dependent allosteric activation mechanism. Structure. 2005;13:1289–1297. doi: 10.1016/j.str.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Buckler DR, Zhou Y, Stock AM. Evidence of intradomain and interdomain flexibility in an OmpR/PhoB homolog from Thermotoga maritima. Structure. 2002;10:153–164. doi: 10.1016/s0969-2126(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 14.Robinson VL, Wu T, Stock AM. Structural analysis of the domain interface in DrrB, a response regulator of the OmpR/PhoB subfamily. J. Bacteriol. 2003;185:4186–4194. doi: 10.1128/JB.185.14.4186-4194.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta N, Kapur V, Singh KK, Das TK, Sachdeva S, Jyothisri K, Tyagi JS. Characterization of a two-component system, devR-devS, of Mycobacterium tuberculosis. Tuber. Lung. Dis. 2000;80:141–159. doi: 10.1054/tuld.2000.0240. [DOI] [PubMed] [Google Scholar]
- 16.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 20.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 2004;279:23082–23087. doi: 10.1074/jbc.M401230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini DK, Malhotra V, Dey D, Pant N, Das TK, Tyagi JS. DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology. 2004;150:865–875. doi: 10.1099/mic.0.26218-0. [DOI] [PubMed] [Google Scholar]
- 23.Saini DK, Malhotra V, Tyagi JS. Cross talk between DevS sensor kinase homologue, Rv2027c, and DevR response regulator of Mycobacterium tuberculosis. FEBS Lett. 2004;565:75–80. doi: 10.1016/j.febslet.2004.02.092. [DOI] [PubMed] [Google Scholar]
- 24.Stock AM, Mottonen JM, Stock JB, Schutt CE. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989;337:745–749. doi: 10.1038/337745a0. [DOI] [PubMed] [Google Scholar]
- 25.Birck C, Mourey L, Gouet P, Fabry B, Schumacher J, Rousseau P, Kahn D, Samama JP. Conformational changes induced by phosphorylation of the FixJ receiver domain. Structure. 1999;7:1505–1515. doi: 10.1016/s0969-2126(00)88341-0. [DOI] [PubMed] [Google Scholar]
- 26.Gouet P, Fabry B, Guillet V, Birck C, Mourey L, Kahn D, Samama JP. Structural transitions in the FixJ receiver domain. Structure. 1999;7:1517–1526. doi: 10.1016/s0969-2126(00)88342-2. [DOI] [PubMed] [Google Scholar]
- 27.Kern D, Volkman BF, Luginbuhl P, Nohaile MJ, Kustu S, Wemmer DE. Structure of a transiently phosphorylated switch in bacterial signal transduction. Nature. 1999;402:894–898. doi: 10.1038/47273. [DOI] [PubMed] [Google Scholar]
- 28.Volkman BF, Nohaile MJ, Amy NK, Kustu S, Wemmer DE. Three-dimensional solution structure of the N-terminal receiver domain of NTRC. Biochemistry. 1995;34:1413–1424. doi: 10.1021/bi00004a036. [DOI] [PubMed] [Google Scholar]
- 29.Lewis RJ, Brannigan JA, Muchova K, Barak I, Wilkinson AJ. Phosphorylated aspartate in the structure of a response regulator protein. J. Mol. Biol. 1999;294:9–15. doi: 10.1006/jmbi.1999.3261. [DOI] [PubMed] [Google Scholar]
- 30.Lewis RJ, Muchova K, Brannigan JA, Barak I, Leonard G, Wilkinson AJ. Domain swapping in the sporulation response regulator Spo0A. J. Mol. Biol. 2000;297:757–770. doi: 10.1006/jmbi.2000.3598. [DOI] [PubMed] [Google Scholar]
- 31.Cho HS, Lee SY, Yan D, Pan X, Parkinson JS, Kustu S, Wemmer DE, Pelton JG. NMR structure of activated CheY. J. Mol. Biol. 2000;297:543–551. doi: 10.1006/jmbi.2000.3595. [DOI] [PubMed] [Google Scholar]
- 32.Lee SY, Cho HS, Pelton JG, Yan D, Berry EA, Wemmer DE. Crystal structure of activated CheY. Comparison with other activated receiver domains. J. Biol. Chem. 2001;276:16425–16431. doi: 10.1074/jbc.M101002200. [DOI] [PubMed] [Google Scholar]
