Abstract
Background
Vector control is facing a threat due to the emergence of resistance to synthetic insecticides. Insecticides of botanical origin may serve as suitable alternative biocontrol techniques in the future. Although several plants have been reported for mosquitocidal activity, only a few botanicals have moved from the laboratory to field use, because they are poorly characterized, in most cases active principals are not determined and most of the works are restricted to preliminary screening. Solanum villosum is a common weed distributed in many parts of India with medicinal properties, but the larvicidal activity of this plant has not been reported so far.
Methods
Aqueous and polar/non-polar solvent extract of fresh, mature, green berries of S. villosum was tested against Stegomyia aegypti, a common vector of dengue fever. A phytochemical analysis of chloroform:methanol extract was performed to search for the active toxic ingredient. The lethal concentration was determined (log probit analysis) and compared with Malathion. The chemical nature of the active substance was also evaluated following ultraviolet-visual (UV-Vis) and infrared (IR) analysis.
Results
In a 72 hour bioassay experiment with the aqueous extract, the highest mortality was recorded in 0.5% extract. When the mortality of different solvent extracts was compared, the maximum (p < 0.05) mortality was recorded at a concentration of 50 ppm of chloroform:methanol extract (1:1, v/v). The larvicidal activity was lower when compared with the chemical insecticide, Malathion (p < 0.05). Results of regression analysis revealed that the mortality rate (Y) was positively correlated with the period of exposure (X) and the log probit analysis (95% confidence level) recorded lowest value (5.97 ppm) at 72 hours of exposure. Phytochemical analysis of the chlororm:methanol extract reported the presence of many bioactive phytochemicals. Two toxic compounds were detected having Rf = 0.82 (70% and 73.33% mortality in 24 and 48 hours, respectively) and Rf = 0.95 (40% and 50% mortality in 24 and 48 hours, respectively). IR analysis provided preliminary information about the steroidal nature of the active ingredient.
Conclusion
S. villosum offers promise as potential bio control agent against S. aegypti particularly in its markedly larvicidal effect. The extract or isolated bioactive phytochemical could be used in stagnant water bodies for the control of mosquitoes acting as vector for many communicable diseases.
Background
Mosquitoes transmit several public health problems, such as malaria, filariasis, dengue and Japanese encephalitis, causing millions of deaths every year [1]. Stegomyia aegypti (= Aedes aegypti) is a vector for an arbovirus responsible for dengue fever, dengue haemorrhagic fever and dengue shock syndrome, and with unusual manifestations such as central nervous system involvement [2,3]. About two-fifths of the world's populations are at risk of catching dengue [4-6].
Mosquitoes in the larval stage are attractive targets for pesticides because they breed in water and, thus, are easy to deal with them in this habitat. The use of conventional chemical pesticides has resulted in the development of resistance [7,8], undesirable effects on non-target organisms and fostered environmental and human health concerns [9]. The use of herbal products is one of the best alternatives for mosquito control. The search for herbal preparations that do not produce any adverse effects in the non-target organisms and are easily biodegradable remains a top research issue for scientists associated with alternative vector control [10].
Solanum villosum (Solanaceae: Solanales), commonly known as red-fruit nightshade, is widely distributed in many parts of India. This is an Ayurvedic herb with multiple medicinal properties [11]. The objective of the present study was to examine the larvicidal activity of aqueous, polar and non-polar solvent extracts of the green berries of this plant against the larvae of S. aegypti mosquitoes and to gather preliminary information about the nature of the active ingredient responsible for larval mortality.
Methods
Test mosquitoes
The present study was conducted at Burdwan (23° 16' N, 87° 54' E), West Bengal, India, during June-August 2006. Larvae of S. aegypti were obtained from a laboratory colony maintained in the Mosquito Research Unit, Department of Zoology, The University of Burdwan. The colony was kept free from exposure to pathogens, insecticides or repellents and maintained at 25–30°C. The larvae were fed on a powdered mixture of dog biscuits and dried yeast powder at a ratio of 3:1. The adult colony was provided with 10% sucrose solution and 10% multivitamin syrup, and was periodically blood-fed on restrained rats.
