Abstract
Inbred mouse strains differ in their preferences for sweeteners, due in part to variations in their T1R3 sweet taste receptor. Recent studies of sweet sensitive C57BL/6J (B6) and subsensitive 129P3/J (129) mice indicate that experiential and post-oral effects of sugar substantially modify sweetener preference. In fact, the strain difference in sucrose preference disappeared after the mice were given 23 h/day tests with sucrose at ascending concentrations (0.5 - 32%). Intragastric infusions of sucrose (16%) also conditioned increased preference for and absolute intake of flavored sweet solutions in B6 and 129 mice. An operant analysis of sweetener appetite revealed, unexpectedly, that sugar-experienced 129 mice respond more vigorously than B6 mice for 16% sucrose rewards. These findings indicate that experiential and nutritional factors can, to some degree, override genetic differences in peripheral taste sensitivity in determining food appetite.
Keywords: Sucrose, Saccharin, Mice, Strain differences, C57BL/6J, 129P3/J, Intragastric conditioning, Operant licking
1. Introduction
The attractiveness of food depends in large part on the stimulation of orosensory receptors that provide food with its flavor, that is, taste, odor, and mouth feel. One of the most potent flavor elements is sweet taste. Our understanding of this taste quality was significantly advanced by the identification of two genes, Tas1r2 and Tas1r3, that code for the taste receptor proteins T1R2 and T1R3 [18]. These two proteins form a heterodimer that functions as a general sweet taste receptor for a variety of sugars and artificial sweeteners. The laboratory mouse has figured prominently in the elucidation of the sweet taste receptor. Early studies revealed that inbred mice differ significantly in their preference for saccharin and other substances that taste sweet to humans [8,11,21,33]. In two-bottle sweetener vs. water tests, some strains significantly prefer saccharin and sugar at low concentrations to which other strains are indifferent. These inbred strains are often classified as sweet “tasters” and “nontasters,” respectively, although most “nontaster” strains prefer saccharin to water at higher concentrations. For this reason, the terms sweet “sensitive” and “subsensitive” are used here to differentiate the various mouse strains. The present paper briefly reviews the avidity for sweets in these inbred strains and highlights recent work from the author’s laboratory demonstrating how sweetener appetite is modified in mice by post-oral nutrient actions.
2. The mouse sweet tooth
Fuller [8] proposed over 30 years ago that a single gene, which he referred to as the Sac locus, accounted for strain differences in saccharin preference. Subsequent studies identified the Sac locus to be the Tas1r3 gene and demonstrated that allelic variation in this gene largely accounted for the strain differences in sweetener preference [1,16,19,24]. Research with gene knockout (KO) mice demonstrated the critical importance of the T1R3 and T1R2 receptors to sweetener preference. Mice with either the Tas1r2 or Tas1r3 gene deleted show no or attenuated gustatory nerve and behavioral responses to sweeteners, and the residual response observed in single KO mice was absent in mice with both genes deleted [5,36].
A number of different behavioral procedures have been used to compare the sweetener response of inbred mice (see [7,9,14,20,23]), but the most common procedure is the 24-h two-bottle sweetener vs. water test. Typically, a number of sweetener concentrations are offered in an ascending series [3,10,15,17,22,33]. This procedure is sometimes referred to as a 48-h test when each concentration is presented on 2 consecutive days so that the left-right positions of sweetener and water can be alternated to control for side biases [33]. A large number of inbred strains (26 or 30) have been tested with a single sweetener solution (1.6 mM saccharin or 50 mM sucrose) [17,24] and a more limited number of strains (2 to 12) have been tested with a wider range of sweeteners and/or concentrations [3,4,10,15,17,22,33].
