Abstract
SR-BI/apoE double knockout (dKO) mice exhibit many features of human coronary heart disease (CHD), including hypercholesterolemia, occlusive coronary atherosclerosis, cardiac hypertrophy, myocardial infarctions, cardiac dysfunction and premature death. Ezetimibe is a FDA-approved, intestinal cholesterol-absorption inhibitor that lowers plasma LDL cholesterol in humans and animals and inhibits aortic root atherosclerosis in apoE KO mice, but has not been proven to reduce CHD. Three week-ezetimibe treatment of dKO mice (0.005% (wt/wt) in standard chow administered from weaning) resulted in a 35% decrease in cholesterol in IDL/LDL-size lipoproteins, but not in VLDL- and HDL-size lipoproteins. Ezetimibe treatment significantly reduced aortic root (57%) and coronary arterial (68%) atherosclerosis, cardiomegaly (24%) and cardiac fibrosis (57%), and prolonged the lives of the mice (27%). This represents the first demonstration of beneficial effects of ezetimibe treatment on CHD. The dKO mice were similarly treated with SC-435 (0.01% (wt/wt)), an apical sodium-codependent bile acid transporter (ASBT) inhibitor, that blocks intestinal absorption of bile acids, lowers plasma cholesterol in animals, and reduces aortic root atherosclerosis in apoE KO mice. The effects of SC-435 treatment were similar to those of ezetimibe: 37% decrease in ILD/LDL-size lipoprotein cholesterol and 57% prolongation in median lifespan. Thus, inhibition of intestinal absorption of either cholesterol (ezetimibe) or bile acids (SC-435) significantly reduced plasma IDL/LDL-size lipoprotein cholesterol levels and improved survival of SR-BI/apoE dKO mice. The SR-BI/apoE dKO murine model of atherosclerotic occlusive, arterial CHD appears to provide a useful system to evaluate compounds that modulate cholesterol homeostasis and atherosclerosis.
Keywords: cholesterol, atherosclerosis, ezetimibe, intestinal lipid absorption, SR-BI, apoE, knockout mice, coronary heart disease
1. Introduction
Statins, cholesterol biosynthesis inhibitors, are effective in treating hypercholesterolemia, particularly elevated LDL cholesterol, and reducing mortality from atherosclerotic coronary heart disease (CHD) 1. Inhibition of intestinal absorption of dietary and biliary cholesterol 2 represents a complementary therapeutic approach. For example, ezetimibe (SCH58235) selectively inhibits the intestinal absorption of dietary and biliary cholesterol 3–5 and has been used successfully as a lipid-lowering agent in a variety of animal models and in humans 3, 5–10. Although clinical trials of ezetimibe’s effects on atherosclerosis and CHD 11 have not been reported, Davis and colleagues have shown that ezetimibe treatment for six months 7 or disruption of the gene encoding the Niemann-Pick C1 Like 1 protein 12, the target of ezetimibe, reduce atherosclerotic lesions in apolipoprotein E (apoE) knockout (KO) mice. Inhibition of intestinal bile acid absorption represents another strategy for treating hypercholesterolemia. For example, bile acid sequestrants induce bile acid excretion, increase hepatic bile acid synthesis and LDL receptor expression and consequently reduce plasma LDL cholesterol and heart attacks, although adverse gastrointestinal effects have limited sequestrant use 13. An alternative approach to lower bile acid recirculation is inhibition of the ileal apical sodium codependent bile acid transporter (ASBT) that mediates reabsorption of bile acids. For example, SC-435, an ASBT inhibitor 14–16, efficiently prevents intestinal absorption of bile acids, leading to increased hepatic bile acid synthesis, increased hepatic LDL receptor activity and reduced plasma LDL cholesterol in several animal models 16–19. SC-435 also reduces aortic root atherosclerosis in apoE KO mice (12 week treatment 17) and cholesterol content in the aortic arch of hypercholesterolemic guinea pigs 18.
One of the most widely used murine models for the study of abnormal lipoprotein metabolism and aortic and carotid atherosclerosis is the apoE KO mouse 20, 21. Unfortunately, apoE KO mice usually do not display many of the cardinal features of human CHD, such as occlusive coronary arterial atherosclerosis or myocardial infarction (MI). However, a derivative of the apoE KO mouse, the SR-BI/apoE double knockout (dKO) mouse, represents an attractive, novel, murine CHD model 22. SR-BI/apoE dKO have homozygous null mutations in the genes for both the HDL receptor SR-BI and apoE. When fed a standard laboratory chow diet, dKO mice exhibit many features of human CHD, including hypercholesterolemia, occlusive coronary arterial atherosclerosis, cardiac hypertrophy, myocardial infarctions, cardiac dysfunction and premature death (mean age of death ~6 weeks) 22–24. Because of the complexity of premature death and the influence of lipoprotein metabolism and cardiac function on many organ systems, we do not know if pathologies other than cardiac dysfunction contribute to the premature death of dKO mice. Here we have examined the effects of oral administration of ezetimibe or SC-435 on lipid metabolism, atherosclerosis and CHD in dKO mice. We found that treatment with ezetimibe or SC-435 significantly reduced cholesterol in plasma IDL/LDL-size lipoprotein particles and improved survival. Additional analysis showed that ezetimibe significantly delayed the onset or progression of atherosclerosis and development of abnormal cardiac phenotypes. Thus, dKO mice appear to provide a useful system to evaluate the potential therapeutic benefits of compounds that modulate cholesterol homeostasis and atherosclerosis.
