Abstract
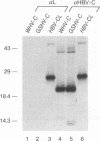
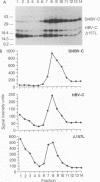
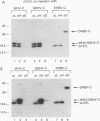
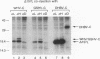
Hepadnaviruses encode a single core (C) protein which assembles into a nucleocapsid containing the polymerase (P) protein and pregenomic RNA during viral replication in hepatocytes. We examined the ability of heterologous hepadnavirus C proteins to cross-oligomerize. Using a two-hybrid assay in HepG2 cells, we observed cross-oligomerization among the core proteins from hepatitis B virus (HBV), woodchuck hepatitis virus, and ground squirrel hepatitis virus. When expressed in Xenopus oocytes, in which hepadnavirus C proteins form capsids, the C polypeptides from woodchuck hepatitis virus and ground squirrel hepatitis virus, but not duck hepatitis B virus, can efficiently coassemble with an epitope-tagged HBV core polypeptide to form mixed capsids. However, when two different core mRNAs are coexpressed in oocytes the core monomers show a strong preference for forming homodimers rather than heterodimers. This holds true even for coexpression of two HBV C proteins differing only by an epitope tag, suggesting that core monomers are not free to diffuse and associate with other monomers. Thus, mixed capsids result from aggregation of different species of homodimers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Fuller S. D. A model for the hepatitis B virus core protein: prediction of antigenic sites and relationship to RNA virus capsid proteins. EMBO J. 1988 Mar;7(3):819–824. doi: 10.1002/j.1460-2075.1988.tb02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beames B., Lanford R. E. Carboxy-terminal truncations of the HBV core protein affect capsid formation and the apparent size of encapsidated HBV RNA. Virology. 1993 Jun;194(2):597–607. doi: 10.1006/viro.1993.1299. [DOI] [PubMed] [Google Scholar]
- Birnbaum F., Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990 Jul;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisova G., Arya B., Dislers A., Borschukova O., Tsibinogin V., Skrastina D., Eldarov M. A., Pumpens P., Skryabin K. G., Grens E. Hybrid hepatitis B virus nucleocapsid bearing an immunodominant region from hepatitis B virus surface antigen. J Virol. 1993 Jun;67(6):3696–3701. doi: 10.1128/jvi.67.6.3696-3701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. E., Newton S. E., Carroll A. R., Francis M. J., Appleyard G., Syred A. D., Highfield P. E., Rowlands D. J., Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. 1987 Nov 26-Dec 2Nature. 330(6146):381–384. doi: 10.1038/330381a0. [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Richmond J. E. Electron microscopy of hepatitis B core antigen synthesized in E. coli. Nature. 1982 Apr 15;296(5858):677–679. doi: 10.1038/296677a0. [DOI] [PubMed] [Google Scholar]
- Deminie C. A., Emerman M. Incorporation of human immunodeficiency virus type 1 Gag proteins into murine leukemia virus virions. J Virol. 1993 Nov;67(11):6499–6506. doi: 10.1128/jvi.67.11.6499-6506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Finkel T., Gillison M. L., Kennedy S. P., Casella J. F., Tomaselli G. F., Morrow J. S., Van Dang C. Karyoplasmic interaction selection strategy: a general strategy to detect protein-protein interactions in mammalian cells. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7958–7962. doi: 10.1073/pnas.89.17.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Hirsch R., Colgrove R., Ganem D. Replication of duck hepatitis B virus in two differentiated human hepatoma cell lines after transfection with cloned viral DNA. Virology. 1988 Nov;167(1):136–142. doi: 10.1016/0042-6822(88)90062-1. [DOI] [PubMed] [Google Scholar]
- Luban J., Alin K. B., Bossolt K. L., Humaran T., Goff S. P. Genetic assay for multimerization of retroviral gag polyproteins. J Virol. 1992 Aug;66(8):5157–5160. doi: 10.1128/jvi.66.8.5157-5160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein R., Carey M., Ptashne M., Harrison S. C. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408–414. doi: 10.1038/356408a0. [DOI] [PubMed] [Google Scholar]
- Miyanohara A., Imamura T., Araki M., Sugawara K., Ohtomo N., Matsubara K. Expression of hepatitis B virus core antigen gene in Saccharomyces cerevisiae: synthesis of two polypeptides translated from different initiation codons. J Virol. 1986 Jul;59(1):176–180. doi: 10.1128/jvi.59.1.176-180.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol. 1992 Jul;66(7):4107–4116. doi: 10.1128/jvi.66.7.4107-4116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Marion P. L., Ganem D., Varmus H. E. In vitro recombinants of ground squirrel and woodchuck hepatitis viral DNAs produce infectious virus in squirrels. J Virol. 1987 Oct;61(10):3241–3247. doi: 10.1128/jvi.61.10.3241-3247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer M., Standring D. N. Recombinant human hepatitis B virus reverse transcriptase is active in the absence of the nucleocapsid or the viral replication origin, DR1. J Virol. 1993 Aug;67(8):4513–4520. doi: 10.1128/jvi.67.8.4513-4520.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer M., Standring D. N. Recombinant human hepatitis B virus reverse transcriptase is active in the absence of the nucleocapsid or the viral replication origin, DR1. J Virol. 1993 Aug;67(8):4513–4520. doi: 10.1128/jvi.67.8.4513-4520.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. J., Murray K. Immunogenicity of peptide fusions to hepatitis B virus core antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6283–6287. doi: 10.1073/pnas.86.16.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Rutter W. J. Assembly of viral particles in Xenopus oocytes: pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9338–9342. doi: 10.1073/pnas.83.24.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. L., Standring D. N. Production of hepatitis B virus nucleocapsidlike core particles in Xenopus oocytes: assembly occurs mainly in the cytoplasm and does not require the nucleus. J Virol. 1991 Oct;65(10):5457–5464. doi: 10.1128/jvi.65.10.5457-5464.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Standring D. N. Hepatitis B virus capsid particles are assembled from core-protein dimer precursors. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10046–10050. doi: 10.1073/pnas.89.21.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Yang S. Q., Standring D. N. Characterization of hepatitis B virus capsid particle assembly in Xenopus oocytes. J Virol. 1992 May;66(5):3086–3092. doi: 10.1128/jvi.66.5.3086-3092.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]