Abstract
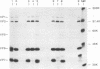
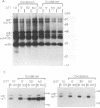
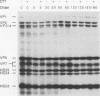
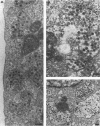
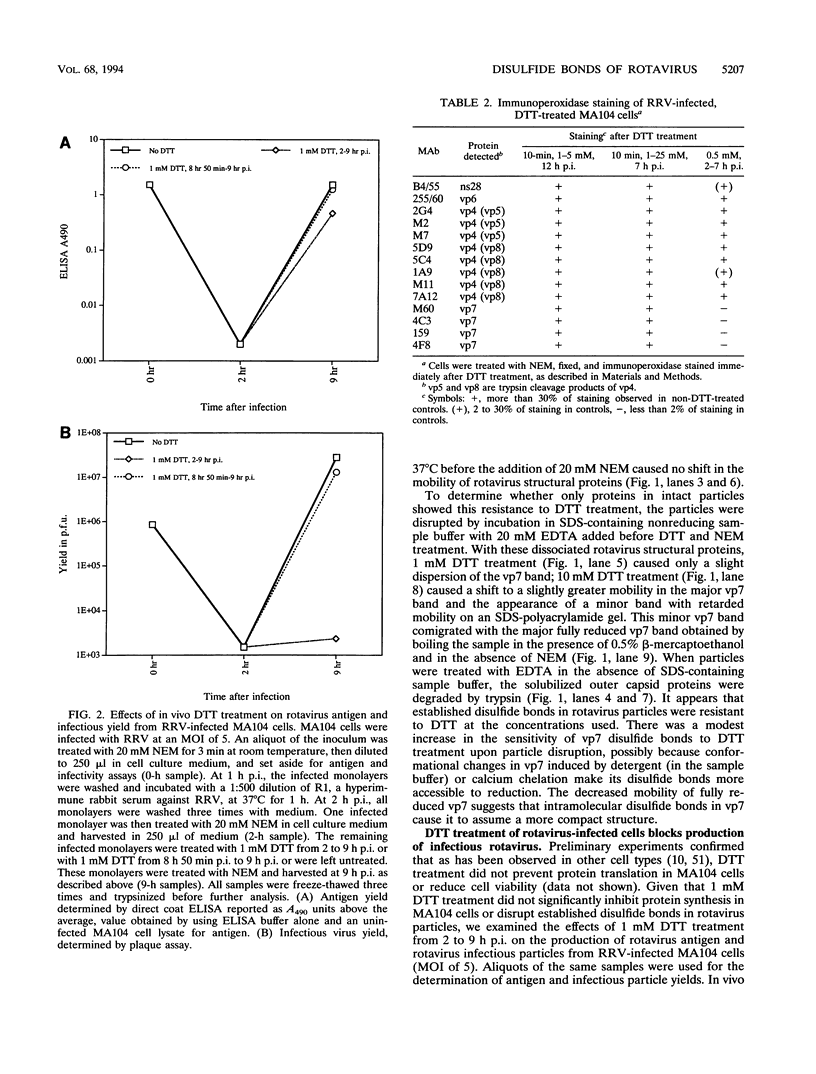
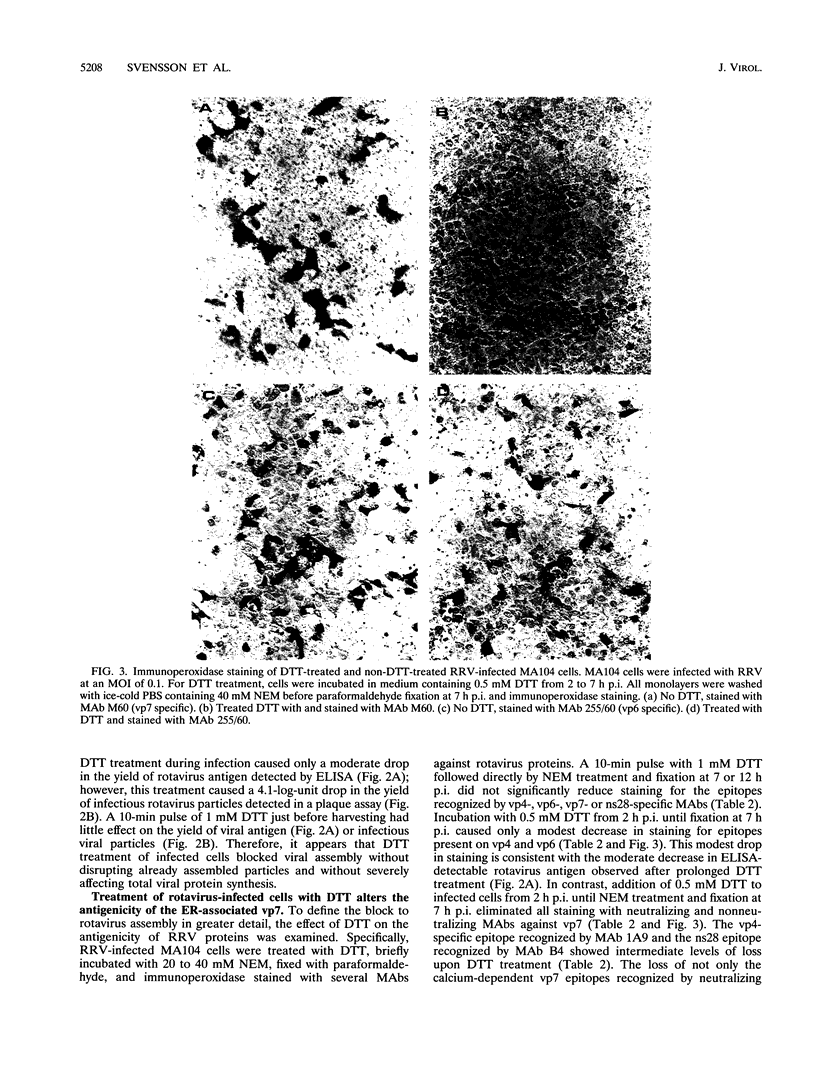
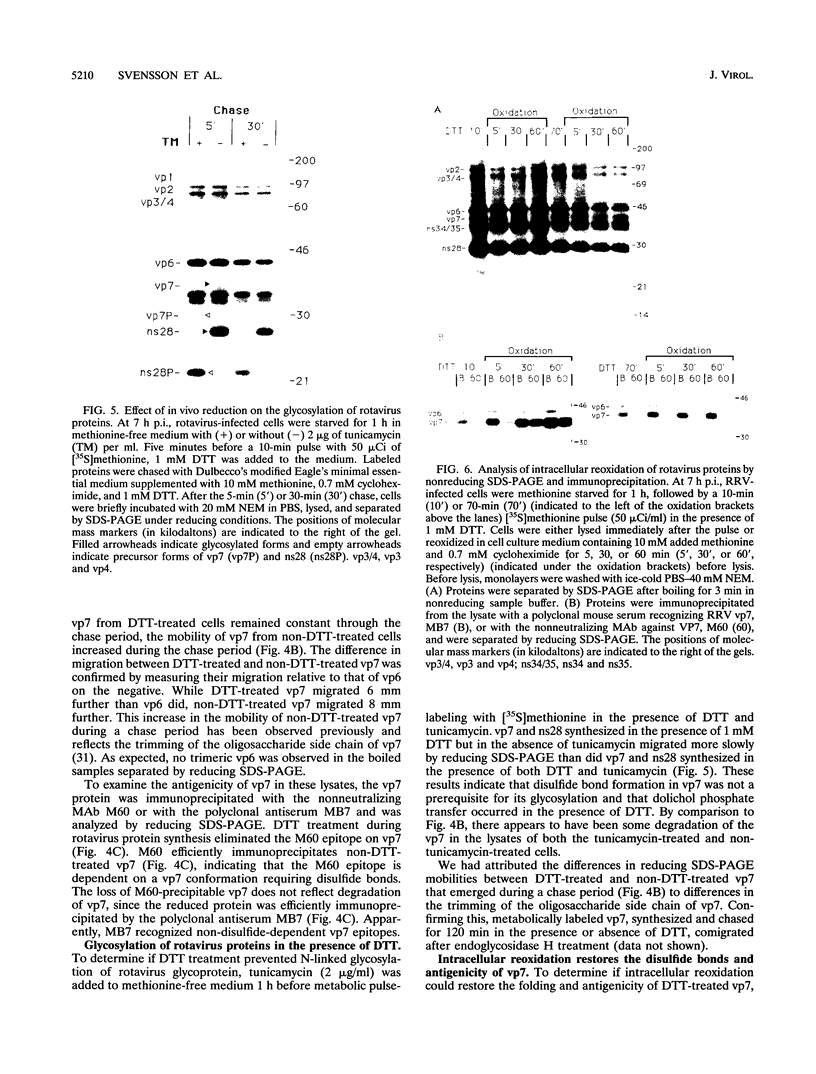
Rotavirus undergoes a unique mode of assembly in the rough endoplasmic reticulum (RER) of infected cells. Luminal RER proteins undergo significant cotranslational and posttranslational modifications, including disulfide bond formation. Addition of a reducing agent (dithiothreitol [DTT]) to rotavirus-infected cells did not significantly inhibit translation or disrupt established disulfide bonds in rotavirus proteins but prevented the formation of new disulfide bonds and infectious viral progeny. In DTT-treated, rotavirus-infected cells, all vp4, vp6, and ns28 epitopes but no vp7 epitopes were detected by immunohistochemical staining with a panel of monoclonal antibodies. When oxidizing conditions were reestablished in DTT-treated cells, intramolecular disulfide bonds in vp7 were rapidly and correctly established with the restoration of antigenicity, although prolonged DTT treatment led to the accumulation of permanently misfolded vp7. Electron microscopy revealed that cytosolic assembly of single-shelled particles and budding into the ER was not affected by DTT treatment but that outer capsid assembly was blocked, leading to the accumulation of single-shelled and enveloped intermediate subviral particles in the RER lumen.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E., SELA M., WHITE F. H., Jr The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1309–1314. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams W. R., Kraft L. M. Electron-Microscopic Study of the Intestinal Epithelium of Mice Infected with the Agent of Epizootic Diarrhea of Infant Mice (EDIM Virus). Am J Pathol. 1967 Jul;51(1):39–60. [PMC free article] [PubMed] [Google Scholar]
- Alberini C. M., Bet P., Milstein C., Sitia R. Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature. 1990 Oct 4;347(6292):485–487. doi: 10.1038/347485a0. [DOI] [PubMed] [Google Scholar]
- Altenburg B. C., Graham D. Y., Estes M. K. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980 Jan;46(1):75–85. doi: 10.1099/0022-1317-46-1-75. [DOI] [PubMed] [Google Scholar]
- Au K. S., Chan W. K., Burns J. W., Estes M. K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J Virol. 1989 Nov;63(11):4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Beckwith J. The bonds that tie: catalyzed disulfide bond formation. Cell. 1993 Sep 10;74(5):769–771. doi: 10.1016/0092-8674(93)90455-y. [DOI] [PubMed] [Google Scholar]