2. Materials and methods
2.1 Animals
All animal experiments, except that in Figure 4C, were conducted at the Biomedical Research Models (BRM, Inc.) facility in Worcester, Massachusetts, using SR-BI/apoE double knockout (dKO) mice on a mixed C57BL/6x129 (75:25) background (strain 2) 22, 24, 25. These dKO mice were generated by mating dKO males, treated with 0.5% (wt/wt) Probucol (Sigma) from weaning and for at least 3 weeks, to SR-BI (+/−)/apoE (−/−) females for 5 days on chow diet without Probucol 26, 27. The dKO male breeders, used repeatedly while healthy, were maintained on probucol-containing diet between matings 27. Genotyping by polymerase chain reaction was performed as previously described 22, 24. At weaning (21 days old), animals were fed standard growth chow (Picolab Mouse diet 20; TestDiet) without or with supplementation of 0.005% (wt/wt) ezetimibe (powdered, Zetia, SCH 58235, 3, 4) or 0.01% (wt/wt) of the ASBT inhibitor SC-435 (pelleted 14, 18, 28), until death or tissue harvesting (3 weeks after weaning). Dose selection of ezetimibe and SC-435 was guided by established responses in murine models to achieve comparable lowering of LDL-cholesterol expected with therapeutic intervention in humans. Based on body weights and food consumption, animals received ~12 mg/kg/day of ezetimibe (standard human dose is 10 mg/day) or ~20 mg/kg/day for SC-435. Mice were housed individually; body weights and apparent food intake (defined as the difference in unconsumed food weight between sequential days) were recorded daily. IACUC animal care guidelines were followed. The survival study in Figure 4C was performed at Pfizer Inc., Saint-Louis, Missouri using strain 1 dKO mice 22, 24, and animals were housed with littermates and fed from weaning a standard chow diet without or with 0.01% (wt/wt) SC-435.
Figure 4. Effects of the ASBT inhibitor SC-435 on plasma lipids, lipoprotein profiles and survival.
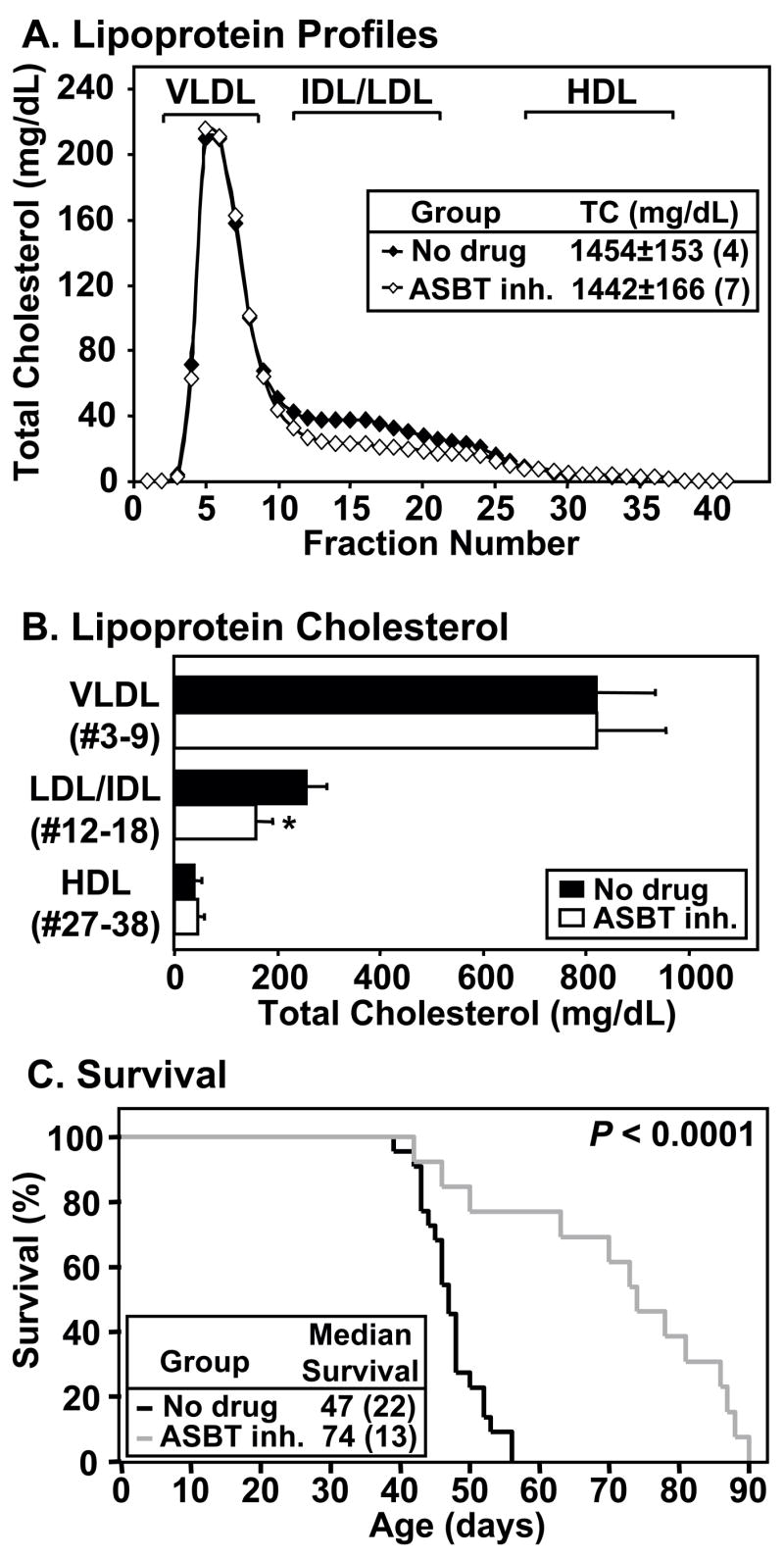
dKO mice were fed standard chow without (‘no drug’; black line, bars or symbols) or with SC-435 (‘ASBT inh.’; grey line or white bars or symbols) from weaning. A and B. Lipoprotein cholesterol profiles, plasma total cholesterol (TC) levels and lipoprotein cholesterol determined at 40–41 days of age. A. Lipoprotein cholesterol profiles (averages from four untreated and seven SC-435-treated mice). Inset shows the average plasma levels of TC. B. For each mouse, TC levels in the indicated pooled fractions corresponding to VLDL-, IDL/LDL- or HDL-size particles were summed and averages of four untreated and seven ASBT inhibitor-treated mice were calculated. * P=0.009 for no drug vs. SC-435-treated. C. Survival curves. Inset indicates median survival time (animal number in parentheses); P < 0.0001. Mice housed at the Massachusetts (panels A and B) or Missouri (panel C) facilities. SC-435 treatment also prolonged the lives of dKO mice in a much smaller scale survival study in the Massachusetts facility (n=3/group), although to a lesser extent (2 week extension; P=0.04).
2.2 Histology, atherosclerosis, and cardiac fibrosis
