Abstract
Objective
The Insulin-like Growth Factor-I (IGF-I) is critically involved in the control of cartilage matrix metabolism. It is well known that its binding protein-3 (IGFBP-3) is increased during osteoarthritis (OA), but its function(s) is not known. In other cells, IGFBP-3 can regulate IGF-I action in the extracellular environment and can also act independently inside the cell; this includes transcriptional gene control in the nucleus. These studies were undertaken to localize IGFBP-3 in human articular cartilage, particularly within cells.
Design
Cartilage was dissected from human femoral heads derived from arthroplasty for OA, and OA grade assessed by histology. Tissue slices were further characterized by extraction and assay of IGFBPs by IGF Ligand blot (LB) and by ELISA. Immunohistochemistry (IHC) for IGF-I and IGFBP-3 was performed on cartilage from donors with mild, moderate and severe OA. Indirect fluorescence and immunogold labeling IHC studies were included.
Results
LBs of chondrocyte lysates showed a strong signal for IGFBP-3. IHC of femoral cartilage sections at all OA stages showed IGF-I and IGFBP-3 matrix stain particularly in the top zones, and closely associated with most cells. A prominent perinuclear/nuclear IGFBP-3 signal was seen. Controls using non-immune sera or antigen-blocked antibody showed negative or strongly reduced stain. In frozen sections of human ankle cartilage, immunofluorescent IGFBP-3 stain co-localized with the nuclear DAPI stain in greater than 90% of the cells. Immunogold IHC of thin sections and TEM immunogold microscopy of ultra-thin sections showed distinct intra-nuclear staining.
Conclusions
IGFBP-3 in human cartilage is located in the matrix and within chondrocytes in the cytoplasm and nuclei. This new data indicates that the range of IGFBP-3 actions in articular cartilage is likely to include IGF independent roles and opens the door to studies of its nuclear actions, including the possible regulation of hormone receptors or transcriptional complexes to control gene action.
Keywords: Insulin-like growth factor-1 (IGF-I), IGF-binding protein-3 (IGFBP-3), human, cartilage, nuclear
INTRODUCTION
The IGF signaling axis is involved in the maintenance of matrix metabolism in articular cartilage1,2. Degenerative arthritis (osteoarthritis) is hallmarked by a demise in the metabolic control of cartilage matrix content3 and therefore, possible aberrations in the IGF signaling system prior to or during OA are of great interest. It is well known that IGF activities are regulated by the high affinity IGF-binding proteins (IGFBPs), a system of 6 homologous proteins encoded by different genes4,5. The IGFBPs are a highly versatile group of proteins with the ability to inhibit or enhance IGF action depending on tissue specific regulation. Several investigators have demonstrated increased IGFBPs, primarily IGFBP-3 in chondrocytes derived from OA patients compared to normal controls6,7 and in cartilage explant cultures8. In addition, others and we have demonstrated that elevated levels of IGFBP-3 can be directly extracted or desorbed from fresh uncultured cartilages of OA vs. normal donors9,10. More recently, a weak but statistically significant correlation of IGFBP-3 levels vs. OA score was found in a group of 35 samples from various OA stages11 (Abstract). Previously, IGFBP-3 was co-localized with fibronectin in the pericellular matrix of the chondrocyte12. This pool of IGFBP-3 may be involved in the modulation of IGF-I activity in the vicinity of the IGF-I signaling receptors4,5.
Interestingly, several of the IGFBPs are also capable of signaling independently of the IGFs presumably through their own cellular receptors13, and/or by direct nuclear interactions14. Consistent with these findings, recent studies have suggested that IGFBP-3 may also have IGF-independent actions in cartilage. Transgenic mice over expressing IGFBP-3 show retarded growth, even though they have increased serum IGF-I levels15. This may be due to a direct action of IGFBP-3 at the level of the growth plate. Further, studies with a chondrogenic cell line (RCJ3.1C5.18) reveal that IGFBP-3 has anti-proliferative actions in chondroprogenitors and early chondrocytes derived from this cell line. This activity is independent of IGF-I and involves activation of STAT-116. These studies opened the question of whether IGFBP-3 also has independent actions within articular cartilage. We now explore the localization of IGFBP-3 in human cartilages, focusing on its intracellular distribution and providing new insights into the likely range of action of IGFBP-3 in health and disease.
MATERIALS AND METHODS
Tissue Sources and Preparation
Human cartilages were obtained as discarded surgical material (under MGH protocol # 000214 for human subjects research). Tissues included the femoral heads of 5 donors that underwent hip arthroplasty for OA (ages 45-55; 1 female, 4 males) and one 70-year-old female donor that underwent knee arthroplasty for OA. For each source that had a defined ulcer and sufficient cartilage left on the joint, two pools of cartilage slices were prepared: fibrillated and non-fibrillated (to the naked eye). The fibrillated cartilage was obtained mostly from the rim of the ulcer, comprising ∼3-4 mm of the surrounding tissue. The unfibrillated cartilage was obtained mostly from sites distal to the ulcer, and included smooth and superficially fibrillated cartilage. Two to 3 slices from each site were used for histological assessment of OA grade by the Mankin method17. This method scores severity of disease by evaluation of tissue integrity (formation of fissures and clefts), cell numbers and clone formation, and proteoglycan matrix levels (Saffranin-O stain). The integrity of the tidemark was not evaluated in this study. The best score obtained with this method is 0 (normal) and the worse is 13 (most severe OA). One blinded evaluator and one unblinded author (TM) scored the samples; the grades were averaged. Additionally, 3 representative slices from each cartilage pool were removed for immunohistochemistry (IHC). Both sets of slices were immediately immersed in cold 4% formaldehyde and fixed overnight at 4 °C. The remainder of the cartilage slices were processed for biochemistry as previously described9, i.e. they were extracted in 8M urea containing buffer, the proteoglycans removed by DEAE-chromatography and the eluted proteins dialyzed and dried by speed vac centrifugation. The buffers used for extraction and chromatography were also enriched in proteinase inhibitors to help protect IGFBP-3 from degradation (5 mM phenylmethylsulfonyl fluoride (PMSF), 3 mM o-phenanthroline (o-phe), 6.5 μM pepstatin and 9.5 μM leupeptin); these inhibitors were also maintained in the acidic 4mM HCl solutions during the extensive dialysis prior to drying9 (0.1 mM PMSF, 1 mM o-phe, 0.15 μM pepstatin and 0.2 μM leupeptin). Aliquots were resuspended in assay buffer and IGFBP-3 measured by ELISA (Diagnostic Systems Laboratories). The Iowa team (JM & JB) also obtained human cartilage from their OR (the University of Iowa protocol # 200502797). Ankle cartilage was obtained from a 19 year-old female donor that underwent an above-knee amputation as treatment for osteosarcoma and used for the immunoflorescense study. Additional tissue from the knee of a 48-year-old male, derived from an arthroplasty for OA, was obtained at MGH, tissue slices immersed in DMEM/F12 media (containing penicillin, streptomycin and Hepes buffer) and sent to IOWA by overnight FEDEX. This tissue was used for preparation of thin sections for immunogold IHC and TEM microscopy.