In a comprehensive study of different sweeteners, Bachmanov et al. [3] compared the 24-h two-bottle preferences of the sweet sensitive C56BL/6ByJ (B6) mouse and sweet subsensitive 129P3/J (129) mouse to 17 different sweeteners at several concentrations. The sweeteners included two sugars (sucrose, maltose), two sugar alcohols (sorbitol, erythritol), four amino acids (d-phenylalanine, D-tryptophan, L-proline, glycine), four artificial sweeteners preferred by mice (saccharin, acesulfame, sucralose, SC 45647), and five artificial sweeteners not typically preferred by mice (glycyrrhizic acid, neohesperidin, thaumatin, cyclamate, aspartame). An important feature of this study was that it reported both the relative intake (preference) and absolute intake of the various sweeteners. Many studies only report percent preference data which can obscure important strain difference in sweetener intake. In his original study, Fuller [8] distinguished between preference ratio measures and absolute intake measures of sweetener intake and suggested that they reflect the same underlying phenotype. Bachmanov et al. [3], however, reported differences between preference and intake measures. With respect to sugar and artificial sweetener preference, at low concentrations the B6 mice displayed stronger preferences than did 129 mice, but at higher concentrations both strains displayed near 100% preferences. However, on the intake measure, the B6 mice consumed more of the artificial sweetener solutions than did the 129 mice at all preferred concentrations. On the other hand, while the B6 mice overconsumed sucrose and maltose solutions at low to intermediate concentrations, the two strains consumed comparable amounts of sugar at the higher concentrations (30 or 32%). The similar intakes of the concentrated sugar solutions could be attributed to the postingestive satiating action of sugars that limits volume intake (but see below). Bachmanov et al. [3] also proposed that the nutritive effects of sugars may explain their higher consumption compared to the non-nutritive sweeteners. Other, non-sweetness sensory factors (e.g., bitterness of artificial sweetener) may have contributed to the preference and intake results, but the authors concluded that “the peripheral perception of sweet taste was a major determinant of the strain difference.” Supporting this view are electrophysiological data showing stronger chorda tympani nerve responses to sweeteners in B6 mice than in 129 mice [12,13].
3. Experiential effects on sweetener preference
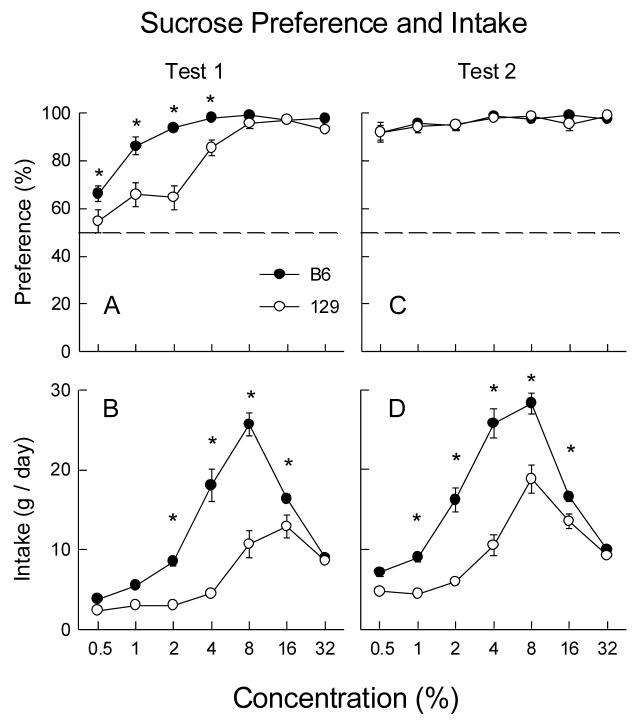
A recent study [28] conducted in the author’s laboratory closely replicated the findings of Bachmanov et al. [3] with regards to sucrose preference and intake. Sugar-naive B6 mice displayed stronger preferences than did 129 mice for sucrose at low concentrations (0.05% - 4%) but the strains did not differ in their sucrose preferences at higher concentrations (8% - 32%; Figure 1A) in sucrose vs. water tests (23 h/day). (The solutions were prepared on a weight/weight basis because intakes were by weight rather than volume.) In addition, sucrose intake of B6 mice was higher than that of 129 mice at intermediate concentrations (2% - 16%) but not at the highest concentration (32%; Figure 1B). The new finding of the study was that the strain difference in sucrose preference disappeared when the same mice where given a second series of tests with ascending sucrose concentrations (0.5% - 32%; Figure 1C). The B6 and 129 mice now displayed nearly identical robust preferences (∼90%) for all sucrose solutions. Experience with sucrose also reduced the strain difference in sucrose intake at some concentrations (8%), but increased it at other concentrations (1% - 4%; Figure 1D). Figures 1C and 1D illustrate the point that preference and intake measures can dramatically differ: whereas the experienced B6 and 129 mice equally preferred sucrose over the range of concentrations they still differed substantially in their absolute intakes of sugar (1% - 16%). Prior sucrose experience also eliminated the difference in the saccharin preferences, but not in absolute intake, of B6 and 129 mice at 0.05% to 0.2% concentrations. Previous exposure only to saccharin solutions did not have this effect on saccharin preference.