All morphological and histochemical analyses were performed as described previously 22 or below. Hearts were collected and transverse cryosections were stained with Oil Red O (to visualize sites of lipid accumulation) and hematoxylin. Aortic root lesions were quantified (SPOT software, Diagnostic Instruments, Inc.) by scoring and averaging lesion areas from 3–5 sections spanning the aortic root region. For each heart, the total number of coronary arteries on 5 cross-sections (2 mid-heart and 3 aortic root) was recorded and each coronary artery was scored and distributed into one of three categories, according to the level of occlusion of the lumen 29: severely occluded (50–100%), partially occluded (10–50%) and no or minor occlusion (0–10%). Quantitative assessments of cardiac fibrosis were made using midheart level transverse cryosections stained with Masson’s Trichrome (Sigma) 22. Digital images of sections collected using a Nikon E600 microscope with a SPOT digital camera and software (Diagnostic Instruments, Inc.) were analyzed using ImageJ software (Wright Cell Imaging Facility, Canada) and color-based thresholding segmentation of the images (32 bits/pixel, RGB). To calculate the percentage of cardiac fibrosis, we defined total ventricular myocardial tissue area as the total number of all blue and red pixels in the image, excluding atrial tissue and all valve leaflets. Fibrotic area was then defined as the total number of blue pixels. % cardiac fibrosis = (fibrotic area/total tissue area) ×100.
2.3 Determination of plasma lipids and lipoprotein cholesterol content
Levels of plasma total cholesterol (TC), unesterified cholesterol and phospholipids (PL) were determined using standard assay kits from Wako Chemicals (Richmond, VA), according to vendor instructions. Plasma triglyceride (TG) levels were determined by Charles River Laboratories. FPLC plasma fractionation was performed as previously described 24.
2.4 Statistical Analysis
A value of P ≤ 0.05 was considered significant (2-tailed, unpaired Student’s t test). Average values are shown ± standard deviation (SD). Error bars represent SD.
3. Results
To determine the influence of drugs that interfere with intestinal lipid absorption on CHD and premature death in SR-BI/apoE dKO mice 22, 24, 25, we fed these mice starting at weaning (21 days old) a standard growth chow diet alone (untreated or ‘no drug’) or supplemented with either the cholesterol absorption inhibitor ezetimibe 3 (0.005% wt/wt) or the bile acid uptake inhibitor (apical sodium codependent bile acid transporter inhibitor (ASBT inh.)) SC-435 14, 15 (0.01% wt/wt), and then examined the effects of the treatments. Blood and heart samples were collected at ~6 weeks of age (range: 39–44 days), after 3 weeks of drug treatment.
3.1 Effects of Ezetimibe on Body Weight and Food Intake
At weaning, body weights were similar for untreated (9.4±1.4 g (n=21)) and ezetimibe-treated (9.8±1.4 g (n=26); P=0.37) dKO mice. Subsequently, all animals gained weight for at least 2 weeks. Untreated dKO mice usually stopped gaining weight, or even lost weight, one to seven days prior to death (22; data not shown). Average body weight throughout the study was higher in the ezetimibe-treated (16.6±4.3 g; n=26) than in the no drug (14.5±3.2 g; n=21; P<0.001) group. Treated mice also exhibited a slightly higher apparent food intake (3.4±1.3 vs. 2.9±1.2 g/day; P<0.001), possibly due to better palatability of the ezetimibe-containing food or to a beneficial effect of ezetimibe on general health. Based on the average body weight and apparent food intake, we estimate that treated dKO mice received an average ezetimibe dose of 12 mg/kg/day.