Immunohistochemistry of Femoral Head Specimens
After overnight fixation in cold 4% formaldehyde, the cartilage samples were embedded in paraffin blocks as previously described. After scoring the OA stage in parallel sections17 of the same donors & sites, paraffin embedded slices from 3 sites representing mild, moderate and severe OA were selected for IHC (54 year old male, 45 year old female and 55 year old male respectively). The blocks were then shipped via priority international carrier (FEDEX) to Dr. Hunziker’s laboratory in Switzerland. Sections were cut to a thickness of 5 μm and then subjected to treatment with Chondroitinase ABC (0.02 U/ml) for 60 minutes. The standard horseradish peroxidase immunohistochemical protocol of Sternberger18 was followed. Briefly, unspecific epitopes in the cartilage sections were blocked by treatment with 1% BSA for 45 minutes prior to the immune reaction. Samples were immunoreactive at 1:100 - 1:200 dilution of anti-human IGFBP-3 (Upstate Biotechnologies). Immune reaction with the IGFBP-3 antibody was monitored by addition of the secondary antibody-peroxidase complex and measurement of peroxidase activity with the 3-amino-9-ethylcarbazole substrate. Sections were counter stained with Meyer’s Hematoxilin at a dilution of 1:5 for 1 min. Negative controls were prepared for all samples; these included (1) samples treated with non-Immune serum instead of the first antibody (anti-IGFBP-3), and (2) samples treated with anti-IGFBP-3 previously blocked by incubation with excess antigen (12:1 molar ratio) and then diluted 1:200. IHC for IGF-I was similarly performed in sections from the same donors using polyclonal anti-IGF-I from GroPep LTD. Signal visualization required a 1:20 antibody dilution. The antigen-blocked controls were also performed with a 1:20 dilution of the antigen-antibody complex.
Frozen sections
Cartilage cryosections (10um-thick) were fixed for 5 minutes in freshly prepared 2% paraformaldehyde. Sections were washed in phosphate buffered saline with 0.3% tween-20 (PBST) and blocked for 1 hour in PBST with 1.0 % bovine serum albumin (BSA). Sections were incubated overnight at 4°C in rabbit polyclonal anti-IGFBP-3 primary antibody (diluted 1:250 in PBST, 1% BSA). After 3 washes in PBST the sections were blocked for 30 minutes with PBST containing 10% normal goat serum. The sections were then incubated for 1 hour at room temperature with a goat anti-rabbit Cy2-conjugated secondary antibody (Jackson Immunoresearch), which was diluted 1:250 in PBST+10% goat serum. After several washes in PBST the sections were mounted in Vectashield (Vector Laboratories) containing DAPI. Stained sections were imaged on an Olympus BX60 epifluorescence microscope using 488 nm illumination for Cy2 and 390 nm illumination for DAPI. One section was used as a negative control (no primary antibody).
Sample Processing for Light and Transmission Electron Microscopy
Pieces of human knee cartilage were fixed overnight in 2% paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA) in phosphate buffered saline pH 7.2 (PBS), rinsed in PBS and dehydrated in a graded ethanol series. Samples were infiltrated with several changes of London Resin White resin (Ted Pella Inc., Redding, CA). The pieces were then placed in flat embedment molds, covered in Aclar to minimize exposure to air and polymerized over night with ultraviolet light. The resulting blocks were sectioned at 1μm using a diamond histology knife (Diatome; Biel, Switzerland) and collected upon pre-cleaned glass slides. Ultra-thin sections for transmission electron microscopy (90nm) were cut using a ultra-diamond knife (Diatome; Biel, Switzerland) on a Leica UC-6 ultramicrotome (Leica Microsystems GmbH Ernst-Leitz-Strasse 17-37).
Immunohistochemistry of Thin Sections
A Pelco Biowave, a laboratory microwave (Ted Pella Inc., Redding, CA) was set to a temperature restriction of 37 °C at 150 watts of power. Slides were placed directly on a Cold-Spot load cooler (Ted Pella Inc., Redding, CA). The sections on the slides were covered in PBST (Dulbecco’s PBS adjusted to pH 7.4; with 0.1% tween 20) and given 30 seconds of microwave power. The solution was drained and replaced with 0.05 M glycine in PBST and given another 30 seconds of microwave power. After 3 five minute rinses in PBS at room temperature, the sections were covered in an incubation solution of PBST containing 1% acetylated bovine serum albumin (Electron Microscopy Sciences, Hatfield, PA), and 0.1% cold water fish skin gelatin (Electron Microscopy Sciences, Hatfield, PA) and micro-waved for 30 seconds. IGFBP-3 antibody was diluted in a 1:200 concentration in the incubation buffer or incubation solution alone and micro-waved for 30 seconds, allowed to rest for 30 seconds, and micro-waved for another 30 seconds. The slides were rinsed in 3 changes of PBST and a solution of .Goat anti-rabbit ultra-Small gold conjugate (Aurion, The Netherlands) was flooded onto the sections in a 1:75 concentration in incubation buffer and micro-waved in the same manner as the primary. The slides were again rinsed in PBST at room temperature 3 times, rinsed in double distilled water and silver enhanced with AURION R-Gent Silver Enhancement Kit (Electron Microscopy Sciences, Hatfield, PA) for 18 minutes. The slides were viewed with a BX-51 Olympus light microscope in both brightfield and darkfield mode.
Transmission Electron Microscopy
The ultra thin sections were placed onto a 2 × 1 slot grid coated with a thin layer of formvar. Immunocytochemistry was performed as above with the sections floating on a 20 μl drop of reagent. Grids were then examined with a JEOL-1230 Transmission Electron Microscope (1-2, Musashino 3-chome Akishima, Tokyo 196-8558, Japan).
IGF Ligand Blots
For comparison of cell lysates and tissue extracts, a sample (coded 10-04D) was divided such that half of the cartilage was extracted in buffers containing urea and proteinase inhibitors and purified by the methods described before9 (summarized above); the other half of the cartilage was subjected to digestion with pronase and collagenase to release cells19. Another sample (coded 11-04D; from knee cartilage) was used only for cell preparation. Isolated chondrocytes were maintained in spinner culture overnight20 and then lyzed with RIPA lysis buffer from Upstate (Lake Placid, N.Y.) (0.05 M Tris-HCl, pH 7.4, 0.15 M NaCl, 0.25% deoxycholic acid, 1% NP-40 and 1 mM EDTA). The lysis buffer was supplemented with additional proteinase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml of each aprotinin, leupeptin and pepstatin) and with phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM sodium fluoride).