Preparation of aqueous extracts
Fresh, mature, green berries of S. villosum were randomly harvested during the study period from plants growing on the outskirts of Burdwan. All the berries were initially rinsed with distilled water and dried on a paper towel. The crude extracts were prepared by grinding the plant material in a mortar and pestle and passing the ground material through Whatman No 1 filter paper. Required concentrations of aqueous extracts were prepared by mixing the crude extract with a suitable amount of sterilized distilled water.
Preparation of plant extracts in different solvent systems
We harvested 25 g of fresh, mature berries, which were rinsed with distilled water and dried in a shed. The dried berries were put in a Soxhlet apparatus and the plant extracts were prepared using five solvents, namely petroleum ether, benzene, chloroform:methanol (1:1, v/v), acetone and absolute alcohol, applying one after another (extraction period 72 hour for each solvent and the temperature was < 40°C). The extracts were collected separately and the column of the Soxhlet apparatus was washed with 200 ml of water and 100 ml of a similar solvent as an eluent after each type of solvent extraction procedure. The eluted materials and each type of extract were concentrated in combination at 40°C to 100 ml of extract by evaporation in a rotary evaporator. Then each of the extracts was filtered, solvents were evaporated and the solid residues were weighed and then dissolved in a suitable amount of sterilized distilled water for the formulation of graded concentrations. The total yield of each extract from 25 g of berries was as follows: petroleum ether extract, 1.26 g; benzene extract, 2.38 g; chloroform:methanol (1:1, v/v) extract, 4.33 g; acetone extract, 3.00 g; and absolute alcohol extract 2.36 g.
Larvicidal bioassay
The larvicidal bioassay followed the World Health Organization (WHO) standard protocols [12] with slight modifications. Each of the concentrations of aqueous berry extract (0.1–0.5%) was transferred into sterile glass Petri dishes (9 cm diameter/150 ml capacity). Ten third instar larval form of S. aegypti were separately introduced into different Petri dishes containing graded concentrations and the mortality were recorded after 24, 48 and 72 hours of the exposure period. The data of mortality in 48 and 72 hours were expressed by the addition of the mortality at 24 and 48 hours, respectively. Dead larvae were identified when they failed to move after probing with a needle in the siphon or cervical region. The experiments were replicated three times and conducted under laboratory conditions at 25–30°C and 80–90% relative humidity. Similar types of bioassay were conducted with different polar and non-polar solvent extracts (concentrations of 50, 25 and 15 ppm) of the green berries and with a chemical insecticide, Malathion, on third instar larval forms, and chloroform:methanol (1:1, v/v) extract on first and fourth instar larval forms. Larval toxicity was also tested according to similar methodologies using the bioactive substances (from chloroform:methanol extract) isolated from thin-layer chromatographic (TLC) plates.
Phytochemical analysis
The phytochemical analysis was carried out using the chloroform:methanol extract (as it exhibited highest mortality against S. aegypti larvae) of the green berries of S. villosum using the standard methods of Harbone [13] and Stahl [14]. One or two drops of the chloroform:methanol extract were applied (using a capillary tube) to the bottom of each of the pre-coated and pre-heated (100°C for 30 minutes) glass plates (eight glass plates), which were prepared with silicagel G using Unoplan coating apparatus (Shadon, London). After 5 minutes of drying, each of the plates was placed in the separate glass chamber for TLC analysis, with different solvent systems as the mobile phase. After the movement of solvent at the top of the plates, each plate was removed from the glass chamber and separately air-dried. After 10 minutes each of plates was sprayed with a different spraying reagent for the identification of appropriate phytochemical. The phytochemicals included in the study were sapogenins, steroid, terpenoids, flavonoids, alkaloid, essential oils, phenolics and amino acids. A qualitative test was carried out to indicate the presence of saponins [15]; the remaining phytochemicals were determined using TLC analysis by the application of suitable solvents and spray reagents and, in each case, Rf values were recorded.