3.2 Effects of Ezetimibe on Plasma Lipids and Lipoproteins
Figure 1A shows that ezetimibe treatment did not alter the steady-state plasma levels of total cholesterol (TC), phospholipids (PL) or triglycerides (TG), nor did treatment alter the abnormally high unesterified cholesterol to TC ratio (not shown) previously reported for untreated dKO mice 27. The lipoprotein cholesterol profiles (FPLC size fractionation, Figure 1B) of untreated and ezetimibe-treated mice were very similar, with no differences in the distribution of cholesterol in the VLDL- and HDL-size ranges. However, ezetimibe treatment induced a significant 35% reduction in cholesterol in the IDL/LDL-size range (no drug: 217±63 mg/dL; ezetimibe: 140±56 mg/dL; P=0.043; Figure 1B and C). These ezetimibe effects on dKO mice were rather modest. Because substantial portions of the lipoproteins in the VLDL and IDL/LDL-size ranges in dKO mice are abnormally large, apoA-I-containing, HDL-like particles 24, and previous reports suggest ezetimibe has only a minor influence on HDL cholesterol 5, the modest effects of ezetimibe observed here are not surprising.
Figure 1. Effects of ezetimibe on plasma lipids and lipoproteins.
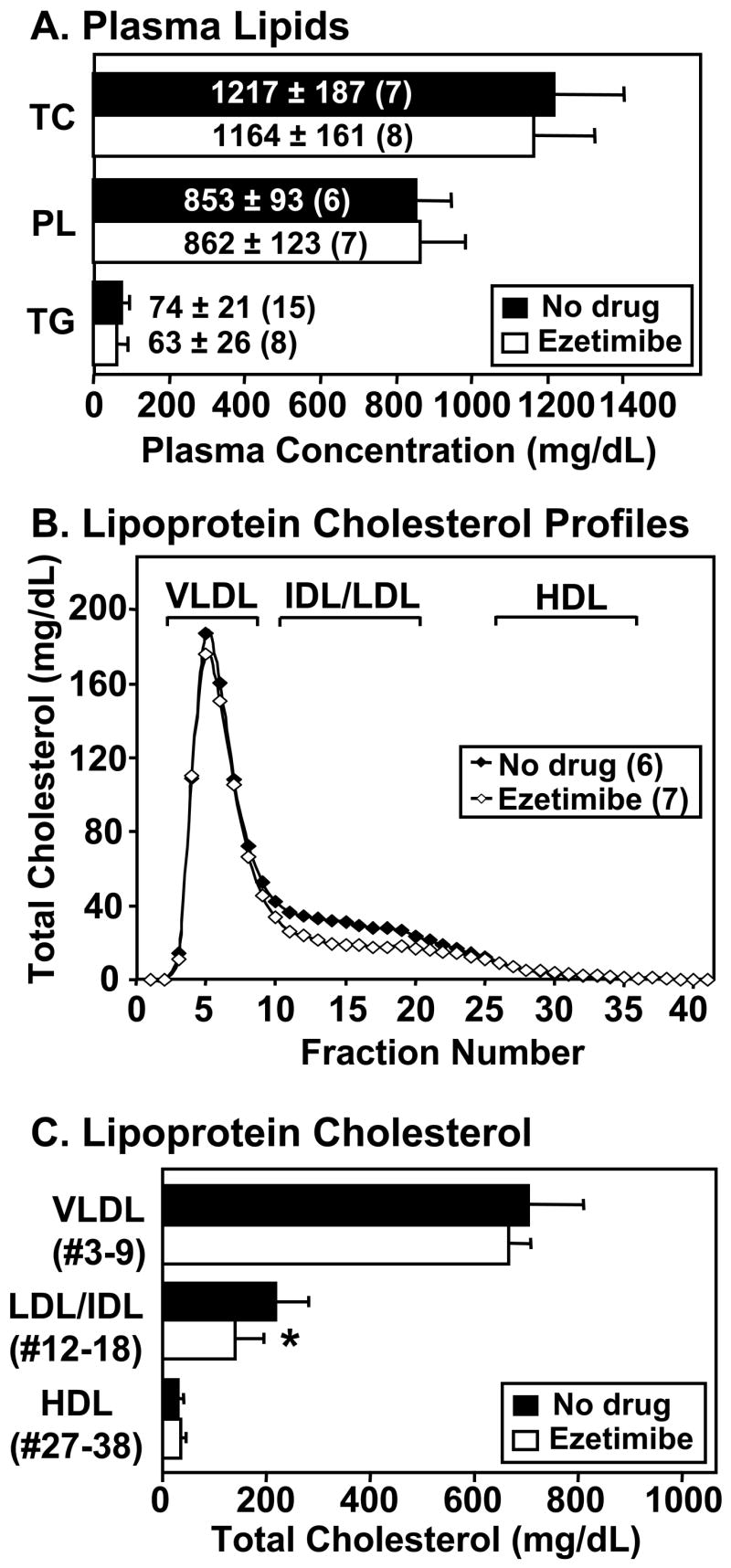
dKO mice (animal number in parentheses) were fed standard chow without (‘no drug’, black bars or symbols) or with ezetimibe (white bars or symbols) from weaning. Plasma was harvested at 39–44 days of age. A. Average plasma lipid concentrations for total cholesterol (TC), phospholipids (PL), and triglycerides (TG). B. Plasma lipoprotein cholesterol profiles determined by FPLC size fractionation (TC, mg/dL plasma, average of profiles from six untreated and seven ezetimibe-treated mice). Brackets indicate sizes of standard human lipoproteins. C. For each mouse, total cholesterol levels in the indicated pooled fractions corresponding to VLDL-, IDL/LDL- or HDL-size particles were summed and averages for six untreated and seven ezetimibe-treated mice were calculated. * P=0.043 for no drug vs. ezetimibe-treated values.