RESULTS
In previous work, the correlation of IGFBP-3 levels with OA was examined closely by determining the relationship between levels of the binding protein and individual OA scores11(Abstract). It could be demonstrated that while the relationship was weak, there was a statistically significant correlation between IGFBP-3 and OA score. There was also a molar dominance of IGF-I over IGFBP-3 in many of the OA samples, irrespective of score11. This led us to ask whether different sub anatomical pools contain high pockets of IGFBP-3 that may be diluted or obscured in the tissue extracts, but which may play different roles during OA.
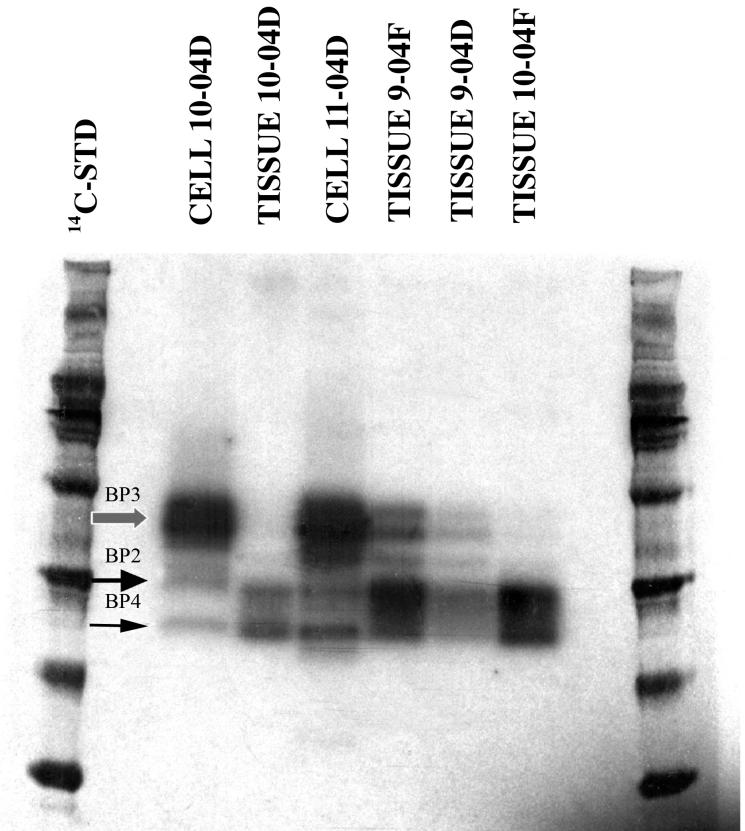
Since there are a number of reports indicating that IGFBP-3 is found within the cell in various cell lines, the question of whether some of the IGFBP-3 is present in chondrocytes was raised. We first addressed this question by preparing fresh chondrocytes and lysing them after minimal culture (overnight in spinner culture). This short incubation period was designed to allow cell recovery from proteolytic matrix excision, while minimizing pericellular protein accumulation. Figure 1 shows an IGF Ligand Blot in which chondrocyte lysates were run alongside cartilage extracts and the membranes probed with a 125I-IGF-II ligand. It can be clearly seen that the cell lysates displayed a very strong positive signal coincident with IGFBP-3. The cell lysate of the 10-04D sample can be directly compared to the tissue extract from the same sample (Figure 1): the lysate showed a much stronger signal even though equal amounts of protein were added to each lane. The more extensive dilution of the whole tissue extracts with matrix protein may have contributed to this. However, the finding that both cell lysates displayed strong signals for IGFBP-3 was consistent with the possibility that a significant portion of the IGFBP-3 in cartilage was cell-associated. We then needed to rule out the possibility that cellular expression was a consequence of the preparative procedures (release of chondrocytes from the matrix by proteolysis) and/or the short incubation period of the cells prior to lysis.
Figure 1. 125IGF-II Western Ligand Blots of Cell Lysates Compared to Total Tissue Extracts.
Sample 10-04 D was divided into two portions; one was extracted (Methods) and the second was subjected to proteolytic digestion to isolate chondrocytes, which were then lysed by RIPA buffer (Methods). Sample 11-04D was used for isolation and lysis of chondrocytes in the same manner. Twenty five μg of tissue extract or cell lysate protein were applied per well. IGF Ligand blot was as described9. The IGFBPs were identified by their molecular weight and previous immunological identification9. BP3 = IGFBP-3, BP2 = IGFBP-2, and BP4 = IGFBP-4. The histological Mankin scores for the samples (from left to right) were: 8.5, 8.5, N.D. (not determined), 7.5, 3, 7.5.
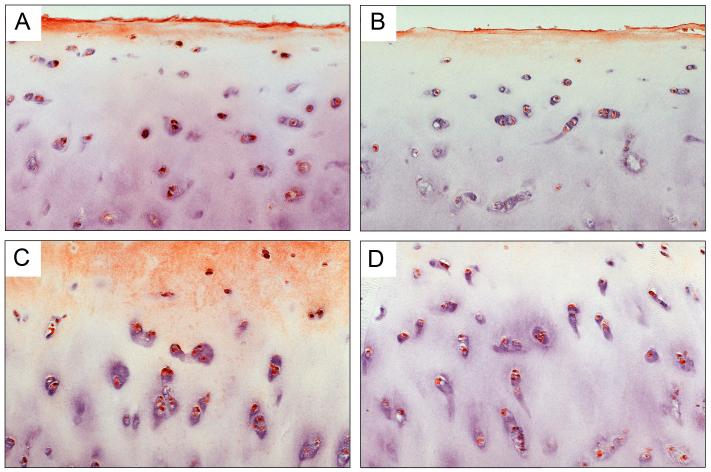
To explore the cellular localization of IGFBP-3 in intact cartilage we carried out immunohistochemistry of representative slices from normal human cartilage (Normal, Mankin grade 1; IGFBP-3 content =6 ng/mg protein), from intermediate (score 6; 15 ng IGFBP-3/mg protein) and advanced OA grades (score 9; 17 ng IGFBP-3/mg protein). Figure 2 clearly shows the presence of cell-associated IGFBP-3 in slices of normal human cartilage (panels A and B) and in sections of cartilage from a donor with intermediate OA (panels C and D). In addition, Figure 3 panels A and B show that cartilage from a donor with severe OA also shows strong cellular immunostain, which includes the cloned cells. Matrix stain was noted in all sections. In the normal cartilage, staining was seen as a thin layer on the top zone of the section (Figure 2A and B, and Figure 4A). In the OA samples, there was more staining, which extended deeper into the section (Figure 2C and 3A and B) but remained mostly confined to ∼the top half of the section.
Figure 2. Immunohistochemistry for IGFBP-3 of Samples with Normal and Intermediate Stage Osteoarthritis.
