Abstract
3′-azido-3′-deoxythymidine (AZT) has been shown to be a potent inhibitor of thymidine kinase 2 in work from this laboratory. Inhibition results in decreased salvage of thymidine to TTP, which may lead to depletion of the TTP pool and result in the mitochondrial dysfunction and mt-DNA depletion observed with AZT toxicity. The effect of AZT on thymidine phosphorylation in growing cells expressing thymidine kinase 1 has not been shown. Three cell lines were used in these experiments: H9c2, derived from rat cardiomyoblasts; U-937, derived from human monocytes; and Raji, derived from human lymphoblasts. AZT inhibited growth in a concentration dependent manner in U-937 cells, but not the other cell lines. The phosphorylation of [3H]-thymidine or [3H]-AZT was determined during log growth. All cell lines salvaged and phosphorylated thymidine to TTP, with TTP the major product. The U-937 cells had a much more active salvage pathway than the other cells. All cell lines phosphorylated AZT to the triphosphate, but the major product was AZTMP. The AZT inhibition of growth of the U-937 cells did not correlate with levels of phosphorylated AZT. In contrast, pro-drug AZT was shown to inhibit thymidine phosphorylation in all lines with 50% inhibition concentrations (IC50) ranging from 4.4–21.9 μM. Since the U-937 cells expressed higher activity of the salvage pathway than the other cell lines, the U-937 cells may rely more heavily on the salvage pathway for TTP synthesis, accounting for AZT inhibition of growth.
1. Introduction
3′-azido-3′-deoxythymidine (AZT) is an analog of the thymidine nucleoside and belongs to the class of drugs called the nucleoside-analog reverse transcriptase inhibitors (NRTIs), used in the treatment of AIDS. NRTIs are all pro-drugs and must be first activated by the cellular deoxynucleotide salvage pathways in order to function against HIV. In particular, AZT utilizes the thymidine salvage enzymes. It is first phosphorylated to AZT-5′-monophosphate (AZTMP) by either cytosolic thymidine kinase 1 or mitochondrial thymidine kinase 2. Thymidine kinase 1 is only expressed during S phase of the cell cycle [1], where as thymidine kinase 2 is constitutively expressed. Next AZTMP is phosphorylated to AZT-5′-diphosphate (AZTDP) by thymidylate kinase. While the cytosolic thymidylate kinase has been characterized, no enzyme has yet been found in the mitochondrial matrix, but the enzymatic activity is known to exist [2, 3]. The final step is the phosphorylation of AZTDP to AZT-5′-triphosphate (AZTTP) by nucleoside diphosphate kinase. AZTTP is the active form of the drug and inhibits the reverse transcriptase of HIV.
In the late 1980’s and early 1990’s, AZT was given as monotherapy with dosage of 1200 mg/day. Under this therapy regimen, AZT was associated with a variety of toxic side effects that included myopathy, cardiomyopathy, hepatotoxicity, and various cytopenias. Tissues experiencing AZT toxicity were found to have mitochondrial damage and mitochondrial DNA depletion. In the more modern highly active antiretroviral therapy (HAART) regimen, AZT is given at a lower dosage of 600 mg/day and in combination with other drugs. This has reduced the prevalence of the toxicities seen with monotherapy, but AZT is now associated with lipodystrophy and anemia.
The current prevailing hypothesis for the mechanism of this toxicity states that mitochondrial damage is mediated through AZTTP inhibition of the mitochondrial DNA polymerase γ (IC50 of >100 μM AZTTP) [4]. However, other research has shown that AZT is slowly phosphorylated, if at all, beyond AZTMP in many tissues [2, 3, 5]. Past research from this laboratory has shown that AZT competitively inhibits thymidine kinase 2 in isolated rat heart and liver mitochondria [6] and in the perfused whole rat heart [7]. In the isolated mitochondria, the Ki values for inhibition of thymidine phosphorylation are 10.6 ± 4.5 μM AZT for heart mitochondria and 14.0 ± 2.5 μM AZT for liver mitochondria [6]. This suggests that AZT inhibits thymidine phosphorylation much more readily than AZTTP inhibits polymerase γ. This has led us to an alternative hypothesis for AZT toxicity in which AZT inhibits thymidine phosphorylation, resulting in the depletion of the intracellular pool of TTP, which could limit mitochondrial DNA replication and cause mitochondrial toxicity. In non-replicating cells, such as those in heart and liver, this inhibition is directed towards the mitochondrial thymidine kinase 2, since the cytoplasmic thymidine kinase 1 is only expressed during S phase of the cell cycle [1]. The effect of AZT on replicating cells, which express thymidine kinase 1, is examined here using three cell lines: H9c2 (rat cardiac myoblasts), U-937 (human monocytes from histiocytic lymphoma) [8], and Raji (human lymphoblasts from Burkitt’s lymphoma).