Ultraviolet-visual and infrared analysis of the active ingredient
The chloroform:methanol extract of the green berries of S. villosum was further chromatogrammed (30 plates) without the application of spraying reagents and each of the spots showed positive activity were separately scrapped according to their respective Rf values. Then each of the spots with their distinguishing Rf value was combined (from 30 plates) and undergoes further bioassay experiment to reveal the nature of active ingredient. As the spots exhibited positive response in Liberman Buchard reagent recorded highest larval mortality during further bioassay experiments, it undergoes spectral analysis by ultraviolet-visual (UV-Vis) and infrared (IR) spectroscopy. The UV-Vis analysis was carried out using a UV-1601 PC, SHIMADZU spectrophotometer with medium scan speed and sampling interval of 0.5 seconds. The IR spectroscopy analysis of the active spot was performed using KBr plates (JASCO FT-IR Model-420) with a scanning speed of 2 mm s-1.
All solvents and reagents used were of analytical grade and purchased from E. Merck, India. The TLC silica gel plates (0.25 mm thickness) were prepared and equilibrated with 2% (w/w) of water before use.
Statistical analysis
The percentage mortality observed (%M) was corrected using Abbott's formula [16] during the observation of the larvicidal potentiality of the plant extracts. A Student's t-test was performed to find the significance between the concentration of plant extract and mortality at different periods with different instars. Statistical analysis of the experimental data was performed using the computer software Statplus 2006 and MS EXCEL 2002 to find the LC50, regression equations (Y = mortality; X = concentrations) and regression coefficient values.
Results
The results of the present study indicate that the mortality rates at a 0.5% concentration were highest amongst all concentrations of the aqueous extracts tested for larval mortality and it was significantly higher (p < 0.05) than the mortality rates at 0.1%, 0.2%, 0.3% and 0.4% concentration of aqueous plant extract at 24, 48 and 72 hours of exposure (Table 1). The mortality of the same instar larval form with different polar and non-polar solvent extracts is presented in Table 2. The larvicidal potentiality of chloroform:methanol (1:1, v/v) extract was further tested for first and fourth instar larvae: the highest mortality was recorded at 15 ppm for first instar larvae and it is statistically more significant (p < 0.05) than both the 5 and 10 ppm concentrations and at both 48 and 72 hours of exposure (Table 3).
Table 1.
The larvicidal activity (mean mortality ± standard error) of different concentrations of aqueous extract of the green berries of S. villosum on third instar larvae of S. aegypti. Student's t-test t = 29.42*, 5.5*, 17.0* (between 0.5% and 0.1%) 12.43*, 3.32*, 14.0* (between 0.5% and 0.2%) and 1.73*, 4.33*, 4.0* (between mortality in 0.5% and 0.3% plant extract at 24, 48 and 72 hours bioassay); * denotes significant (p < 0.05); table value = 2.92 at five degrees of freedom. M, mortality (%); SE, standard error.
| Period of exposure (hours) | |||
| Concentration (%) | 24 | 48 | 72 |
| 0.1 | 20 ± 5.77 | 26.67 ± 8.67 | 30 ± 8.81 |
| 0.2 | 30 ± 7.69 | 36.67 ± 5.77 | 40 ± 7.69 |
| 0.3 | 60 ± 5.57 | 70 ± 1.92 | 73.33 ± 3.84 |
| 0.4 | 66.66 ± 1.92 | 70 ± 1.92 | 76.66 ± 5.57 |
| 0.5 | 76.66 ± 1.92 | 86.66 ± 5.77 | 90 ± 1.92 |
Table 2.
Efficacy of different concentrations of polar and non-polar solvent extracts of the green berries of S. villosum on third instar larvae of S. aegypti. M, mortality (%); S, survivality (%).