Panels A and B show slices from a normal donor (Mankin grade 1). The superficial zone is facing the top of the figure and approximately the top half of the cartilage section is shown. Panels C and D show tissue from a donor with intermediate grade OA (Mankin grade 6). The photo in C shows approximately the lower 2/3 of the section, and D shows a middle section. Magnification is ∼200x. Sections were counter stained with Meyer’s Hematoxilin at a dilution of 1:5 for 1 min
Figure 3. Immunohistochemistry for IGFBP-3 of Samples with Advanced Osteoarthritis.
Panels A and B show cartilage from a donor with a Mankin grade 9. Panel C shows a control of the same cartilage treated with antigen-blocked primary antibody (AG:AB molar ratio = 12:1), and D shows a second control in which the primary antibody was omitted. Full cartilage depth is shown in the sections, which were counter stained as in Figure 2. Magnification is ∼200x.
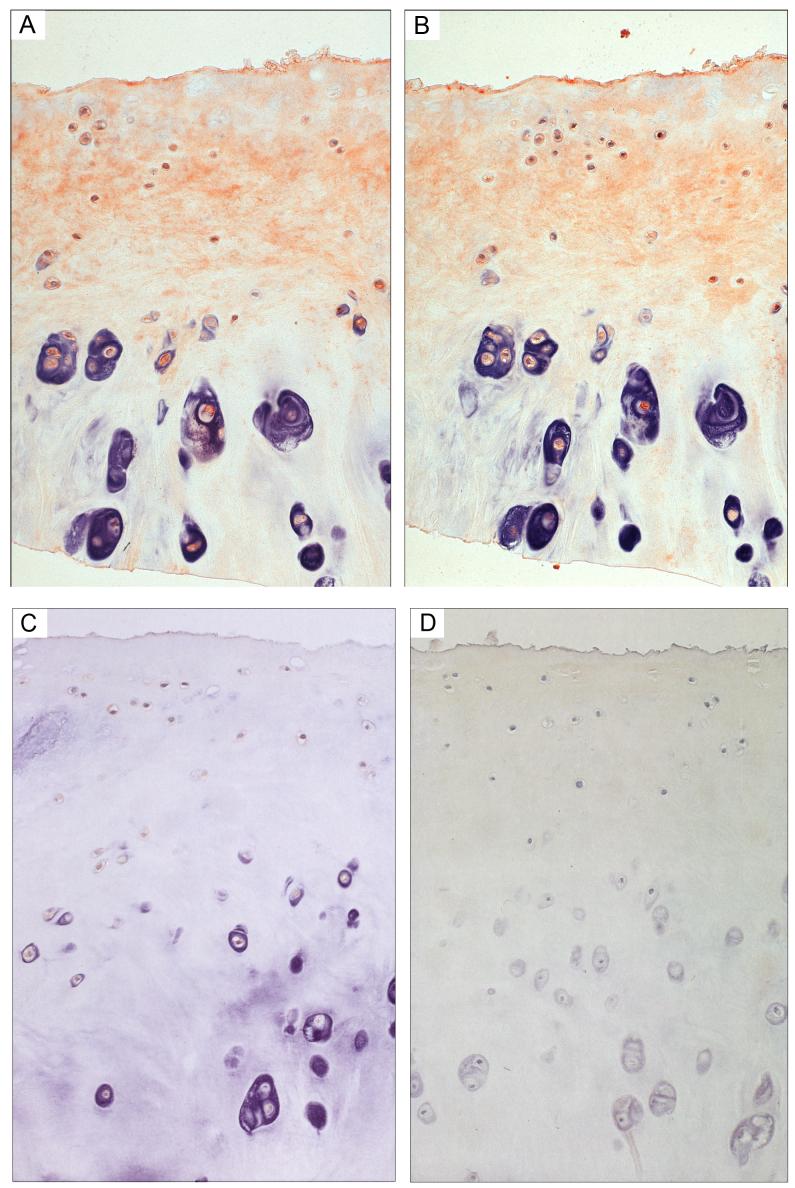
Figure 4. Immunohistochemistry of Nuclei for IGFBP-3 in Normal Cartilage.
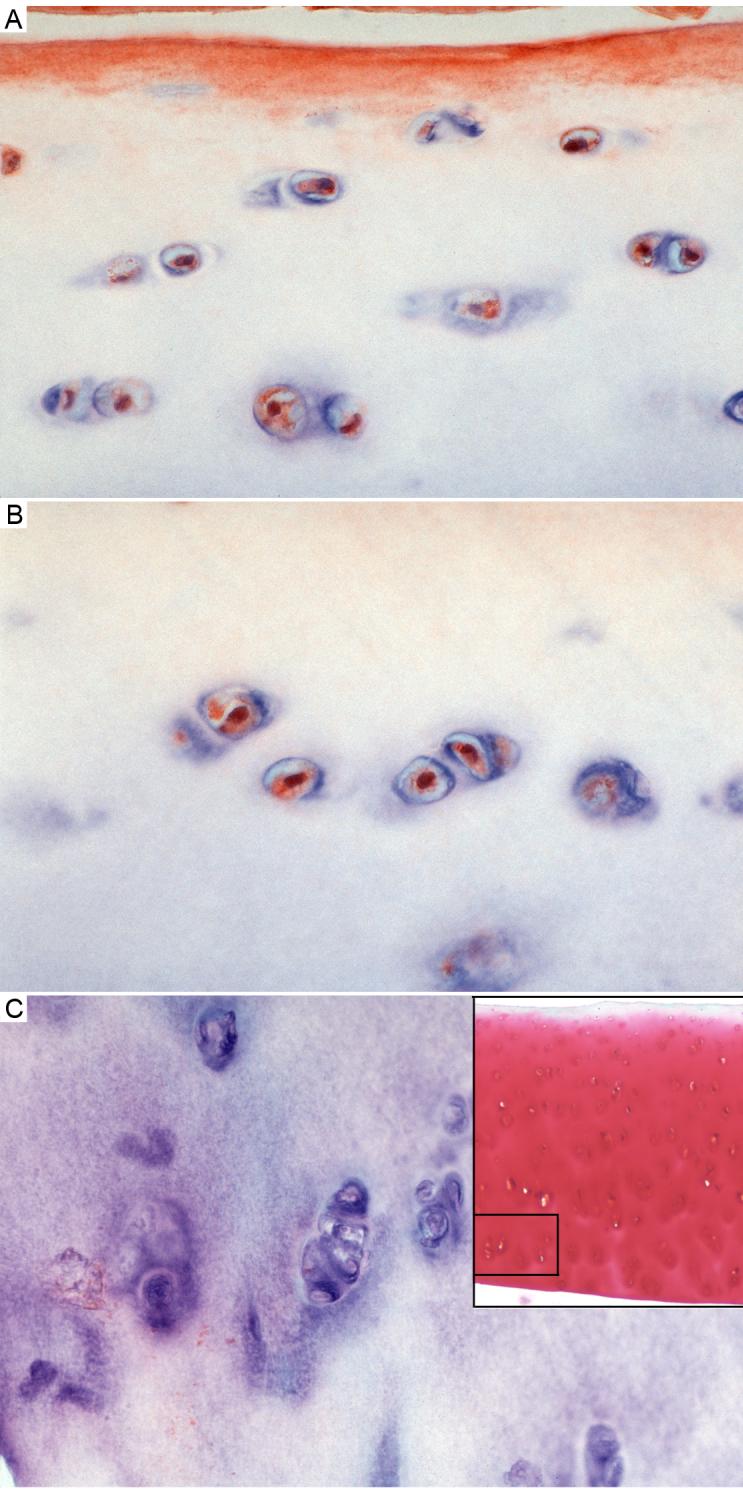
Panels A, B and C show the normal cartilage (Mankin grade 1) immunostained for IGFBP-3. Panel A shows the approximate upper third, panel B shows another view of a similar section just under the superficial zone and panel C shows the ∼ lower third of cartilage (deep zone). Antibody was used at 1:100 dilution. Sections were counter stained as in figure 2. Magnification is approximately 600x. The right hand inset in panel C shows the full-depth section, stained with saffranin-O; the rectangle in this inset shows the approximate location of the IHC section shown in panel C.