3.3 Effects of Ezetimibe on Atherosclerosis
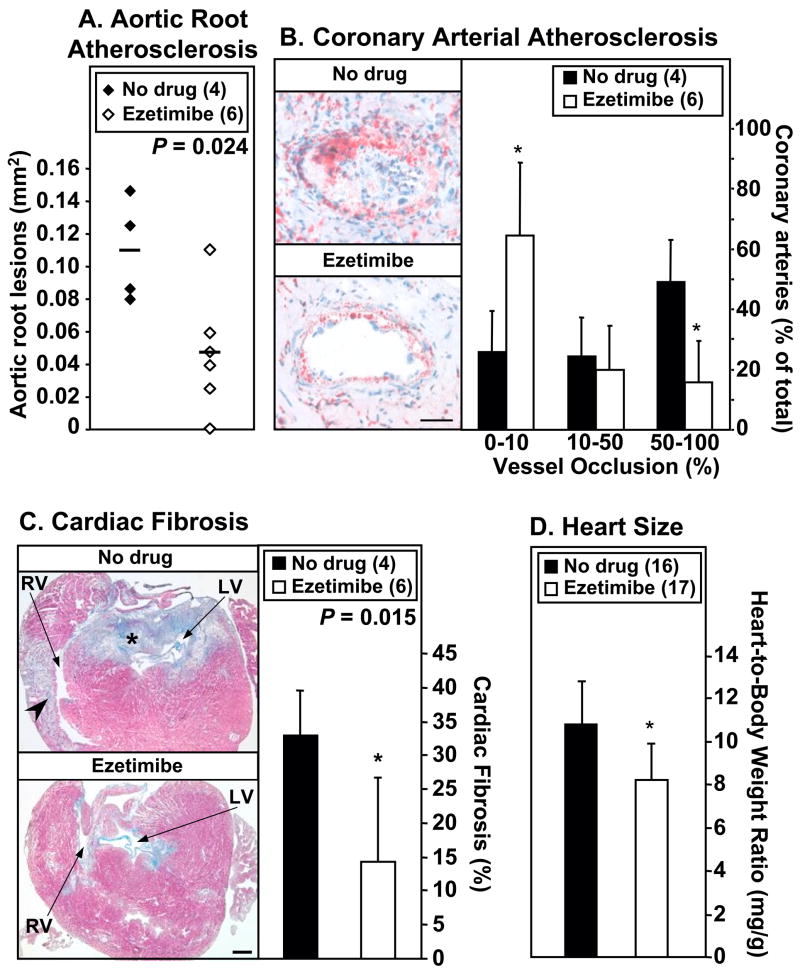
We then assessed the effects of ezetimibe on atherogenesis. Quantitative analysis of aortic sinus atherosclerosis showed ezetimibe treatment decreased aortic sinus plaque areas by 57% (Figure 2A). Figure 2B (left panels) shows representative coronary arteries in right ventricular walls in hearts from untreated (top, virtually completely occluded) or ezetimibe-treated (bottom, less than 10% of the vessel lumen occluded by plaque) mice. Extents of occlusion in coronary arteries were quantified by distributing scored coronary arteries into three categories: severely occluded (50–100%), partially occluded (10–50%) and no or minor occlusion (0–10%). Figure 2B (right panel) shows that ezetimibe treatment resulted in a 68% decrease in the percentage of severely occluded coronary arteries (P ≤ 0.01) with a corresponding 147% increase in the percentage of vessels showing no or minor lesions (P ≤ 0.01). Thus, ezetimibe treatment significantly reduced atherosclerosis in both the aortic sinus and the coronary arteries.
Figure 2. Effects of ezetimibe on atherosclerosis and heart disease.
dKO mice (animal number in parentheses) were fed standard chow without (‘no drug’, black bars or symbols) or with ezetimibe (white bars or symbols) from weaning. Hearts were harvested at 39–44 days of age. A. Oil red O-stained lesions in the aortic root. Average lesion areas (mm2, horizontal lines) were: no drug, 0.109±0.032 (n=4); ezetimibe 0.047±0.037 (n=6, P=0.024). B. Coronary arterial atherosclerosis. Left: Representative oil red O stained sections of coronary arteries (neutral lipid stains red) from untreated (no drug, top) and ezetimibe-treated (bottom) mice. Bar: 50 μm. Right: Extent of coronary arterial occlusions (average percent of coronary vessels with 0–10%, 10–50% or 50–100% occlusions). *P ≤ 0.01 for no drug vs. ezetimibe-treated. C. Cardiac fibrosis. Left: Representative trichrome-stained (healthy myocardium, red; fibrotic tissue, blue) transverse cryosections of hearts from untreated (no drug; top) or ezetimibe-treated (bottom). RV: right ventricle; LV: left ventricle; arrowhead: myocardial infarct; * extensive fibrosis in the outflow tract area. Bar: 500 μm. Right: Quantitative analysis of cardiac fibrosis. *P = 0.015 for no drug vs. ezetimibe-treated values. D. Heart-to-Body Weight Ratios. * P<0.001 for comparison to no drug control.