| Type of solvents | Concentrations (ppm) | Period of exposure (hours) | |||||
| 24 | 48 | 72 | |||||
| M | S | M | S | M | S | ||
| Petroleum ether | 50 | 3.33 | 96.67 | 6.67 | 93.33 | 13.33 | 86.67 |
| 25 | 3.33 | 96.67 | 3.33 | 96.67 | 10 | 90 | |
| 15 | 0 | 100 | 3.33 | 96.67 | 6.67 | 93.33 | |
| Benzene | 50 | 6.67 | 93.33 | 13.33 | 86.67 | 23.33 | 76.67 |
| 25 | 3.33 | 96.67 | 6.67 | 93.33 | 16.67 | 83.33 | |
| 15 | 3.33 | 96.67 | 6.67 | 93.33 | 13.33 | 86.67 | |
| Chloroform: methanol | 50 | 70 | 30 | 73.33 | 26.66 | 76.66 | 23.33 |
| 25 | 53.33 | 46.66 | 56.66 | 43.33 | 56.66 | 43.33 | |
| 15 | 40 | 60 | 43.33 | 56.66 | 43.33 | 56.66 | |
| Acetone | 50 | 6.67 | 93.33 | 10 | 90 | 13.33 | 86.67 |
| 25 | 6.67 | 93.33 | 10 | 90 | 10 | 90 | |
| 15 | 3.33 | 96.67 | 6.67 | 93.33 | 10 | 90 | |
| Absolute alcohol | 50 | 30 | 70 | 36.67 | 63.63 | 50 | 50 |
| 25 | 13.33 | 86.67 | 23.33 | 76.67 | 33.33 | 66.67 | |
| 15 | 10 | 90 | 16.67 | 83.33 | 26.67 | 73.33 | |
Table 3.
The larvicidal potentiality (mean mortality ± standard error) of different concentrations of chloroform:methanol (1:1, v/v) extract of the green berries of S. villosum and a synthetic insecticide, Malathion, on first and fourth instars larvae of S. aegypti. For first instar larvae: t = 2.07NS, 3.14*, 7.56* (between 15 and 10 ppm at 24, 48 and 72 hours); t = 5.2*, 26.62*, 13.99* (between 15 and 5 ppm at 24, 48 and 72 hours). For fourth instar larvae: t = 2NS, 1.99NS, 0.91NS (between 15 and 10 ppm at 24, 48 and 72 hours); t = 1.73NS, 1.89NS, 1.82NS (between 15 and 5 ppm at 24, 48 and 72 hours). * denotes significant (p < 0.05); NS, not significant (p > 0.05). Table value = 2.92 at five degrees of freedom. M, mortality (%); SE, standard error.
| Instars of mosquito larvae | Concentrations (ppm) | Period of exposure (hours) | ||
| 24 | 48 | 72 | ||
| First | 15 | 60 ± 1.92 | 66.67 ± 5.67 | 70 ± 2.93 |
| 10 | 40 ± 5.57 | 43.33 ± 3.84 | 46.66 ± 3.84 | |
| 5 | 40 ± 5.57 | 40 ± 5.67 | 53.33 ± 1.92 | |
| Malathion (5 ppm) | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | |
| Fourth | 15 | 40 ± 1.92 | 43.33 ± 3.84 | 46.66 ± 5.57 |
| 10 | 33.33 ± 3.84 | 40 ± 1.92 | 43.33 ± 5.56 | |
| 5 | 30 ± 5.57 | 33.33 ± 2.93 | 36.67 ± 2.84 | |
| Malathion (5 ppm) | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | |
However, no significant difference was recorded for fourth instar larvae between 15 and 10 ppm concentrations and 15 and 5 ppm concentrations. An absolute mortality (100%) was observed within 24 hours during the exposure to the chemical insecticide, Malathion (5 ppm concentration). The results of regression analysis revealed that the mortality rate (Y) is positively correlated with the period of exposure (X) having a regression coefficient close to one in each case (Table 4). The results of log probit analysis (95% confidence level) revealed that LC50 values gradually decreased with the exposure periods having the lowest value at 72 hours of exposure to third instar larvae, followed by first and fourth instar larvae. The results of preliminary phytochemical analysis of the chloroform:methanol extract of the green berries of S. villosum are presented in Table 5. A qualitative test indicated the presence of saponins and chromatographic analysis revealed the presence of steroids, alkaloids, terpenoids, saponins, amino acids, phenolics, flavonoids and essential oil as major phytochemicals and the absence of the sapogenins following the application of different solvent systems and spraying reagents. When the isolated compounds from the TLC plates were further bio-assayed against the third instar larvae, the mortality was recorded in two compounds. The highest mortality (at a concentration of 50 ppm) was recorded in the first compound having Rf = 0.818 (70% and 73.33% in 24 and 48 hours, respectively) followed by a second compound having Rf = 0.946 (40% and 50% in 24 and 48 hours, respectively) with maximum absorption at 297.50 and 361.00 nm, respectively, during UV-Vis analysis. IR analysis of two compounds and their respective functional groups are shown in Figures 1 and 2.