2. Materials and Methods
2.1 Cell Culture
U-937, Raji, and H9c2 cells were obtained from American Type Culture Collection. U-937 and Raji cells were grown in RPMI 1640 media with 2 mM L-glutamine, 1.5 g/L NaHCO3, 4.5 g/L glucose, 10 mM HEPES, 1 mM sodium pyruvate, and 10% v/v fetal bovine serum. H9c2 cells were grown in Dulbecco’s modified Eagle’s media with 4 mM L-glutamine, 1.5 g/L NaHCO3, 4.5 g/L glucose, and 10% v/v fetal bovine serum. All cells were cultured at 37° C in 5% CO2. H9c2 cells were subcultured (1:4) at 70% confluence in order to prevent any differentiation.
2.2 Determination of Thymidine Concentration in Growth Media
Growth media (10 ml) that reflects the condition of the media at the time of the experiments detailed in the following sections (supporting cell growth for two days for U-937 and Raji or fresh media unexposed to cells for H9c2) was removed from the flask and lyophilized in order to concentrate it. It was then diluted back to 1 ml. The remainder of this protocol was developed from the one described by Grem et al. [9]. Briefly, 50 μl of glacial acetic acid and 2 ml of acetonitrile were added to each sample. The samples were then vortexed and spun at 2000 × g for 20 minutes. The supernatant was removed and then dried using a Speedvac. Each sample was then resuspended in 0.5 ml water and analyzed using reverse phase HPLC with an in-line UV detector (254 nm) with the method described previously [2]. In order to determine thymidine concentration, the samples were compared to a standard curve constructed using dialyzed fetal bovine serum spiked with 0.2 to 50 nmol of thymidine and treated in the same fashion as the samples.
2.3 Detection of Thymidine and AZT Phosphorylation
Flasks of cells were incubated in normal growth media supplemented with thymidine and/or AZT. Concentrations of thymidine and AZT, specific radioactivities of [3H]-thymidine and [3H]-AZT, and time frames of incubations are described in the figure legends.
For Raji and U-937 cells, thymidine and/or AZT was added directly to the media two days after seeding at 150,000 cells/ml (U-937) or 300,000 cells/ml (Raji). At the end of the incubation, the cell suspension was removed and centrifuged to pellet the cells. The supernatant was removed and saved for later analysis. The pellet was resuspended in 5% trichloroacetic acid and put on ice for 10 minutes. This was then centrifuged for 3 minutes, and the acid soluble supernatant was neutralized with AG-11A8 resin.
For H9c2 cells, the cells were grown to 70–80% confluence. The media was removed, and the cells then were washed with phosphate buffered saline. Thymidine and/or AZT were mixed with fresh media, and this media was then added to the growth flask at the beginning of the incubation. At the end of the incubation, the media was removed and saved for later analysis, and 5% trichloroacetic acid was added to the flask. The flask was put on ice and gently rocked for 20 minutes. The acid soluble fraction was pipetted off and centrifuged for 3 minutes. The supernatant was then removed and neutralized with AG-11A8 resin.
The media removed at the end of incubation from the H9c2 flask and the supernatant removed after the centrifugation of the U-937 and Raji cell suspension were mixed with and equal volume of 10% trichloroacetic acid and put on ice for 10 minutes. This was then centrifuged, and the acid soluble supernatant removed and neutralized with AG-11A8 resin.
A separate, but identical plate of cells was counted using a hemacytometer for each cell line to determine cell number at the time of experiment.
2.4 HPLC Analysis of Acid Soluble Extracts
The neutralized extracts from the media and cellular samples described above were analyzed by reverse phase HPLC with an Alltech absorbosphere nucleoside/nucleotide column coupled to an in-line UV monitor (254 nm) and an in-line scintillation counter used methods previously described [2, 3].