Figure 1.
Mean (±SEM) sucrose preference (panels A and C) and intakes (panels B and D) of C57BL/6J (B6) and 129P3/J (129) mice in two-bottle tests (23 h/day) with sucrose vs. water. The sucrose concentrations were each presented for two consecutive days in an ascending series. Tests 1 and 2 were separated by 4 days of water only. Significant (P<0.05) between-strain differences are indicated by an asterisk (*). Data are from Sclafani [28] which presents results of additional sucrose tests.
Taken together, these findings indicate that experience with the orosensory and post-oral actions of sucrose conditions an increase in the attraction of B6 and 129 mice to sucrose solutions such that the strain difference in sugar preference is virtually eliminated and difference in sucrose intake is reduced at higher concentrations. This post-oral conditioning interpretation implies that 129 mice can taste dilute sucrose solutions despite their subsensitive T1R3 receptor. It may be that dilute sucrose solutions generate taste responses that are too weak to stimulate a sugar preference in naive 129 mice but are effective in mice that have previously experienced the oral and post-oral effects of more concentrated solutions. Alternatively, 129 mice may use nongustatory cues to detect dilute sugar solutions. Some data indicate that rats and mice can smell sucrose solutions [26,34], although the effectiveness of odor cues (or texture cues) to mediate the preference for dilute sucrose solutions remains to be demonstrated. The finding that prior sucrose exposure increased the preference for dilute saccharin solutions as well as for dilute sucrose solutions also suggests gustatory mediation of the experiential effect.
Another dramatic example of an experientially-induced increase in sucrose preference was reported by Zhao et al. [36] in T1R3 KO mice. These mice displayed only a minimal response to sucrose at high concentrations during brief access (5 sec) lick tests. However, when given 24-h sucrose vs. water tests with ascending sucrose concentrations, the T1R3 KO mice displayed an ∼82% preference for 1 M (34.2%) sucrose which was close to the ∼92% preference displayed by normal B6 mice. In contrast, other T1R3 KO mice that were not tested with ascending concentration of sucrose showed virtually no preference (∼55%) for 1 M sucrose. The authors hypothesized that this experientially-induced sucrose preference in the T1R3 KO mice was mediated by a learned response to the odor and/or texture of the sugar solutions. However, an involvement of the animals’ remaining functional T1R2 sweet receptor cannot be excluded.
4. Sugar conditioned flavor preference
The experiential effects on sweetener preference summarized above strongly suggest that the postoral effects of sugars enhance sweetener preference in mice. This raises the possibility that the difference in sugar intake among inbred mouse strains may be due not only to differences in oral sweet taste sensitivity, but also to differences in the post-oral conditioning response to sugars. Consistent with this idea, recent studies have identified T1R3 and T1R2 sweet receptor proteins in the rodent intestine [6], although the role of these gut receptor proteins in the post-oral action of sugars is unknown. However, it is well established that carbohydrates infused directly into the stomach or intestines condition robust flavor preferences in rats [27]. Sclafani and Glendinning [31] have adapted the intragastric (IG) conditioning procedure to the mouse and recently used this technique to compare sucrose conditioning in B6 and 129 mice [32]. The mice were trained to drink one flavored solution (the CS+, e.g., grape Kool-Aid) paired with IG infusions of 16% sucrose and another flavored solution (the CS-, e.g., cherry Kool-Aid) paired with IG water infusions during alternate 22 h/day sessions. A two-bottle choice test was then conducted with the CS+ vs. CS-still paired with their respective infusions. Chow was available ad libitum throughout training and testing. The oral intakes and IG infusions were matched in volume so that on CS+ training days the 16% sugar infusate was diluted to 8% in the stomach by the orally consumed CS solution. This net 8% concentration was selected because peak differences in 24-h sugar intakes of B6 and 129 mice occur at or near this concentration [3,15,28].