Table 4.
Log probit analysis of the larvicidal activity of chloroform:methanol extract of the green berries of S. villosum on different instar larvae of S. aegypti. LC, lethal concentration; R, coefficient of regression equations.
| Type of instars of mosquito larvae | Period of bioassay (hours) | Regression equations | R2 | LC50 values (ppm) | Lower and upper fiducidal limits (ppm) |
| First | 24 | Y = 29.82 + 0.820x | 0.97 | 22.06 | 16.05–27.66 |
| 48 | Y = 33.16 + 0.820x | 0.97 | 19.58 | 14.36–24.20 | |
| 72 | Y = 31.19 + 0.923x | 0.98 | 19.19 | 13.97–23.45 | |
| Third | 24 | Y = 29.83 + 0.820x | 0.97 | 11.67 | 8.49–14.84 |
| 48 | Y = 33.16 + 0.820x | 0.97 | 9.54 | 6.82–12.25 | |
| 72 | Y = 31.19 + 0.923x | 0.98 | 5.97 | 2.15–9.79 | |
| Fourth | 24 | Y = 25.98 + 0.282x | 0.99 | 49.84 | 44.53–54.77 |
| 48 | Y = 31.19 + 0.256x | 0.82 | 21.22 | 13.30–29.13 | |
| 72 | Y = 34.53 + 0.256x | 0.82 | 21.02 | 15.97–73.94 | |
Table 5.
Phytochemical analysis of the chloroform:methanol extract of the green berries of S. villosum
| Solvents used | Spraying reagents | Rf values (and appearance) of the positive spot | Presence/absence of phytochemicals |
| Acetone-hexane (4:1) | Antimony chloride in concentrated hydrochloric acid | - | Absence of sapogenins |
| Methanol-concentrated ammonium hydroxide (200:3) | Dragendorff | 0.95, 0.96 (green) | Presence of alkaloids |
| Chloroform | Libermann-Buchard | 0.95, 0.82, 0.68 (reddish pink) | Presence of steroids |
| Chloroform:benzene (1:1) | Vanillin-sulphuric acid | 0.99 (violet blue) | Presence of essential oil |
| Chloroform:acetic acid:water (90:45:6) | Saturated alcoholic sodium acetate | 0.98 (green) | Presence of flavonoids |
| Ethyl acetate:benzene (1:1) | Folin reagent | 0.98, 0.94 (blue) | Presence of phenolics |
| Ninhydrin | 0.78 | Presence of protein | |
| Chloroform on silica gel plate treated with silver nitrate | Antimony chloride in chloroform | 0.97, 0.48 (green) | Presence of terpenoids |
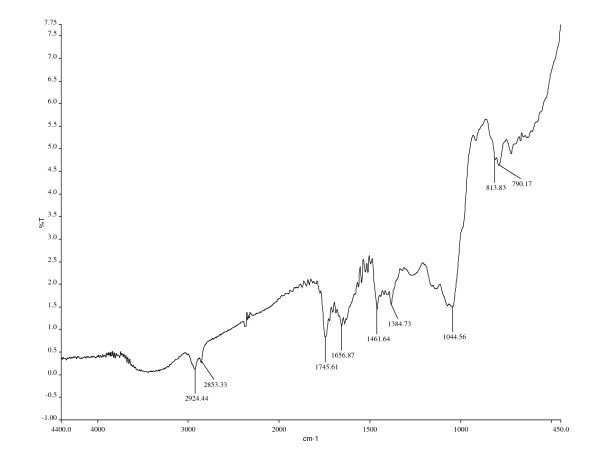
Figure 1.
Interpretation of IR spectra of the compound having Rf = 0.95. Frequency range and probable functional groups of the compound (Rf = 0.946): 2,924.44 and 2,853.33 cm-1, C-H (S) group; 1,745.61 cm-1, C = O (S) stretch; 1,656.87 cm-1, asymmetrical stretch of NO2 compounds (S); 1,461.64 cm-1, scissoring and bending of C-H compounds (V); 1,384.73 cm-1, symmetrical stretches of NO2B Bcompounds (S); 1,044.56 cm-1, C-O stretch (S); 813.83 cm-1, phenyl ring substitution bands (S); 790.17 cm-1, C-H bend (B). V, variable; M, medium; S, strong; Br, broad; W, weak.