- Bastardo J. W., Holmes I. H. Attachment of SA-11 rotavirus to erythrocyte receptors. Infect Immun. 1980 Sep;29(3):1134–1140. doi: 10.1128/iai.29.3.1134-1140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Hoover-Litty H., Wagner K. R., Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991 Aug;114(3):401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Mottet G. Homooligomerization of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3 occurs before the acquisition of correct intramolecular disulfide bonds and mature immunoreactivity. J Virol. 1991 May;65(5):2362–2371. doi: 10.1128/jvi.65.5.2362-2371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Doig A. J., Williams D. H. Is the hydrophobic effect stabilizing or destabilizing in proteins? The contribution of disulphide bonds to protein stability. J Mol Biol. 1991 Jan 20;217(2):389–398. doi: 10.1016/0022-2836(91)90551-g. [DOI] [PubMed] [Google Scholar]
- Dormitzer P. R., Greenberg H. B. Calcium chelation induces a conformational change in recombinant herpes simplex virus-1-expressed rotavirus VP7. Virology. 1992 Aug;189(2):828–832. doi: 10.1016/0042-6822(92)90616-w. [DOI] [PubMed] [Google Scholar]
- Dormitzer P. R., Ho D. Y., Mackow E. R., Mocarski E. S., Greenberg H. B. Neutralizing epitopes on herpes simplex virus-1-expressed rotavirus VP7 are dependent on coexpression of other rotavirus proteins. Virology. 1992 Mar;187(1):18–32. doi: 10.1016/0042-6822(92)90291-v. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Hanssen H. H., Estes M. K. Two types of glycoprotein precursors are produced by the simian rotavirus SA11. Virology. 1983 Jun;127(2):320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Freedman R. Protein chemistry. Folding into the right shape. Nature. 1987 Sep 17;329(6136):196–197. doi: 10.1038/329196a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gilbert H. F. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Larrea C., Liprandi F., Esparza J. Biochemical evidence for the oligomeric (possibly trimeric) structure of the major inner capsid polypeptide (45K) of rotaviruses. J Gen Virol. 1985 Sep;66(Pt 9):1889–1900. doi: 10.1099/0022-1317-66-9-1889. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. E., Gorziglia M., Woode G. N. Amino acid sequence analysis of bovine rotavirus B223 reveals a unique outer capsid protein VP4 and confirms a third bovine VP4 type. Virology. 1992 Nov;191(1):291–300. doi: 10.1016/0042-6822(92)90191-q. [DOI] [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991 Apr 2;30(13):3147–3161. doi: 10.1021/bi00227a001. [DOI] [PubMed] [Google Scholar]