3.4 Effects of Ezetimibe on Cardiac Fibrosis and Cardiomegaly
Occlusive coronary arterial lesions are likely to contribute to the development of MI and cardiac dysfunction in dKO mice and to their premature death (22, 29 and unpublished data). To determine if the ezetimibe-induced reduction in atherosclerosis was accompanied by reduced severity of abnormal cardiac phenotypes, we evaluated cardiac fibrosis (MI) and heart size. Cardiac fibrosis was assessed in trichrome-stained cryosections (Figure 2C). Untreated dKO mice exhibited extensive fibrosis in the outflow tract area of all hearts (4/4), and right ventricular wall MI in 3/4 of the hearts (Figure 2C, top left panel, asterisk and arrowhead respectively; also see 22). The effect of ezetimibe treatment on cardiac fibrosis was variable. Outflow tract fibrosis was drastically reduced in 3/6 hearts from ezetimibe-treated mice and none exhibited extensive right ventricular wall MI (Figure 2C, bottom left panel). Overall, ezetimibe treatment reduced myocardial fibrosis by 57% (33.1±6.4% to 14.3±12.4%; P=0.015; Figure 2C, right panel).
Cardiomegaly is one indicator of abnormal heart function 30. Ezetimibe treatment reduced the abnormally large heart weights of dKO mice by 12% (0.17±0.02 g (n=16) to 0.15±0.03 g (n=17); P=0.04) even though the treated mice had significantly higher body weight (see paragraph 3.1 above). The heart-to-body weight ratio (Figure 2D) of untreated dKO mice (10.8±2.0 mg/g; n=16, ~1.7-times that of control mice 22 was reduced by 24% by ezetimibe treatment (8.2±1.7 mg/g; n=17; P<0.001). Thus, ezetimibe treatment significantly reduced cardiac pathology in the dKO mice.
3.5 Effects of Ezetimibe on Survival
Figure 3 shows the survival curves for untreated (black) and ezetimibe-treated (grey) dKO mice. The median age of death (44 days) for untreated dKO mice was increased to 56 days by ezetimibe treatment (P<0.0001). Thus, ezetimibe’s inhibition of cholesterol intestinal absorption prolonged survival by 27%. The increased survival time could not be attributed to an improvement in the anemia of untreated dKO animals 31, as hematocrits for treated (31.0 ± 3.5 %) and untreated (33.2 ± 5.6%) animals did not differ (P=0.46).
Figure 3. Effect of ezetimibe on survival.
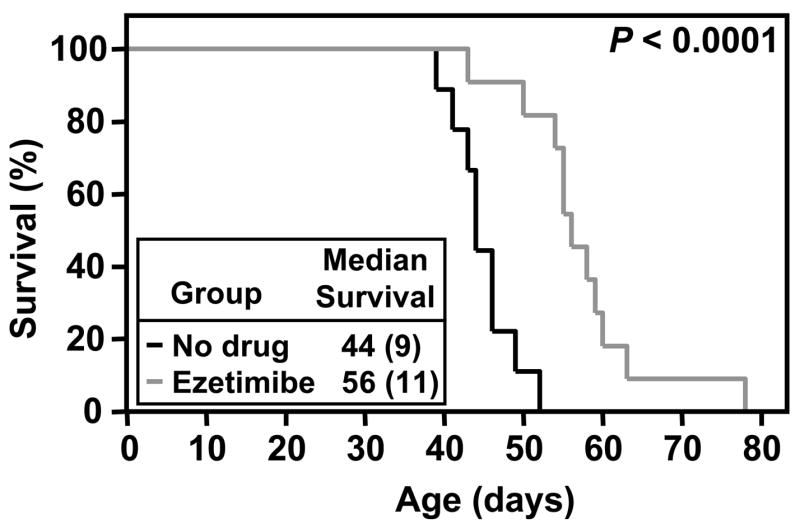
Survival curves for dKO mice fed standard chow without (‘no drug’, black line) or with ezetimibe (grey line) from weaning. Inset indicates median survival time (animal number in parentheses); P < 0.0001.
3.6 Effects of ASBT inhibitor (SC-435) on Survival and Plasma Lipids and Lipoproteins
To further study the effects of intestinal lipid absorption inhibition on lipoprotein metabolism and survival in dKO mice, we fed dKO mice a diet containing 0.01% (wt/wt) SC-435, an ASBT inhibitor that efficiently prevents intestinal absorption of bile acids 14–16. The effects of SC-435 treatment on plasma lipids and lipoproteins were measured in 40–41 day old dKO mice. As was the case for ezetimibe treatment (Figure 1), a 3-week treatment with SC-435 did not alter plasma total cholesterol levels (Figure 4A, inset), unesterified cholesterol (not shown), nor the levels of cholesterol in VLDL- or HDL-size lipoproteins (Figure 4A and B). However, SC-435 treatment significantly reduced the cholesterol in IDL/LDL-size particles by 37% (no drug: 256±41 mg/dL; ASBT inh.: 160±30 mg/dL; P=0.009).
Figure 4C shows that the median age of death (47 days) for untreated dKO mice (black) was increased by almost 4 weeks to 74 days by SC-435 treatment (grey; P<0.0001), a 57% increase. The survival data in Figure 4C (SC-435) and Figure 3 (ezetimibe) were generated in different facilities (see Materials and Methods) and thus cannot be used to draw conclusions about the relative effects of these two compounds on dKO survival. However, these data show that both lipid absorption inhibitors markedly prolong the lives of dKO mice. Therefore, inhibitors of both cholesterol and bile acid absorption resulted in a reduction in the amount of cholesterol in the IDL/LDL size lipoprotein particles. accompanied by prolonged lifespan.