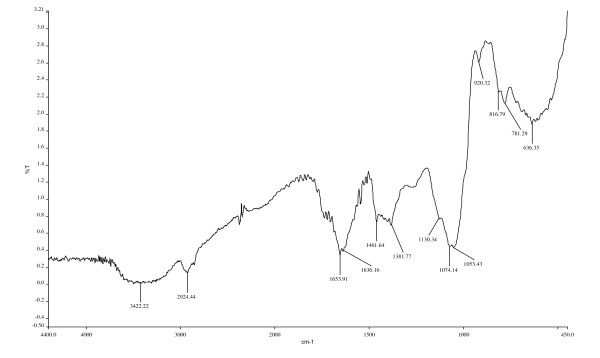
Figure 2.
Interpretation of IR spectra of the compound having Rf = 0.82. Frequency range and probable functional groups of the compound (Rf = 0.818): 3,422.22 cm-1, H bonded OH stress (B); 2,924.44 cm-1, CH (S) stretch; 1,653.91 cm-1, asymmetrical stretch of NO2 compounds (S); 1,636.16 cm-1, NH (M) bond; 1,461.64 cm-1, scissoring and bending of C-H compounds (V); 1,381.77 cm-1, doublet isopropyl (M-W); 1,130.34, 1,074.14, 1,053.43 cm-1, CO group (S) stretch; 920.32 cm-1, alkenes (S) bend; 816.79, 781.29 cm-1, CH phenyl ring (S) substitution bend; 636.35 cm-1, alkynes bend (B). V, variable; M, medium; S, strong; Br, broad; W, weak.
Discussion
Nowadays, mosquito control is mostly directed against larvae and only against adults when necessary. This is because the fight against adult is temporary, unsatisfactory and polluting for the environment, while larval treatment is more localized in time and space resulting in less-dangerous outcomes. Larval control can be an effective control tool due to the low mobility of larval mosquitoes, especially where the principal breeding habitats are man-made and can be easily identified [17].
The secondary compounds of plants make up a vast repository of compounds with a wide range of biological activities. Most studies report active compounds as steroidal saponins. Saponins are freely soluble in both organic solvents and water, and they work by interacting with the cuticle membrane of the larvae, ultimately disarranging the membrane, which is the most probable reason for larval death [18]. Wiesman and Chapagain [19] reported that saponin extracted from the fruit of Balanites aegyptica showed 100% mortality against larvae of S. aegypti. The larvicidal property of a saponin mixture isolated from Cestrum diurnum was also evaluated against Anopheles stephensi mosquito by Ghosh and Chandra [20]. The impact of phenolic compounds on the mosquito larvae has also been reported by many authors [21,22]. Aluminium chloride obtained from alder leaf, known for its phenolic complexing activity, is also reported to have the larvicidal activity against S. aegypti [23]. Isoflavonoids from tubers of Neorautanenia mitis had a larvicidal effect against the malaria and filariasis transmitting mosquitoes, Anopheles gambiae and Cx. quinquefaciatus, respectively [24]. Essential oils extracted from Brazilian plants exhibited larvicidal activity against S. aegypti, with LC50 values ranging from 60 to 538 ppm (see [25]). Studies with Lippia sidoides [26] and Cymbopogon citrates [27] essentials oils suggested that they are a promising biocontrol agent against S. aegypti. Rohini et al [28] isolated D-pinitol, from the EtOH extract of Acacia nilotica, which showed larvicidal activity. Alkaloids derived from Piper longum fruit [29] and Triphyophyllum pellatum [30] showed larvicidal activity against C. pipiens and A. stephensi, respectively. Khanna and Kannabiran [31] reported the role of tannin compounds extracted from Hemidesmus indicus, Gymnema sylvestre and Eclipta prostrate that causes mortality in Cx. quinquefasciatus larvae.