2.5 Determination of Cell Growth Curve
U-937 and Raji cells were seeded at 150,000 cells/ml and 300,000 cells/ml, respectively. Various concentrations of AZT (0–200 μM) were added to the media at the time of seeding. Every 24 hr for the following 7 days, a sample was taken from each growth flask, and the cells counted using a hemacytometer. H9c2 cells tend to differentiate if allowed to approach confluence, thus as noted above these cells were subcultured at 70% confluence. Freshly subcultured H9c2 cells were incubated with various concentrations of AZT (0–100 μM). The cells were subcultured (1:4) when they reached ~70% confluence (~72 hr) and exposure was maintained for 21 days. While cells numbers were not directly determined, the time required to reach ~70% confluence was recorded (~72 hr) and did not vary between the control and the AZT treated through 7 passages over 21 days.
3. Results
3.1 Thymidine Concentration in Growth Media
Since thymidine is present in fetal bovine serum, the thymidine concentration was determined in media that had been exposed to growing cells to provide accurate measures of specific radioactivities in the labeling experiments. The measured thymidine concentrations were 0.40 ± 0.01 μM in H9c2 media, 0.13 ± 0.01 μM in Raji media, and 0.10 ± 0.01 μM in U-937 media (mean ± SEM of three to four samples).
3.2 Time courses of thymidine and AZT phosphorylation
Flasks of cells were incubated for up to 90 minutes in the presence of growth media containing [3H]-thymidine as described in the Figure legend. To compare thymidine phosphorylation between different cell lines, the labeling concentration of thymidine was normalized to 1 μM. Thymidine was readily phosphorylated in all three cell lines with TTP as the predominant product together with a small amount of TDP, while levels of TMP were negligible. These data indicate that the rate-limiting step of conversion of thymidine to TTP is the initial phosphorylation by thymidine kinase. The conversion of labeled thymidine to TDP/TTP reached a steady-state plateau of 35–40 pmol/106 cells within 15 min in H9c2 and Raji cell cultures. The U-937 cells made about twice as much TDP/TTP as the H9c2 and Raji cells, with TDP/TTP levels still increasing somewhat after 90 min of incubation, suggesting a more active salvage pathway in U-937 cells. In the media from all three cell lines, no phosphorylated thymidine was detected, and the amount of thymidine present in the media did not drop significantly over the time course (data not shown).
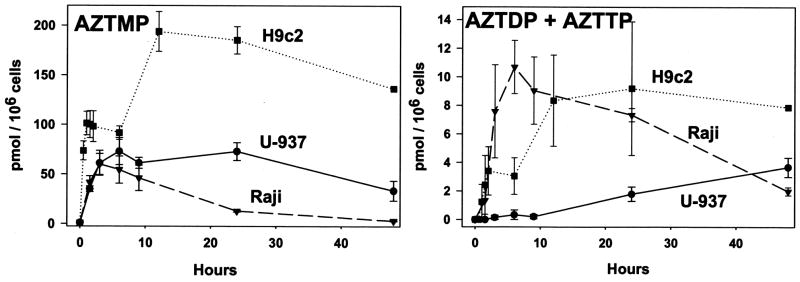
Flasks of cells were incubated for up to 48 hours with the addition of 1 μM [3H]-AZT to the growth media. All three cell lines readily phosphorylated AZT to AZTMP (Figure 2, left panel). The AZTMP levels in U-937 and Raji cells reached a maximum of about 60 pmol/106 cells within 3 hr of incubation. This level was maintained in the U-937 cells, but steadily decreased in the Raji cells over the next 24 hr. The H9c2 cells reached a maximum level of AZTMP of nearly 200 pmol/106 cells after 12 hr of incubation followed by a gradual decrease over the next 12–36 hr. AZTDP and AZTTP were also produced, albeit at a much slower rate, in all three cell lines (Figure 2, right panel), with AZTTP as the predominant product. These data clearly indicate that TMP kinase is the rate-limiting reaction for production of AZTTP as has already been well-noted in the literature [5]. The Raji and the H9c2 cell line synthesized 9–11 pmol/106 cells of AZTDP/AZTTP, but with different kinetics. The Raji cell line produced 11 pmol AZTDP/AZTTP by 9 hr of incubation followed by a steady decline over the next two days to a level of 2 pmol. The H9c2 cell line required 12 hr to reach about 9 pmol of AZTDP/AZTTP, but maintained that amount for the next 2 days. The time-course of synthesis of the di- and tri-phosphate of AZT mirrors the time-course of synthesis of the mono-phosphate for these two cell lines. In comparison, the U-937 cell line produced AZTDP/AZTTP in a much slower, but steady fashion, eventually reaching about 3 pmol/106 cells of AZTDP/AZTTP after 2 days of incubation. Interestingly, while the U-937 cells produced the most TTP, the H9c2 cells produced the most phosphorylated AZT. Since TK1 phosphorylates AZT relative to thymidine much better than TK2 [10], these data suggest that the thymidine phosphorylation activity of U-937 depends more on TK2 than TK1 relative to the other cell lines. The amount of AZT in the media did not change significantly over time, and no phosphorylated AZT was detected in the media (data not shown).