In all samples at all OA stages, >90% of the cells were clearly positive for IGFBP-3 content. Importantly, many of the cells in all samples had a strong nuclear IGFBP-3 signal. This can be appreciated best in high-resolution photographs of normal cartilage shown in Figure 4 panels A and B, which show intense but discreet immunoreactivity associated with the nucleus. In addition, many of the cells had a web-like IGFBP-3 pattern outside of the nucleus (Figure 4 A and B). Chondrocytes in the deep zone of the normal cartilage specimens were negative while the OA samples showed a positive cellular signal in this zone (compare Fig. 4C to Figure 3A and B). Controls were prepared for each of the samples either without anti-IGFBP-3 or with anti-BP-3 previously saturated with IGFBP-3 antigen. The negative antibody controls were negative for cells in all the samples (see Figure 3D for example), and the antigen-blocked antibody controls consistently showed very reduced cell immunoreactivity (e.g. Figure 3C). The matrix stain was also blocked or largely reduced in all control samples. However, it needs to be pointed out that the intermediate OA sample (Fig 2C) had particularly intense matrix stain in the top zone (partially shown in the top of Fig 2C) and diffuse stain remained in the antigen-blocked controls (not shown).
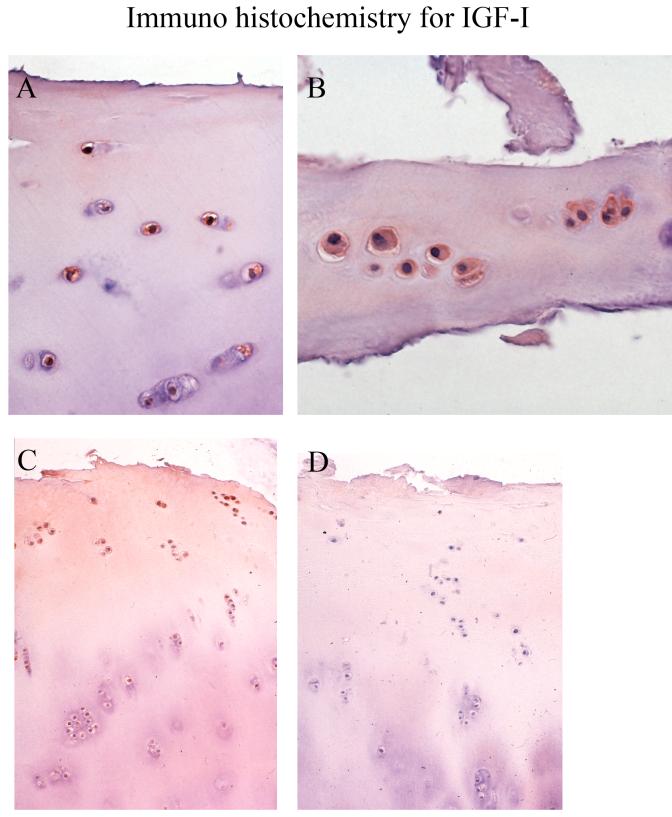
We then asked whether the IGF-I ligand showed a similar distribution in sections from the same samples examined by IGFBP-3 IHC. Figure 5 shows the close association of IGF-I with the chondrocyte in the normal (panel A) and severe OA (panel B) cartilages. Panel C shows a fuller view of the cartilage with intermediate OA; there is more intense IGF-I stain in the upper half of the cartilage in both cells and matrix. This general distribution pattern was also observed in the normal and severe OA samples. Panel D shows the negative control performed without antibody with greatly diminished stain in both the matrix and the cell compartments. Similarly, antigen-blocked controls showed greatly reduced immunostain. In contrast to the IGFBP-3 distribution, IGF-I did not show a dominant association with the nucleus.
Figure 5. Immunohistochemical staining for IGF-I.
Panel A shows a normal cartilage, Panel B a severe OA cartilage and panels C and D are intermediate OA samples, with D being the minus antibody negative control. Panel A shows the approximate upper third of the cartilage, B is a full section of very thin residual cartilage in this severe OA sample, and panels C and D are full depth figures. Sections were counterstained as described in the legend to Figure 2. Magnification is 400x for panels A and B and 200x for C and D.
The apparent nuclear localization of IGFBP-3 deserved closer scrutiny. Frozen sections of 19-year old human cartilage from an ankle joint were examined. Figure 6 shows the immunofluorescent IGFBP-3 stain in the left side panels (A & C) and the DAPI stain in the right side panels (B & D) for adjacent sections. The coincidence between the staining patterns can be seen, indicative of immunoreactive IGFBP-3 in association with the nucleus. Figure 6 E shows the overlay of the two images from panels C & D. Extra nuclear stain can also be appreciated. Negative controls (without primary antibody) were blank (not shown).
Figure 6. Fluorescent immunomicroscopy of IGFBP-3 and DAPI Stained Nuclei in Young Human Cartilage.