The present study indicates that green berries of S. villosum had biocontrol activity against S. aegypti. The highest mosquitocidal activity was noted in chloroform:methanol extract. The qualitative and chromatographic study of green berries of S. villosum revealed the presence of several bioactive compounds. However, the IR spectra of the bioactive compounds during the present study also indicated that any steroid compound(s) is responsible for larval toxicity.
Conclusion
In conclusion, S. villosum offers promised as a potential bio control agent against S. aegypti particularly in its markedly larvicidal effect. The biocontrol potentiality was lower than chemical insecticides such as Malathion. The extract or isolated bioactive phytochemical from the plant could be used in stagnant water bodies which are known to be the breeding grounds for mosquitoes. However, further studies on the identification of the active principals involved and their mode of action and field trials are needed to recommend S. villosum as an anti-mosquito product used to combat and protect from mosquitoes in a control program.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
NC carried out the laboratory bioassay experimentation and phytochemical analysis of the extract. AG participated in the statistical and spectroscopic analysis and drafted the manuscript. GC participated in the conception, design of experiments, critical revision of the manuscript and coordination. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We are grateful to Dr S Laskar, Professor, Department of Chemistry, The University of Burdwan, West Bengal, India, for his contribution in analysis and interpretation of data. We extend our gratitude to Dr GG Maity, Department of Botany, Kalyani University, West Bengal, for the taxonomic identification of the selected plant.
Contributor Information
Nandita Chowdhury, Email: cnandita_2006@yahoo.co.in.
Anupam Ghosh, Email: anupamghosh75@yahoo.co.in.
Goutam Chandra, Email: goutamchandra63@yahoo.co.in.
References
- Vatandoost H, Vaziri M. Larvicidal activity of neem extract (Azadirachta indica) against mosquito larvae in Iran. Pestology. 2001;25:69–72. [Google Scholar]
- Hendarto SK, Hadinegoro SR. Dengue encephalopathy. Acta Paediatr Jpn. 1992;34:350–357. doi: 10.1111/j.1442-200x.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Pancharoen C, Kulwichit W, Tantawichien T, Thisyakorn U, Thisyakorn C. Dengue infection: a global concern. J Med Assoc Thai. 2002;85:25–33. [PubMed] [Google Scholar]
- Kautner I, Robinson M, Kuhnle U. Dengue virus infection: epidemiology, pathogenesis, clinical presentation, diagnosis, and prevention. J Pediatr. 1997;131:516–524. doi: 10.1016/S0022-3476(97)70054-4. [DOI] [PubMed] [Google Scholar]
- Rigau P. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/S0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization Dengue http://www.who.int/inf-fs/en/fact117.html
- Severini C, Rom R, Marinucci M, Rajmond M. Mechanisms of insecticide resistance in field populations of Culex pipiens from Italy. J Am Mosq Control Assoc. 1993;9:164–168. [PubMed] [Google Scholar]
- World Health Organization Insecticide resistance and vector control. XVII Report of WHO expert Committee on Insecticides. World Health Organ Tech Rep Ser. 1970;443:279. [PubMed] [Google Scholar]
- Forget O. Pesticides, necessary but dangerous poisons. The IDRC Reports. 1989;18:7–13. [Google Scholar]
- Redwane A, Lazrek HB, Bouallam S, Markouk M, Amarouch H, Jana M. Larvicidal activity of extracts from Querus lusitania var infectoria galls (oliv) J Ethnopharmacol. 2002;79:261–263. doi: 10.1016/S0378-8741(01)00390-7. [DOI] [PubMed] [Google Scholar]
- Edmonds JM, Chweya JA. Promoting the Conservation and Use of Underutilized and Neglected Crop; Black Night Shades (Solanum nigrum L) and Related Species. Rome: International Plant Genetic Resources Institute; 1977. pp. 40–46. [Google Scholar]