- 33.Stock AM, Martinez-Hackert E, Rasmussen BF, West AH, Stock JB, Ringe D, Petsko GA. Structure of the Mg(2+)-bound form of CheY and mechanism of phosphoryl transfer in bacterial chemotaxis. Biochemistry. 1993;32:13375–13380. doi: 10.1021/bi00212a001. [DOI] [PubMed] [Google Scholar]
- 34.Meyer MG, Park S, Zeringue L, Staley M, McKinstry M, Kaufman RI, Zhang H, Yan D, Yennawar N, Yennawar H, Farber GK, Nixon BT. A dimeric two-component receiver domain inhibits the sigma54-dependent ATPase in DctD. FASEB J. 2001;15:1326–1328. doi: 10.1096/fj.00-0516fje. [DOI] [PubMed] [Google Scholar]
- 35.Park S, Meyer M, Jones AD, Yennawar HP, Yennawar NH, Nixon BT. Two-component signaling in the AAA + ATPase DctD: binding Mg2+ and BeF3− selects between alternate dimeric states of the receiver domain. FASEB J. 2002;16:1964–1966. doi: 10.1096/fj.02-0395fje. [DOI] [PubMed] [Google Scholar]
- 36.Hastings CA, Lee SY, Cho HS, Yan D, Kustu S, Wemmer DE. High-resolution solution structure of the beryllofluoride-activated NtrC receiver domain. Biochemistry. 2003;42:9081–9090. doi: 10.1021/bi0273866. [DOI] [PubMed] [Google Scholar]
- 37.Feher VA, Zapf JW, Hoch JA, Whiteley JM, McIntosh LP, Rance M, Skelton NJ, Dahlquist FW, Cavanagh J. High-resolution NMR structure and backbone dynamics of the Bacillus subtilis response regulator, Spo0F: implications for phosphorylation and molecular recognition. Biochemistry. 1997;36:10015–10025. doi: 10.1021/bi970816l. [DOI] [PubMed] [Google Scholar]
- 38.Gardino AK, Volkman BF, Cho HS, Lee SY, Wemmer DE, Kern D. The NMR solution structure of BeF3−-activated Spo0F reveals the conformational switch in a phosphorelay system. J. Mol. Biol. 2003;331:245–254. doi: 10.1016/s0022-2836(03)00733-2. [DOI] [PubMed] [Google Scholar]
- 39.Madhusudan, Zapf J, Whiteley JM, Hoch JA, Xuong NH, Varughese KI. Crystal structure of a phosphatase-resistant mutant of sporulation response regulator Spo0F from Bacillus subtilis. Structure. 1996;4:679–690. doi: 10.1016/s0969-2126(96)00074-3. [DOI] [PubMed] [Google Scholar]
- 40.Varughese KI, Tsigelny I, Zhao H. The crystal structure of beryllofluoride Spo0F in complex with the phosphotransferase Spo0B represents a phosphotransfer pretransition state. J Bacteriol. 2006;188:4970–4977. doi: 10.1128/JB.00160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riepl H, Scharf B, Schmitt R, Kalbitzer HR, Maurer T. Solution structures of the inactive and BeF3-activated response regulator CheY2. J. Mol. Biol. 2004;338:287–297. doi: 10.1016/j.jmb.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 42.Toro-Roman A, Mack TR, Stock AM. Structural analysis and solution studies of the activated regulatory domain of the response regulator ArcA: a symmetric dimer mediated by the alpha4-beta5-alpha5 face. J. Mol. Biol. 2005;349:11–26. doi: 10.1016/j.jmb.2005.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachhawat P, Swapna GV, Montelione GT, Stock AM. Mechanism of activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. Structure. 2005;13:1353–1363. doi: 10.1016/j.str.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doucleff M, Chen B, Maris AE, Wemmer DE, Kondrashkina E, Nixon BT. Negative regulation of AAA + ATPase assembly by two component receiver domains: a transcription activation mechanism that is conserved in mesophilic and extremely hyperthermophilic bacteria. J. Mol. Biol. 2005;353:242–255. doi: 10.1016/j.jmb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Cho HS, Pelton JG, Yan D, Kustu S, Wemmer DE. Phosphoaspartates in bacterial signal transduction. Curr. Opin. Struct. Biol. 2001;11:679–684. doi: 10.1016/s0959-440x(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 46.Birck C, Malfois M, Svergun D, Samama J. Insights into signal transduction revealed by the low resolution structure of the FixJ response regulator. J. Mol. Biol. 2002;321:447–457. doi: 10.1016/s0022-2836(02)00651-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhang JH, Xiao G, Gunsalus RP, Hubbell WL. Phosphorylation triggers domain separation in the DNA binding response regulator NarL. Biochemistry. 2003;42:2552–2559. doi: 10.1021/bi0272205. [DOI] [PubMed] [Google Scholar]