4. Discussion
The very rapid onset of atherosclerosis and CHD, as well as premature death in SR-BI/apoE dKO mice, raised the possibility that they may be useful for studying the mechanisms underlying these pathologies and for testing the efficacy of pharmacologic agents 22. To date the only pharmacologic agent reported to influence pathology in dKO mice is probucol 27. Probucol treatment virtually completely reverses early onset of all of the pathologies seen in untreated dKO animals and dramatically prolongs their lives (mean age of death increased to ~30 weeks). Unfortunately, it is difficult to draw mechanistic conclusions from the striking effects of probucol on dKO mice, because of its many varied reported activities, including lipid-lowering, anti-atherosclerosis, anti-oxidation, anti-inflammatory, gene regulatory and other activities 32, 33.
In the current study, we examined the effects on dKO mice of oral administration starting at weaning of two highly specific inhibitors of intestinal lipid absorption: the FDA approved cholesterol absorption inhibitor ezetimibe 3–5 and the conjugated bile acid absorption inhibitor SC-435 14–16. Both inhibitors reduce plasma cholesterol levels in various animals and aortic root atherosclerotic lesions in apoE KO mice 3, 5–10, 16–19. In dKO mice, ezetimibe and SC-435 treatments significantly lowered plasma cholesterol in the IDL/LDL-size range (35 and 37%, respectively), but did not alter the levels of cholesterol in the VLDL-size particles that carry the bulk of the cholesterol or in HDL-size particles, and thus total plasma cholesterol. Similarly, Bhat et al. 17 reported that a 3-week treatment of apoE single KO mice fed a Western diet with doses of SC-435 comparable to those used here, had no effect on total plasma cholesterol, and extension of treatment to 12 weeks resulted in a 35% reduction, primarily in the VLDL- and IDL/LDL-size fractions. Davis et al 7 have reported that a 6-month treatment of Western diet-fed apoE single KO mice with 0.005% ezetimibe substantially reduced total plasma cholesterol (61%), primarily due to major reduction in the VLDL/chylomicron cholesterol (89%) together with a reduction in IDL/LDL cholesterol and an increase in the relatively low basal level of HDL cholesterol. It is not clear if the different effects of ezetimibe treatment on plasma lipoproteins in apoE single KO mice 7 and dKO mice (this study) were due to the substantial differences in treatment times (~3 weeks vs 6 months), diets (standard chow used here) or differences in the compositions and structures of the lipoproteins in apoE single KO and dKO mice 24, 27.
Inhibition of lipid absorption across the intestinal mucosa by ezetimibe (cholesterol) or SC-435 (bile acids) significantly prolonged the lives of the dKO mice. Detailed analyses of the effects of ezetimibe showed that at six weeks of age, ezetimibe treatment significantly reduced aortic root (57%) and occlusive coronary arterial (68%) atherosclerosis, cardiomegaly (24%) and cardiac fibrosis (57%). Thus, these results suggest that ezetimibe increased the lifespan of dKO mice because it slowed the onset of occlusive coronary arterial atherosclerosis and accompanying MI and cardiac dysfunction.
These findings provide the first evidence of beneficial effects of ezetimibe on coronary atherosclerosis and CHD. Lipoprotein metabolism in dKO mice and most other murine models of atherosclerosis differs from that in humans (e.g., mice do not naturally express cholesteryl ester transfer protein (CETP; see 34); thus caution must be exercised in comparing lipoprotein-associated pathology in such models to human disease. Nevertheless, the results reported here suggest that the SR-BI/apoE dKO murine model of atherosclerotic occlusive CHD appears to provide a useful and very rapid system to evaluate compounds that modulate cholesterol homeostasis and atherosclerosis, and may be helpful for the analysis of new therapeutic agents.