Figure 2. Time course of AZT phosphorylation in cultured cells.
Flasks of cells (U-937, Raji, and H9c2) were incubated for up to 48 hours with the addition of 1 μM [methyl-3H]-AZT (2200 DPM/pmol) to the growth media. Samples were prepared as described in Materials and Methods and the DPM s obtained converted to pmoles by dividing by the specific radioactivity of the AZT pool for that experiment. Data represent the mean ± SEM of three to four trials. AZTMP (left) and the sums of AZTDP and AZTTP (right) from the cellular extracts are shown as pmol per 106 cells versus hours of incubation.
3.3 Effect of AZT on thymidine phosphorylation
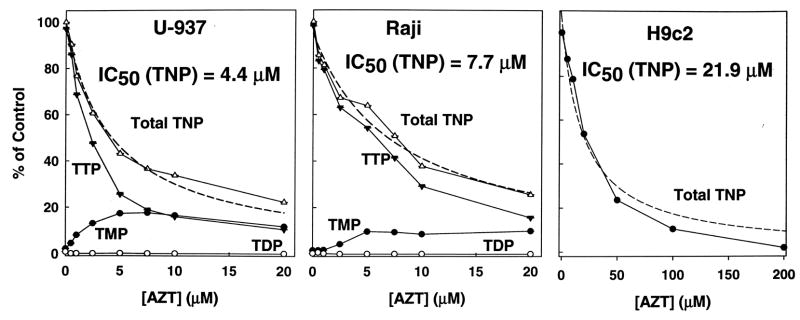
The data from Figure 1 indicated that most of the cellular [3H]-TTP was synthesized from [3H]-thymidine within 10 min of incubation. The effect of AZT on this synthesis was determined by incubating flasks of cells for 10 minutes in growth media with [3H]-thymidine (0.2 μM for U-937 and Raji and 1.4 μM for H9c2) and with unlabeled AZT (0–200 μM) (Figure 3). AZT inhibited thymidine phosphorylation in all three cell lines. The 50% inhibitory concentration (IC50) for production of all phosphorylated forms of thymidine is 4.4, 7.7, and 21.9 μM AZT for U-937, Raji, and H9c2 respectively. In H9c2 cells (right), TTP was the only phosphorylated form of thymidine observed. However, TMP, which is not observed in the control, increased in the presence of AZT in U-937 (left) and Raji (center), suggesting that AZT or a small amount of AZTMP inhibited thymidylate kinase. Earlier work by Furman et. al., [11], has shown AZTMP is a competitive inhibitor of human thymidylate kinase (Ki = 8.6 μM). However, the highest concentrations of AZTMP were observed in H9c2 cells in which TMP was not observed. This discrepancy may be related to the relative activities, and/or cellular localization of thymidine versus thymidylate kinases.
Figure 1. Time course of thymidine phosphorylation in cultured cells.