- 48.Da Re S, Schumacher J, Rousseau P, Fourment J, Ebel C, Kahn D. Phosphorylation-induced dimerization of the FixJ receiver domain. Mol. Microbiol. 1999;34:504–511. doi: 10.1046/j.1365-2958.1999.01614.x. [DOI] [PubMed] [Google Scholar]
- 49.Leoni L, Ascenzi P, Bocedi A, Rampioni G, Castellini L, Zennaro E. Styrene-catabolism regulation in Pseudomonas fluorescens ST: phosphorylation of StyR induces dimerization and cooperative DNA-binding. Biochem. Biophys. Res. Commun. 2003;303:926–931. doi: 10.1016/s0006-291x(03)00450-9. [DOI] [PubMed] [Google Scholar]
- 50.Wyman C, Rombel I, North AK, Bustamante C, Kustu S. Unusual oligomerization required for activity of NtrC, a bacterial enhancer-binding protein. Science. 1997;275:1658–1661. doi: 10.1126/science.275.5306.1658. [DOI] [PubMed] [Google Scholar]
- 51.Blat Y, Eisenbach M. Phosphorylation-dependent binding of the chemotaxis signal molecule CheY to its phosphatase, CheZ. Biochemistry. 1994;33:902–906. doi: 10.1021/bi00170a008. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Swanson RV, Simon MI, Weis RM. The response regulators CheB and CheY exhibit competitive binding to the kinase CheA. Biochemistry. 1995;34:14626–14636. doi: 10.1021/bi00045a003. [DOI] [PubMed] [Google Scholar]
- 53.Welch M, Oosawa K, Aizawa S, Eisenbach M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc. Natl. Acad. Sci. USA. 1993;90:8787–8791. doi: 10.1073/pnas.90.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welch M, Oosawa K, Aizawa SI, Eisenbach M. Effects of phosphorylation, Mg2+, and conformation of the chemotaxis protein CheY on its binding to the flagellar switch protein FliM. Biochemistry. 1994;33:10470–10476. doi: 10.1021/bi00200a031. [DOI] [PubMed] [Google Scholar]
- 55.Wisedchaisri G, Wu M, Rice AE, Roberts DM, Sherman DR, Hol WG. Structures of Mycobacterium tuberculosis DosR and DosR-DNA Complex Involved in Gene Activation during Adaptation to Hypoxic Latency. J. Mol. Biol. 2005;354:630–641. doi: 10.1016/j.jmb.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 56.Ducros VM, Lewis RJ, Verma CS, Dodson EJ, Leonard G, Turkenburg JP, Murshudov GN, Wilkinson AJ, Brannigan JA. Crystal structure of GerE, the ultimate transcriptional regulator of spore formation in Bacillus subtilis. J. Mol. Biol. 2001;306:759–771. doi: 10.1006/jmbi.2001.4443. [DOI] [PubMed] [Google Scholar]
- 57.Kurashima-Ito K, Kasai Y, Hosono K, Tamura K, Oue S, Isogai M, Ito Y, Nakamura H, Shiro Y. Solution Structure of the C-Terminal Transcriptional Activator Domain of FixJ from Sinorhizobium meliloti and Its Recognition of the fixK Promoter. Biochemistry. 2005;44:14835–14844. doi: 10.1021/bi0509043. [DOI] [PubMed] [Google Scholar]
- 58.Maris AE, Kaczor-Grzeskowiak M, Ma Z, Kopka ML, Gunsalus RP, Dickerson RE. Primary and Secondary Modes of DNA Recognition by the NarL Two-Component Response Regulator. Biochemistry. 2005;44:14538–14552. doi: 10.1021/bi050734u. [DOI] [PubMed] [Google Scholar]
- 59.Maris AE, Sawaya MR, Kaczor-Grzeskowiak M, Jarvis MR, Bearson SM, Kopka ML, Schroder I, Gunsalus RP, Dickerson RE. Dimerization allows DNA target site recognition by the NarL response regulator. Nat. Struct. Biol. 2002;9:771–778. doi: 10.1038/nsb845. [DOI] [PubMed] [Google Scholar]