Acknowledgments
We thank James Januzzi for helpful discussions and scientific advice; Dennis Guberski, Ed Kislauskis, Mary Gardner, Chris Hogan and Don Winans (BRM, Inc.), for helpful discussions, scientific advice and/or technical assistance. This work was supported by grants from the US National Institute of Health Heart Lung and Blood Institute to SA (5R43HL81038) and MK (HL66105, HL52212).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kreisberg RA, Oberman A. Clinical review 141: lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J Clin Endocrinol Metab. 2002;87:423–437. doi: 10.1210/jcem.87.2.8057. [DOI] [PubMed] [Google Scholar]
- 2.Burnett JR, Huff MW. Cholesterol absorption inhibitors as a therapeutic option for hypercholesterolaemia. Expert Opin Investig Drugs. 2006;15:1337–1351. doi: 10.1517/13543784.15.11.1337. [DOI] [PubMed] [Google Scholar]
- 3.Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR., Jr In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- 4.Rosenblum SB, Huynh T, Afonso A, Davis HR, Jr, Yumibe N, Clader JW, Burnett DA. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- 5.Gazi IF, Mikhailidis DP. Non-low-density lipoprotein cholesterol-associated actions of ezetimibe: an overview. Expert Opin Ther Targets. 2006;10:851–866. doi: 10.1517/14728222.10.6.851. [DOI] [PubMed] [Google Scholar]
- 6.Davis HR, Jr, Pula KK, Alton KB, Burrier RE, Watkins RW. The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor, ezetimibe, in combination with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors in dogs. Metabolism. 2001;50:1234–1241. doi: 10.1053/meta.2001.26737. [DOI] [PubMed] [Google Scholar]
- 7.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 8.van Heek M, Compton DS, Davis HR. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol. 2001;415:79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- 9.van Heek M, Austin TM, Farley C, Cook JA, Tetzloff GG, Davis HR. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 2001;50:1330–1335. doi: 10.2337/diabetes.50.6.1330. [DOI] [PubMed] [Google Scholar]
- 10.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res. 2005;46:779–789. doi: 10.1194/jlr.M400475-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J. 2005;149:234–239. doi: 10.1016/j.ahj.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Davis HR, Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 13.Insull W., Jr Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006;99:257–273. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 14.Rapp B. Beaudry Napawan and Keller, SC-435 is a potent inhibitor of the apical sodium co-dependent bile acid transporter (ASBT) in mice, rats, hamsters and dogs and reduces atherosclerotic lesions in apoE −/−mice. XIV International Symposium on Drugs Affecting Lipid Metabolism; Fondazione Giovanni Lorenzini, Milan. 2001. [Google Scholar]
- 15.West KL, Ramjiganesh T, Roy S, Keller BT, Fernandez ML. 1-[4-[4[(4R,5R)-3,3-Dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy -1,1-dioxido-1-benzothiepin-5-yl]phenoxy]butyl]-4-aza-1-azoniabicyclo[2.2. 2]octane methanesulfonate (SC-435), an ileal apical sodium-codependent bile acid transporter inhibitor alters hepatic cholesterol metabolism and lowers plasma low-density lipoprotein-cholesterol concentrations in guinea pigs. J Pharmacol Exp Ther. 2002;303:293–299. doi: 10.1124/jpet.102.038711. [DOI] [PubMed] [Google Scholar]
- 16.Huff MW, Telford DE, Edwards JY, Burnett JR, Barrett PH, Rapp SR, Napawan N, Keller BT. Inhibition of the apical sodium-dependent bile acid transporter reduces LDL cholesterol and apoB by enhanced plasma clearance of LDL apoB. Arterioscler Thromb Vasc Biol. 2002;22:1884–1891. doi: 10.1161/01.atv.0000035390.87288.26. [DOI] [PubMed] [Google Scholar]
- 17.Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J Lipid Res. 2003;44:1614–1621. doi: 10.1194/jlr.M200469-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.West KL, Zern TL, Butteiger DN, Keller BT, Fernandez ML. SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis. 2003;171:201–210. doi: 10.1016/j.atherosclerosis.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Xu G, Shang Q, Pan L, Shefer S, Batta AK, Bollineni J, Tint GS, Keller BT, Salen G. Inhibition of ileal bile acid transport lowers plasma cholesterol levels by inactivating hepatic farnesoid X receptor and stimulating cholesterol 7 alpha-hydroxylase. Metabolism. 2004;53:927–932. doi: 10.1016/j.metabol.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 21.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 22.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, Krieger M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation. 2005;111:3457–3464. doi: 10.1161/CIRCULATIONAHA.104.523563. [DOI] [PubMed] [Google Scholar]
- 24.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karackattu SL, Picard MH, Krieger M. Lymphocytes are not required for the rapid onset of coronary heart disease in scavenger receptor class B type I/apolipoprotein E double knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:803–808. doi: 10.1161/01.ATV.0000158310.64498.ac. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest. 2001;108:1717–1722. doi: 10.1172/JCI13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun A, Zhang S, Miettinen HE, Ebrahim S, Holm TM, Vasile E, Post MJ, Yoerger DM, Picard MH, Krieger JL, Andrews NC, Simons M, Krieger M. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc Natl Acad Sci U S A. 2003;100:7283–7288. doi: 10.1073/pnas.1237725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telford DE, Edwards JY, Lipson SM, Sutherland B, Barrett PH, Burnett JR, Krul ES, Keller BT, Huff MW. Inhibition of both the apical sodium-dependent bile acid transporter and HMG-CoA reductase markedly enhances the clearance of LDL apoB. J Lipid Res. 2003;44:943–952. doi: 10.1194/jlr.M200482-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Karackattu SL, Trigatti B, Krieger M. Hepatic lipase deficiency delays atherosclerosis, myocardial infarction, and cardiac dysfunction and extends lifespan in SR-BI/apolipoprotein E double knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:548–554. doi: 10.1161/01.ATV.0000202662.63876.02. [DOI] [PubMed] [Google Scholar]
- 30.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 31.Holm TM, Braun A, Trigatti BL, Brugnara C, Sakamoto M, Krieger M, Andrews NC. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood. 2002;99:1817–1824. doi: 10.1182/blood.v99.5.1817. [DOI] [PubMed] [Google Scholar]
- 32.Deng YM, Wu BJ, Witting PK, Stocker R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation. 2004;110:1855–1860. doi: 10.1161/01.CIR.0000142610.10530.25. [DOI] [PubMed] [Google Scholar]
- 33.Heinecke JW. Lipoprotein oxidation in cardiovascular disease: chief culprit or innocent bystander? J Exp Med. 2006;203:813–816. doi: 10.1084/jem.20060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barter PJ, Rye KA. Cholesteryl ester transfer protein, high density lipoprotein and arterial disease. Curr Opin Lipidol. 2001;12:377–382. doi: 10.1097/00041433-200108000-00002. [DOI] [PubMed] [Google Scholar]