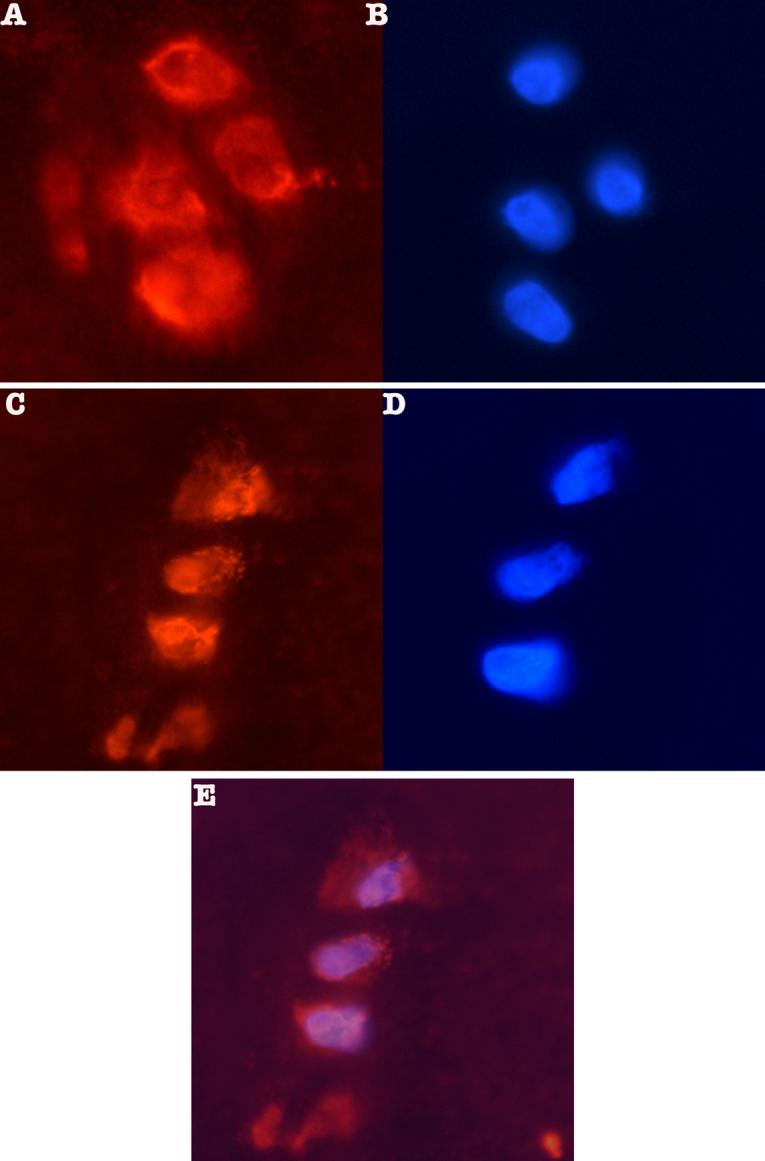
Panels A and C show the orange fluorescent stain for IGFBP-3 detected with Upstate anti-IGFBP-3 (1:250 dilution) and a CY-2 conjugated goat anti-rabbit secondary antibody (Jackson Immunoreasearch). Panels B and D show adjacent sections stained with DAPI to reveal the nuclei. Panel E shows an overlay of panels C & D demonstrating co-localization of IGFBP-3 and DAPI stain.
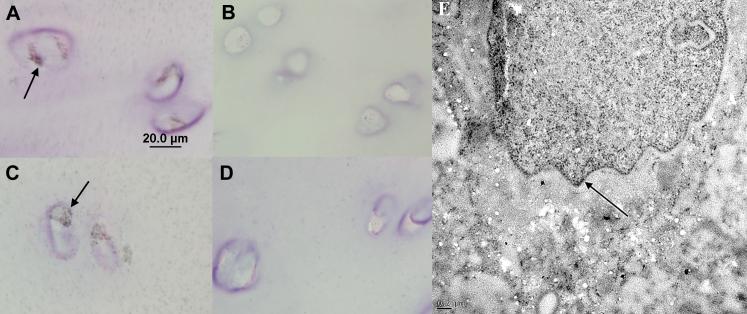
We then prepared thin sections and used immunogold-labeling techniques in order to ascertain if the immunoreactivity associated with the nucleus was intra-nuclear. The tissue source for these studies was a human knee from an OA arthroplasty; the histological OA grade (7.5) indicated that this was an intermediate-to-Severe OA sample. Figure 7, panels A and C shows thin sections (1 μm) of chondrocytes with stained nuclei (arrows) in the transitional zone and the superficial zone respectively, examined by light microscopy. Figure 7, panel E shows the immunogold TEM, which further supports the intra-nuclear location of IGFBP-3.
Figure 7. Immunogold and Immunohistochemical Staining on Thin Sections of Human Cartilage.
Panels A through D show light micrographs of 1 μm sections. Panels A and C show staining of nuclei in chondrocytes (arrows) in the transitional zone and in the superficial zone respectively. Panels B and D show negative controls (without antibody). Panel F shows intra-nuclear immunogold labeling (arrow) for IGFBP-3 in a transitional zone chondrocyte imaged by TEM.
DISCUSSION
The possibility that the IGF-I axis members are segregated within tissue and sub cellular compartments was considered. Tardif et al7 had observed that lysates from monolayer-cultured chondrocytes contained IGFBP-3. We decided to examine freshly isolated chondrocytes maintained in suspension culture for a short overnight period to minimize cellular changes from culture conditions. These experiments indicated that human chondrocyte lysates contained significant levels of IGFBP-3, as assessed by IGF Ligand Blot analysis. We next examined the presence of cell associated IGFBP-3 in intact cartilage slices representing mild, intermediate and severe OA stages by IHC. Positive cellular stain was observed with IGFBP-3 antibodies. A previous report of IHC in human cartilage showed low-resolution immunostaining of IGFBP-3 that seemed associated with cells and matrix21. Our studies extend these previous observations by showing high-resolution microscopy and presenting results of two types of negative controls. As a result, we were able to detect immunoreactive IGFBP-3 in the nucleus. This finding was verified and extended by (1) immunofluorescent co-localization of IGFBP-3 stain and nuclear DAPI stain in frozen sections, (2) immunogold label light microscopy of thin sections and (3) immunogold TEM. The nuclear localization of IGFBP-3 is a new finding in chondrocyte biology, and our study presents one of the most comprehensive evaluations of the association of this protein with the nucleus of any primary cell type. A related paper authored by one of us (TM) describes the sub cellular fractionation of bovine chondrocytes (Morales et al, 2007 in preparation). In this work, we prepared enriched cytoplasmic and nuclear fractions and characterized them by protein patterns, GAPDH, histone and DNA localization, as well as by microscopy of the isolated nuclei. We observed immunoreactive IGFBP-3 in both the cytoplasmic and nuclear fractions. These findings complement and extend the present work by using a completely independent approach to demonstrate the presence of IGFBP-3 in the nuclei of chondrocytes from another species.