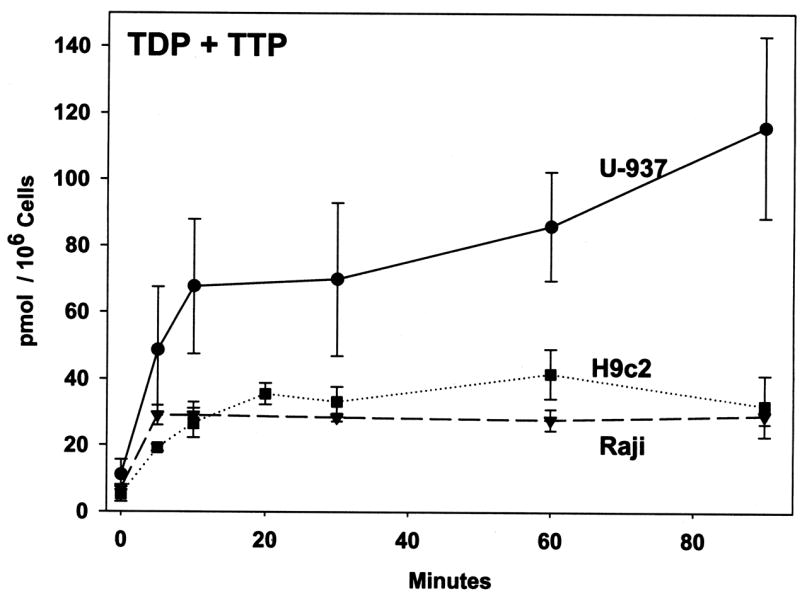
Flasks of cells (U-937, Raji, and H9c2) were incubated for up to 90 minutes in the presence of growth media with a total concentration of [methyl-3H]-thymidine of 0.12 μM for U-937 and Raji cells (1550 DPM/pmol) and 1.4 μM for H9c2 cells (4000 DPM/pmol). Samples were prepared as described in Materials and Methods and analyzed by reverse phase HPLC with an in-line scintillation counter. The DPM data obtained from each experiment was converted to pmoles by dividing by the specific radioactivity of the thymidine pool for that experiment and normalized to 1 μM thymidine. Data represent the mean ± SEM of three to four independent trials. The sums of TDP and TTP from the cellular extracts are shown as pmol per 106 cells versus minutes of incubation. TMP levels were negligible.
Figure 3. Effect of AZT on thymidine phosphorylation in cultured cells.
Flasks of cells were incubated in growth media with unlabeled AZT (0–200 μM) and a total [methyl-3H]-thymidine concentration of 0.12 μM in U-937 and Raji (1550 DPM/pmol) and 1.4 μM in H9c2 (4000 DPM/pmol). Cellular extracts were prepared after 10 min of incubation as described in Materials and Methods. Data represents the mean ± SEM of three to four trials, shown as the percent of the 0 μM AZT control versus AZT concentration. Total TNP represents the sum of TMP, TDP, and TTP. Both TTP and TMP were detected in the U-937 (left) and Raji (center) trials. However, TTP was the only phosphorylated form of thymidine seen with the H9c2 cells (right), so only the Total TNP line is shown. The 50% inhibitory concentration (IC50) for AZT inhibition of total thymidine phosphorylation was calculated from the best fit line (dashed lines) (SigmaPlot 10).
3.4 Effect of AZT on growth rates
Flasks of U-937 (left) and Raji (right) cells were incubated in growth media containing AZT (0–200 μM). Cells were counted every 24 hours through 7 days of incubation. AZT inhibited the growth of U-937 cells in a dose-dependent manner (Figure 4). The CD50 for the effect of AZT calculated for each day from day 3 through day 7 stayed fairly constant and averaged 54 ± 8 μM. However, even at high concentrations, AZT had no effect on the Raji cell line. As noted in the Methods section above, a 21 day exposure of H9c2 cells to varying concentrations of AZT (0–100 μM) had no effect on the time required to reach ~70% confluence, even through 7 passages over 21 days. Under the light microscope, the control and treated cells maintained an identical appearance thorughout the 21 day exposure period. These data suggest that the Raji and H9c2 cell lines may have a more active de novo pathway for TTP synthesis that allows the cells to compensate for inhibition of thymidine salvage.
Figure 4. Effect of AZT on cell growth.
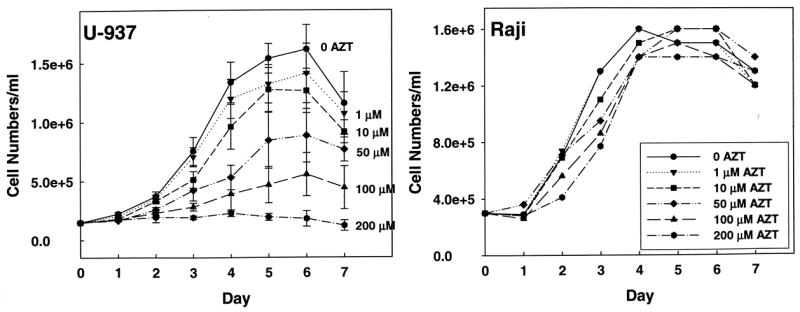
Flasks of U-937 (left) and Raji (right) cells were incubated in growth media containing AZT (0–200 μM). Cells were counted every 24 hours through 7 days of incubation. Data represents the mean of three trials ± SEM. The CD50 for AZT on growth of U-937 cells was calculated for each of the 3 through 7 day periods (Sigma Plot 10) and averaged giving a value of 54 ± 8 μM. The lack of effect of AZT on growth of H9c2 cells is described in the text.