- World Health Organization Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO/VBC. 1981;81:807. [Google Scholar]
- Harborne JB. Phytochemical Methods, A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall; 1984. pp. 49–188. [Google Scholar]
- Stahl E. Thin Layer Chromatography – A Laboratory Handbook. 2. Berlin: Springer; 1989. [Google Scholar]
- Kokate A. Phytochemical methods. Phytotherapy. 1999;78:126–129. [Google Scholar]
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Ento. 1925;18:265–267. [PubMed] [Google Scholar]
- Howard AFB, Zhou G, Omlin FX. Malaria mosquito control using edible fish in western Kenya: preliminary findings of a controlled study. BMC Public Health. 2007;7:199–204. doi: 10.1186/1471-2458-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostettmann K, Marston A. Saponins (Chemistry and Pharmacology of Natural Products) Cambridge: Cambridge University Press; 1995. p. 132. [Google Scholar]
- Wiesman Z, Chapagain BP. Larvicidal effects of aqueous extracts of Balanites aegyptiaca (desert date) against the larvae of Culex pipiens mosquitoes. Afr J Biotechnol. 2005;4:1351–1354. [Google Scholar]
- Ghosh A, Chandra G. Biocontrol efficacy of Cestrum diurnum (L.) (Solanales: Solanaceae) against the larval forms of Anopheles stephensi. Nat Prod Res. 2006;20:371–379. doi: 10.1080/14786410600661575. [DOI] [PubMed] [Google Scholar]
- Tripathi YC, Rathore M. Role of lipids in natural defense and plant protection. Indian J Forestry. 2001;24:448–455. [Google Scholar]
- Marston A, Maillard M, Hostettmann K. Search for antifungal, molluscicidal and larvicidal compounds from African medicinal plants. J Ethnopharmacol. 1993;38:215–223. doi: 10.1016/0378-8741(93)90018-Z. [DOI] [PubMed] [Google Scholar]
- David JP, Rey D, Meyran JC, Marigo G. Involvement of lignin like compounds in toxicity of dietary alder leaf litter against mosquito larvae. J Chem Ecol. 2000;27:161–174. doi: 10.1023/A:1005632403561. [DOI] [PubMed] [Google Scholar]
- Joseph CC, Ndoile MM, Malima RC, Nkunya MH. Larvicidal and mosquitocidal extracts, a coumarin, isoflavonoids and pterocarpans from Neorautanenia mitis. Trans R Soc Trop Med Hyg. 2004;98:451–455. doi: 10.1016/j.trstmh.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Cavalcanti ESB, Morais SM, Lima MAA, Santana EWP. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz. 2004;99:541–544. doi: 10.1590/S0074-02762004000500015. [DOI] [PubMed] [Google Scholar]
- Carvalho AFU, Melo AA, Craveiro MIL, Machado MB, Rabelo EFB. Larvicidal activity of the essential oil from Lippia sidoides Cham. against Aedes aegypti L. Mem Inst Oswaldo Cruz. 2003;98:569–571. doi: 10.1590/s0074-02762003000400027. [DOI] [PubMed] [Google Scholar]
- Sukumar K, Perich MJ, Boobar LR. Botanical derivatives in mosquito control: A Review. J Am Mosq Control Assoc. 1991;7:210–237. [PubMed] [Google Scholar]
- Rohini C, Pushpa PV, Geeta HD, Vijay TB, Vedavati GP, Vishnu DH, Nirmala DR. Larvicidal activity of Acacia nilotica extracts and isolation of D-pinitol: A bioactive carbohydrate. Chem Biodivers. 2005;2:684–688. doi: 10.1002/cbdv.200590044. [DOI] [PubMed] [Google Scholar]
- Lee SE. Mosquito larvicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum. J Am Mosq Control Assoc. 2000;16:245–247. [PubMed] [Google Scholar]
- Francois G, Looveren MV, Timperman G, Chimanuka B, Assi LA, Holenz J, Bringmann G. Larvicidal activity of the naphthylisoquinoline alkaloid dioncophylline-A against the malaria vector Anopheles stephensi. J Ethnopharmacol. 1996;54:125–130. doi: 10.1016/S0378-8741(96)01459-6. [DOI] [PubMed] [Google Scholar]
- Khanna VG, Kannabiran K. Larvicidal effect of Hemidesmus indicus, Gymnema sylvestre, and Eclipta prostrata against Culex qinquifaciatus mosquito larvae. Afr J Biotechnol. 2007;3:307–311. [Google Scholar]