The IGFBP-3 immunostain observed in the matrix in the upper zone of many sections was blocked in most of the controls but diffuse residual stain remained in some sections, particularly in the more intensely stained intermediate OA cartilage. Thus, the largest fraction of the immunostaining reaction was of a specific nature, but there was a small non-specific component to the (interterritorial) matrix stain in the top cartilage zones. It is likely that matrix IGFBP-3 in this top zone of cartilage is derived from diffusion from synovial fluid22. This later statement is consistent with the work of Schneiderman et al22, who carried out a study in which IGF-I and its complexes were desorbed from the superficial (top 200 μm) Vs middle to deep zones of human articular cartilage, using 5 normal and 9 OA samples. These authors showed a dominance of IGF-I and its IGFBP complexes in the superficial zone vs. the middle and deep zones of normal and OA human cartilages, with higher levels in the OA cartilage. Of particular interest was their finding of large complexes composed of the IGF-ligand, the IGFBP and the serum ALS molecule in OA cartilage, with 5-fold more of the complex in the superficial zone Vs the middle-deep zone. This large complex was not detectable in normal cartilage. Their IGF ligand blot also shows IGFBP-3 mostly in the top zones of OA cartilage. In its matrix localization, IGFBP-3 may present a diffusion barrier to IGF-I and limit its access to signaling receptors. Consistent with this general view, a previous study of IGF-I transport through bovine cartilage indicated that IGF-I diffusion is impeded by IGFBPs23. Sequestered IGF-I may then be released upon demand. Our present findings that the IGF-I ligand is present in the matrix of the top OA cartilage zones, and is closely associated with the chondrocyte, are consistent with this view.
Taken together, our previous and current data suggests the following novel hypothesis. The tissue segregation of IGFBP-3 may reflect different loci of activity: (1) Matrix-associated IGFBP-3 may be involved in the control of IGF-I diffusion and cell receptor signaling; (2) intracellular and particularly nuclear IGFBP-3 may deliver independent signals to the gene activating machinery. An observation originating in this study is that matrix IGFBP-3 appears most prominent in the top half of cartilage, particularly in normal cartilage. Likewise, normal cartilage showed cellular stain in the upper zones, but none was detectable in the deep zone cells. Future studies will need to dissect the contributions of different autocrine and paracrine sources to IGFBP-3 levels in the different tissue compartments. In addition to the chondrocyte, both the synovial fluid and underlying bone could contribute signals; additionally mechanical factors may contribute to the autocrine or paracrine regulation of this binding protein.
The association of immunoreactive IGFBP-3 with chondrocyte nuclei is consistent with the presence of a bipartite nuclear localization sequence in the carboxyl terminal domain of this binding protein24. Indeed IGFBP-3 has been shown to be transported to or to be present in the nucleus of several cell lines25-28, and its nuclear presence has been associated with the regulation of cell division and apoptosis29-31. Few studies have been carried out on intact tissue sections, but immunostaining of human colonic epithelial sections revealed strong cytoplasmic and nuclear IGFBP-3 in malignant and benign tumors compared to normal epithelium32. Recent studies suggest that endogenous IGFBP-3 may exit the cell before it is re-Internalized and translocated to the nucleus30. The cellular uptake may take place via the transferin receptor (a clathrin pathway) and the caveolin routes27,28, and the nuclear uptake occurs in association with the importin β subunit26. The regulation of IGFBP-3 re-entry into the cell may depend on its ability to bind IGF-I or additional posttranslational modifications27 but the precise mechanisms underlying this important dynamic remain under investigation. Importantly, it has been shown that once in the nucleus, IGFBP-3 may have the ability to regulate gene expression, a case that has been well illustrated with the retinoid receptor system, which regulates apoptosis14,33. The case for nuclear regulatory actions of IGFBP-3 has also been recently supported by the identification of a direct physical interaction between the binding protein and Rpb3 (ribonucleic acid polymerase II binding subunit 3)34. In summary, our study opens new avenues of exploration into the role of IGFBP-3 at the level of gene activation in cartilage and in human osteoarthritis.
ACKNOWLEDGEMENTS
This work was supported by NIH grant AR48304. In addition, the work of Drs Buckwalter and Martin was supported by an NIH P50 award (Specialized Center for Research on OA) AR48939 and by the Veterans Administration. Because of the international nature of the work, we note the following contributions. Dr. Hunziker and Buckwalter were senior authors at the ITI Research Institute (Bern) and the University of Iowa respectively, Dr Martin designed and executed the fluorescence and immunogold labeling studies and Dr. Morales was the lead investigator/ coordinator for the project and senior author at MGH (Boston). The expertise and technical assistance of Carol Trahan (MGH) with the OA scoring is greatly appreciated, as is the technical help of Lihua Zhang (MGH).
Supported by: National Institute of Arthritis, Musculoskeletal and Skin Diseases, NIH, and in part+ by the Veterans Administration (Iowa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington A. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:423–30. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–25. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- (3).Mankin HJ, Mow VC, Buckwalter JA. Articular cartilage repair and osteoarthritis. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. American Academy of Orthopaedic Surgeons; Chicago: 2000. pp. 471–88. [Google Scholar]
- (4).Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocrine Reviews. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- (5).Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinology. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- (6).Olney RC, Tsuchiya K, Wilson DM, Mohtai M, Maloney WJ, Schurman DJ, et al. Chondrocytes from osteoarthritic cartilage have increased expression of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3) and -5, but not IGF-II or IGFBP-4. J Clinical Endocrinology and Metabolism. 1996;81:1096–03. doi: 10.1210/jcem.81.3.8772582. [DOI] [PubMed] [Google Scholar]
- (7).Tardif G, Roboul P, Pelletier J-P, Geng C, Cloutier J-M, Martel-Pelletier J. Normal expression of type 1 insulin-like growth factor receptor by human osteoarthritic chondrocytes with increased expression and synthesis of insulin-like growth factor binding proteins. Arthritis Rheum. 1996;39:968–78. doi: 10.1002/art.1780390614. [DOI] [PubMed] [Google Scholar]