4. Discussion
Cells in culture have two potential pathways for the synthesis of TTP. The cells may salvage thymidine from the medium by TK1 (cytosolic, expressed only in S phase) or TK2 (mitochondrial, constitutive), or the cells may utilize the de novo pathway and convert dUMP to TMP. Each cell line may express these two pathways differently. In this work, we have examined the ability of three cell lines, U-937, Raji, and H9c2, to salvage and phosphorylate thymidine and AZT. Additionally, we examined the effect of AZT on thymidine phosphorylation. All three cell lines were able to salvage thymidine and AZT and make the respective triphosphates to some extent. However, differences between the cell lines are worth noting. The U-937 cells made considerably more TTP than the other two cell lines, suggesting that U-937 cells have greater activity of TK1 and/or TK2. However, the U-937 cell line did not phosphorylate AZT nearly as well as the H9c2 cell line. This discrepancy is likely to be related to the relative amounts of TK1 versus TK2 expressed. Both enzymes function well with thymidine as substrate, but TK1 is much better at phosphorylating AZT than TK2 [10]. Thus, it is possible that there is relatively more TK2 activity in the U-937 cell line while there is relatively more TK1 activity in the H9c2 cell line.
Nucleotide reverse transcriptase inhibitors such as AZT are known to cause mitochondrial toxicities in long term therapy in humans, animal models, and a variety of cell lines. The most commonly cited mechanism for this toxicity is the presumed inhibition of the mitochondrial polymerase γ by AZTTP. In this study, AZT appeared to be toxic only to the U-937 cell line and had no effect, even at high concentrations, in H9c2 or Raji cells. However, both of H9c2 and the Raji cells made substantially more AZTTP than the U-937 cell line, suggesting that AZTTP levels do not account for toxicity. AZTMP has also been suggested to be potentially the toxic component by inhibiting the proofreading ability of the mitochondrial polymerase, albeit at relatively high concentrations. The H9c2 cells had a much higher level of AZTMP than the U-937 cells but were not affected by AZT. In previous work with isolated rat heart and liver mitochondria and the isolated perfused rat heart, we have shown that AZT is a potent inhibitor of thymidine phosphorylation. From this work, we have suggested an alternative to the mitochondrial polymerase inhibition hypothesis for AZT toxicity in which AZT inhibition of thymidine phosphorylation leads to a depletion of the TTP pool needed for mitochondrial DNA replication. In the present work, we have shown that AZT is a potent inhibitor of thymidine phosphorylation in all three of the cell lines used in this study, suggesting that both TK1 and TK2 are inhibited by the drug. However, the U-937 line was the only one to display toxicity. We believe this is related to the degree in which a cell depends on the thymidine salvage pathway. Cells capable of expressing ribonucleotide reductase, thymidylate synthase, and dihydrofolate reductase could salvage uridine or convert de novo synthesized UMP to dUMP and TMP for provision of the TTP pool. It is possible that both the Raji and H9c2 cells are more capable of obtaining TTP from these pathways while the U-937 cells may rely more exclusively upon thymidine salvage for supplying TTP. Inhibition of the salvage pathway by AZT is thus more devastating in U-937 cells and results in slowed growth or death of cells. Other investigators have demonstrated an initial decrease in TTP pools in a variety of cultured cells upon treatment with AZT [11–13]. Fridland, et. al. demonstrated a dramatic fall in TTP (~75% from control) in the first 4 hours following treatment of a human lymphoid cell line (CCRF-CEM) with 200 μM AZT. However, the TTP level rebounded to normal following 24 hr of exposure to the drug. Interestingly, the CD50 for inhibition of cell growth in this study by AZT was 50 μM if measured in the first 24 hours, but rose to 225 μM if measured over 48 hours. One interpretation of the results reported in this study is that growth in this cell line was initially compromised by a high reliance on the salvage pathway with AZT limiting TTP synthesis. However, upon continued exposure the cell line became more resistant to AZT perhaps by inducing the expression of enzymes that can make TTP from the ribonucleoside/ribonucleotide pools (ribonucleotide reductase, thymidylate synthase, etc). In summary, these data demonstrate that the relative expression of the salvage pathway versus the de novo pathway maybe a key factor in determining whether a cell line or tissue will experience adverse effects from exposure to AZT.