- 60.Pristovsek P, Sengupta K, Lohr F, Schafer B, von Trebra MW, Ruterjans H, Bernhard F. Structural analysis of the DNA-binding domain of the Erwinia amylovora RcsB protein and its interaction with the RcsAB box. J. Biol. Chem. 2003;278:17752–17759. doi: 10.1074/jbc.M301328200. [DOI] [PubMed] [Google Scholar]
- 61.Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, De Francesco R, Neddermann P, Marco SD. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 2002;21:4393–4401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang RG, Pappas T, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 63.Jones S, Thornton JM. Protein-protein interactions: a review of protein dimer structures. Prog. Biophys. Mol. Biol. 1995;63:31–65. doi: 10.1016/0079-6107(94)00008-w. [DOI] [PubMed] [Google Scholar]
- 64.Jones S, Thornton JM. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA. 1996;93:13–20. doi: 10.1073/pnas.93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ames SK, Frankema N, Kenney LJ. C-terminal DNA binding stimulates N-terminal phosphorylation of the outer membrane protein regulator OmpR from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1999;96:11792–11797. doi: 10.1073/pnas.96.21.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simonovic M, Volz K. A distinct meta-active conformation in the 1.1-A resolution structure of wild-type ApoCheY. J. Biol. Chem. 2001;276:28637–28640. doi: 10.1074/jbc.C100295200. [DOI] [PubMed] [Google Scholar]
- 67.Volkman BF, Lipson D, Wemmer DE, Kern D. Two-state allosteric behavior in a single-domain signaling protein. Science. 2001;291:2429–2433. doi: 10.1126/science.291.5512.2429. [DOI] [PubMed] [Google Scholar]
- 68.Madhusudan M, Zapf J, Hoch JA, Whiteley JM, Xuong NH, Varughese KI. A response regulatory protein with the site of phosphorylation blocked by an arginine interaction: crystal structure of Spo0F from Bacillus subtilis. Biochemistry. 1997;36:12739–12745. doi: 10.1021/bi971276v. [DOI] [PubMed] [Google Scholar]
- 69.Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 70.Schneider TR, Sheldrick GM. Substructure solution with SHELXD. Acta Crystallogr. D. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- 71.Howell PL, Blessing RH, Smith GD, Weeks CM. Optimizing DREAR and SnB parameters for determining Se-atom substructures. Acta Crystallogr D. 2000;56(Pt 5):604–617. doi: 10.1107/s0907444900002936. [DOI] [PubMed] [Google Scholar]
- 72.Terwilliger TC. Automated structure solution, density modification and model building. Acta Crystallogr. D. 2002;58:1937–1940. doi: 10.1107/s0907444902016438. [DOI] [PubMed] [Google Scholar]
- 73.Bricogne G, Vonrhein C, Flensburg C, Schiltz M, Paciorek W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D. 2003;59:2023–2030. doi: 10.1107/s0907444903017694. [DOI] [PubMed] [Google Scholar]
- 74.La Fortelle E, Bricogne G. Maximum-likelyhood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 1997;276:472–494. doi: 10.1016/S0076-6879(97)76073-7. [DOI] [PubMed] [Google Scholar]
- 75.Terwilliger TC. Improving macromolecular atomic models at moderate resolution by automated iterative model building, statistical density modification and refinement. Acta Crystallogr. D. 2003;59:1174–1182. doi: 10.1107/S0907444903009922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J. Appl. Cryst. 1997;30:1022–1025. [Google Scholar]
- 77.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 78.Murshudov GN, Vagin AA, Dodson EJ. Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta Crystallogr. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 79.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 80.Winn MD, Murshudov GN, Papiz MZ. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 2003;374:300–321. doi: 10.1016/S0076-6879(03)74014-2. [DOI] [PubMed] [Google Scholar]