- (8).Chevalier X, Tyler JA. Production of binding proteins and role of the insulin-like growth factor I binding protein 3 in human articular cartilage explants. Br J Rheum. 1996;35:515–22. doi: 10.1093/rheumatology/35.6.515. [DOI] [PubMed] [Google Scholar]
- (9).Morales TI. The insulin-like growth factor binding proteins in uncultured human cartilage. Increases in insulin-like growth factor binding protein 3 during osteoarthritis. Arthritis Rheum. 2002;46:2358–67. doi: 10.1002/art.10482. [DOI] [PubMed] [Google Scholar]
- (10).Eviatar T, Kauffman H, Maroudas A. Synthesis of insulin-like binding protein 3 in vitro in human articular cartilage cultures. Arthritis Rheum. 2003;48:410–7. doi: 10.1002/art.10761. [DOI] [PubMed] [Google Scholar]
- (11).Hunziker EB, Kapfinger E, Morales TI. The Insulin-like growth factor binding protein-3: Relation to IGF levels and localization during human osteoarthritis (Abstract) OA and Cartilage. 2005;13(Supplement A):A32. [Google Scholar]
- (12).Martin JA, Miller MB, Scherb MB, Lebke LA, Buckwalter JA. Co-localization of insulin-like growth factor binding protein 3 and fibronectin in human articular cartilage. Osteoarthritis Cartilage. 2002;10:556–63. doi: 10.1053/joca.2002.0791. [DOI] [PubMed] [Google Scholar]
- (13).Youngman O, Rosenfeld RG. IGF-independent actions of the IGF binding proteins. In: Rosenfeld R, Roberts C Jr., editors. Contemporary Endocrinology: The IGF System. Humana Press; Totowa, N.J.: 1999. pp. 257–72. [Google Scholar]
- (14).Lee KW, Cohen P. Beyond carrier proteins. Nuclear effects: Unexpected intracellular actions of insulin-like growth factor binding protein-3. J Endocrinology. 2002;2002;175:33–40. doi: 10.1677/joe.0.1750033. [DOI] [PubMed] [Google Scholar]
- (15).Modric R, Silha JV, Shi Z, Gui Y, Suwanichkul A, Durham SK, et al. Phenotypic Manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology. 2001;142:1958–67. doi: 10.1210/endo.142.5.8165. [DOI] [PubMed] [Google Scholar]
- (16).Spagnoli A, Torello M, Nagalla SR, Horton WA, Pattee P, Hwa V, et al. Identification of STAT-1 as a molecular target of IGFBP-3 in the process of chondrogenesis. J Biol Chem. 2002;277:18860–7. doi: 10.1074/jbc.M200218200. [DOI] [PubMed] [Google Scholar]
- (17).Mankin HJ, Dorfman HD, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II Correlation of morphology with biochemical and metabolic data. J Bone and Joint Surg. 1971;53A:523–37. [PubMed] [Google Scholar]
- (18).Sternberger LA, Sternberger NH. The unlabeled antibody method: Comparison of peroxidase-antiperoxidase with avidin-biotin complex by a new method of quantification. J Histochem Cytochem. 1986;34:599–605. doi: 10.1177/34.5.3517144. [DOI] [PubMed] [Google Scholar]
- (19).Kuettner KE, Pauli BU, Gall G, Memoli A, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982;93:743–50. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: Migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11:603–12. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- (21).Iwanaga H, Matsumoto T, Enomoto H, Okano K, Hishikawa Y, Hiroyuki S, et al. Enhanced expression of insulin-like growth factor-binding proteins in human osteoarthritic cartilage detected by immunohistochemistry and in situ hybridization. Osteoarthritis and Cartilage. 2005;13:439–48. doi: 10.1016/j.joca.2004.12.006. [DOI] [PubMed] [Google Scholar]
- (22).Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hintz RL, et al. Concentration and size distribution of insulin-like growth factor-I in human normal and osteoarthritic synovial fluid and cartilage. Archives of Biochem and Biophys. 1995;324:173–88. doi: 10.1006/abbi.1995.9913. [DOI] [PubMed] [Google Scholar]
- (23).Bhakta NR, Garcia AM, Frank EH, Grodzinsky AJ, Morales TI. The insulin-like growth factors (IGFs) I and II bind to articular cartilage via the IGF-binding proteins. J Biol Chem. 2000;275:5860–6. doi: 10.1074/jbc.275.8.5860. [DOI] [PubMed] [Google Scholar]
- (24).Radulescu RT. Nuclear localization signal in insulin-like growth factor-binding protein type 3. Trends in Biochemical Sciences. 1994;19:278. doi: 10.1016/0968-0004(94)90004-3. [DOI] [PubMed] [Google Scholar]
- (25).Schelich LJ, Young TF, Firth SM, Baxter RC. Insulin-like growth factor-binding protein (IGFBP-3) and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem. 1998;273:18347–52. doi: 10.1074/jbc.273.29.18347. [DOI] [PubMed] [Google Scholar]
- (26).Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC. Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin β subunit. J Biol Chem. 2000;275:23462–70. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- (27).Singh B, Charkowicz D, Mascarenhas D. Insulin-like growth factor-independent effects mediated by a C-terminal metal-binding domain of insulin-like growth factor binding protein-3. J Biol Chem. 2004;279:477–87. doi: 10.1074/jbc.M307322200. [DOI] [PubMed] [Google Scholar]
- (28).Lee K-W, Liu B, Ma L, Li H, Bang P, Koeffler HP, et al. Cellular internalization of insulin-like growth factor binding protein-3. J Biol Chem. 2004;279:469–76. doi: 10.1074/jbc.M307316200. [DOI] [PubMed] [Google Scholar]
- (29).Li W, Fawcet J, Widmer HR, Fielder PJ, Rabkin R, Keller G-A. Nuclear Transport of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in opossum kidney cells. Endocrinology. 1997;138:1763–6. doi: 10.1210/endo.138.4.5176. [DOI] [PubMed] [Google Scholar]
- (30).Wraight CJ, Liepe IJ, White PJ, Hibbs AR, Werther GA. Intranuclear localization of insulin-like growth factor binding protein-3 (IGFBP-3) during cell division in human keratinocytes. J. Investigative Dermatology. 1998;111:239–42. doi: 10.1046/j.1523-1747.1998.00258.x. 1998. [DOI] [PubMed] [Google Scholar]
- (31).Santer FR, Bacher N, Moser B, Morandell D, Ressler S, Firth SM, et al. Nuclear insulin-like growth factor binding protein-3 induces apoptosis and is targeted to ubiquitin/proteasome-dependent proteolysis. Cancer Res. 2006;66:3024–33. doi: 10.1158/0008-5472.CAN-05-2013. [DOI] [PubMed] [Google Scholar]
- (32).Miraki-Moud F, Jenkins PJ, Fairclough PD, Jordan S, Bustin SA, Jones AM, et al. Increased levels of insulin-like growth factor binding protein-2 in sera and tumours from patients with colonic neoplasia with and without acromegaly. Clin Endocrinology. 2001;54:499–508. doi: 10.1046/j.1365-2265.2001.01221.x. [DOI] [PubMed] [Google Scholar]
- (33).Liu B, Lee H-Y, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, et al. Direct Functional Interactions between Insulin-like Growth Factor-Binding Protein-3 and Retinoid X Receptor-α Regulate Transcriptional Signaling and Apoptosis. J Biol Chem. 2000;275:33607–13. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- (34).Oufattole M, Wan-Jung Lin S, Liu B, Mascarenhas D, Cohen P, Rodgers BD. Ribonucleic acid polymerase II binding subunit 3 (Rpb3), a potential nuclear target of insulin-like growth factor binding protein-3. Endocrinology. 2006;147:2138–46. doi: 10.1210/en.2005-1269. [DOI] [PubMed] [Google Scholar]