Acknowledgments
The authors MDL and BKK contributed equally to this work. This work was supported by a pre-doctoral fellowship from the American Heart Association Greater Midwest Affiliate to MDL and by a grant from the National Institute of Health, HL 72710 to EEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coppock DL, Pardee AB. Regulation of thymidine kinase activity in the cell cycle by a labile protein. J Cell Physiol. 1985;124:269–74. doi: 10.1002/jcp.1041240215. [DOI] [PubMed] [Google Scholar]
- 2.McKee EE, Bentley AT, Hatch M, Gingerich J, Susan-Resiga D. Phosphorylation of thymidine and AZT in heart mitochondria: elucidation of a novel mechanism of AZT cardiotoxicity. Cardiovasc Toxicol. 2004;4:155–67. doi: 10.1385/ct:4:2:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynx MD, Bentley AT, McKee EE. 3′-Azido-3′-deoxythymidine (AZT) inhibits thymidine phosphorylation in isolated rat liver mitochondria: A possible mechanism of AZT hepatotoxicity. Biochem Pharmacol. 2006;71:1342–8. doi: 10.1016/j.bcp.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JL, Brown CE, Matthews-Davis N, Reardon JE. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob Agents Chemother. 1994;38:2743–9. doi: 10.1128/aac.38.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie A, Schlichting I, Vetter IR, Konrad M, Reinstein J, Goody RS. The bottleneck in AZT activation. Nat Med. 1997;3:922–4. doi: 10.1038/nm0897-922. [DOI] [PubMed] [Google Scholar]
- 6.Lynx MD, McKee EE. 3′-Azido-3′-deoxythymidine (AZT) is a competitive inhibitor of thymidine phosphorylation in isolated rat heart and liver mitochondria. Biochem Pharmacol. 2006;72:239–43. doi: 10.1016/j.bcp.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susan-Resiga D, Bentley AT, Lynx MD, LaClair DD, McKee EE. Zidovudine inhibits thymidine phosphorylation in the isolated perfused rat heart. Antimicrob Agents Chemother. 2007;51:1142–9. doi: 10.1128/AAC.01227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–77. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 9.Grem JL, Danenberg KD, Behan K, Parr A, Young L, Danenberg PV, et al. Thymidine kinase, thymidylate synthase, and dihydropyrimidine dehydrogenase profiles of cell lines of the National Cancer Institute’s Anticancer Drug Screen. Clin Cancer Res. 2001;7:999–1009. [PubMed] [Google Scholar]
- 10.Arner ESJ, Spasokoukotskaja T, Eriksson S. Selective assays for thymidine kinase 1 and 2 and deoxycytidine kinase and their activities in extracts from human cells and tissues. Biochemical and Biophysical Research Communications. 1992;188:712–8. doi: 10.1016/0006-291x(92)91114-6. [DOI] [PubMed] [Google Scholar]
- 11.Furman PA, Fyfe JA, St Clair MH, Weinhold K, Rideout JL, Freeman GA, et al. Phosphorylation of 3′-azido-3v-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986;83:8333–7. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frick LW, Nelson DJ, St Clair MH, Furman PA, Krenitsky TA. Effects of 3′-azido-3′-deoxythymidine on the deoxynucleotide triphosphate pools of cultured human cells. Biochem Biophys Res Commun. 1988;154:124–9. doi: 10.1016/0006-291x(88)90659-6. [DOI] [PubMed] [Google Scholar]
- 13.Fridland A, Connelly MC, Ashmun R. Relationship of deoxynucleotide changes to inhibition of DNA synthesis induced by the antiretroviral agent 3′-azido-3′-deoxythymidine and release of its monophosphate by human lymphoid cells (CCRF-CEM) Mol Pharmacol. 1990;37:665–70. [PubMed] [Google Scholar]