- Kabcenell A. K., Atkinson P. H. Processing of the rough endoplasmic reticulum membrane glycoproteins of rotavirus SA11. J Cell Biol. 1985 Oct;101(4):1270–1280. doi: 10.1083/jcb.101.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., James J. D., Jr, Kapikian A. Z. Hemagglutination by simian rotavirus. J Clin Microbiol. 1978 Mar;7(3):314–315. doi: 10.1128/jcm.7.3.314-315.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liprandi F., Lopez G., Rodriguez I., Hidalgo M., Ludert J. E., Mattion N. Monoclonal antibodies to the VP6 of porcine subgroup I rotaviruses reactive with subgroup I and non-subgroup I non-subgroup II strains. J Gen Virol. 1990 Jun;71(Pt 6):1395–1398. doi: 10.1099/0022-1317-71-6-1395. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):12753–12760. [PubMed] [Google Scholar]
- Maass D. R., Atkinson P. H. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures. J Virol. 1990 Jun;64(6):2632–2641. doi: 10.1128/jvi.64.6.2632-2641.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. C., Bergmann C. C., Bellamy A. R. Interaction of rotavirus cores with the nonstructural glycoprotein NS28. Virology. 1989 Jul;171(1):98–107. doi: 10.1016/0042-6822(89)90515-1. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Ruiz M. C., del Castillo J. R., Ludert J. E., Liprandi F. Effect of rotavirus infection on intracellular calcium homeostasis in cultured cells. Virology. 1991 Apr;181(2):520–527. doi: 10.1016/0042-6822(91)90884-e. [DOI] [PubMed] [Google Scholar]
- Opstelten D. J., de Groote P., Horzinek M. C., Vennema H., Rottier P. J. Disulfide bonds in folding and transport of mouse hepatitis coronavirus glycoproteins. J Virol. 1993 Dec;67(12):7394–7401. doi: 10.1128/jvi.67.12.7394-7401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Hua J., Mansell E. A. Location of intrachain disulfide bonds in the VP5* and VP8* trypsin cleavage fragments of the rhesus rotavirus spike protein VP4. J Virol. 1993 Aug;67(8):4848–4855. doi: 10.1128/jvi.67.8.4848-4855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Estes M. K., Graham D. Y. Effects of tunicamycin on rotavirus morphogenesis and infectivity. J Virol. 1983 Apr;46(1):270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Greenberg H. B., Graham D. Y., Estes M. K. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1984;1(2):133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Poruchynsky M. S., Maass D. R., Atkinson P. H. Calcium depletion blocks the maturation of rotavirus by altering the oligomerization of virus-encoded proteins in the ER. J Cell Biol. 1991 Aug;114(4):651–656. doi: 10.1083/jcb.114.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Ready K. F., Frenchick P. J., Babiuk L. A. Biochemical evidence for the oligomeric arrangement of bovine rotavirus nucleocapsid protein and its possible significance in the immunogenicity of this protein. J Gen Virol. 1987 Jan;68(Pt 1):123–133. doi: 10.1099/0022-1317-68-1-123. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Babiuk L. A., Lee P. W. Further analysis of the role of calcium in rotavirus morphogenesis. Virology. 1987 May;158(1):103–111. doi: 10.1016/0042-6822(87)90242-x. [DOI] [PubMed] [Google Scholar]
- Shaw A. L., Rothnagel R., Chen D., Ramig R. F., Chiu W., Prasad B. V. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell. 1993 Aug 27;74(4):693–701. doi: 10.1016/0092-8674(93)90516-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Konno T., Numazaki Y. Electron microscopic evidence for budding process-independent assembly of double-shelled rotavirus particles during passage through endoplasmic reticulum membranes. J Gen Virol. 1993 Sep;74(Pt 9):2015–2018. doi: 10.1099/0022-1317-74-9-2015. [DOI] [PubMed] [Google Scholar]
- Svensson L., Sheshberadaran H., Vene S., Norrby E., Grandien M., Wadell G. Serum antibody responses to individual viral polypeptides in human rotavirus infections. J Gen Virol. 1987 Mar;68(Pt 3):643–651. doi: 10.1099/0022-1317-68-3-643. [DOI] [PubMed] [Google Scholar]
- Taniyama Y., Kuroki R., Omura F., Seko C., Kikuchi M. Evidence for intramolecular disulfide bond shuffling in the folding of mutant human lysozyme. J Biol Chem. 1991 Apr 5;266(10):6456–6461. [PubMed] [Google Scholar]
- Tatu U., Braakman I., Helenius A. Membrane glycoprotein folding, oligomerization and intracellular transport: effects of dithiothreitol in living cells. EMBO J. 1993 May;12(5):2151–2157. doi: 10.1002/j.1460-2075.1993.tb05863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Hu Y., Schilling W. P., Lindsay D. A., Eiden J., Estes M. K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994 Jan;68(1):251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A., Braakman I., Helenius A. Posttranslational folding of vesicular stomatitis virus G protein in the ER: involvement of noncovalent and covalent complexes. J Cell Biol. 1993 Feb;120(3):647–655. doi: 10.1083/jcb.120.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]