In the first experiment, the mice were trained with unsweetened CS flavors followed by additional training with the solutions sweetened with 0.2% saccharin. Overall, the mice developed significant preferences for the CS+ over the CS- that were stronger when the solutions were sweetened with saccharin. There was a significant strain difference and the B6 mice displayed stronger preferences than did the 129 mice. However, the B6 mice also drank more of the CS solutions during training, particularly of the saccharin-sweetened CS solutions, than did the 129 mice. This provided them with a greater opportunity to learn the CS+/sucrose association.
In a second experiment CS training intakes of the B6 and 129 mice were equated by using “isosweet” mixtures of dilute sugar and saccharin. A preliminary study determined that giving 129 mice a more concentrated mixture than B6 mice compensated for their reduced sweet taste sensitivity and produced comparable intakes in the two strains. New mice were trained with CS+ and CS- flavored versions of these mixtures paired with IG sucrose and water infusions, respectively. The 129 mice were trained with 2% sucrose + 0.2% saccharin mixtures and the B6 mice were trained with 0.4% sucrose + 0.04% saccharin mixtures, and the concentration of the IG sucrose infusions was adjusted so that net sugar concentration in the stomach was 8% for both strains. The overall intakes of the B6 and 129 mice were very similar and both strains strongly preferred (96-98%) the CS+ to the CS- in the two bottle test. Furthermore, during the one-bottle training sessions both strains consumed about twice as much of CS+ paired with IG sucrose infusions as of the CS- paired with water infusions which documents the postingestive stimulation of sugar intake in mice. The training and test data indicate that 129 and B6 are equally sensitive to the postingestive reinforcing actions of sucrose (at least at the concentration tested) when the sweetness level of the CS solutions is equated for the two strains.
Thus, the fact that B6 mice overconsume 8% sucrose compared to 129 mice in 24-h intake tests does not result because 129 mice are less sensitive than B6 mice to the post-oral effects of the sugar. Nevertheless, post-oral feedback may indirectly contribute to sucrose overconsumption by B6 mice. That is, B6 mice may initially overconsume sucrose because they perceive it as having a sweeter taste than do 129 mice, and then their elevated intake produces a stronger postingestive response that further stimulates sugar consumption. This oral/post-oral positive feedback model can explain why strain differences in sugar intake are greater than those observed with nonnutritive sweeteners, as originally noted by Bachmanov et al. [3].
Note that the nature of the post-oral feedback produced by sugar that reinforces sugar preference and intake remains to be clarified. A recent report has identified T1R2 and T1R3 sweet receptor proteins in the rodent stomach and intestine [6], but the role of these or other gut “taste” receptors in post-oral sugar conditioning is not certain. What is clear is that post-oral sugar reinforcement is not directly related to the satisfaction of energy need since IG sugar infusions condition flavor preferences in freely-fed as well as food restricted animals [35]. Thus, the oral and post-oral actions of sugar are similar in having positive reinforcing actions in sated and deprived animals alike.
5. Operant Analysis of Sweetener Avidity
While B6 mice drink more sugar than 129 mice at low to intermediate concentrations, the strains do not differ in their intakes of concentrated sugar solutions (16% and/or 32%) [3,28]. It may be that the strains do not differ in their taste response to concentrated sucrose solutions but this seems unlikely because chorda tympani nerve recordings indicate that 1 M (34.2%) sucrose stimulates a stronger neural response in B6 mice than in 129 mice [12]. An alternative explanation is that the post-oral satiating effect of concentrated sugar solutions limits the intakes of both strains and thereby masks an increased avidity of B6 mice for sucrose. Various behavioral procedures are available to measure food motivation that minimize the impact of post-oral satiety on performance. One such procedure is the progressive ratio (PR) operant task in which the amount of effort required to obtain successive food rewards increases during a session such that the animal stops responding before it is physiologically satiated. Studies of sucrose motivation in rats indicate that PR responding increases monotonically as sugar concentration increases over a wide range of concentrations [25,30]. This contrasts with results obtained in one- or two-bottle tests in which sucrose consumption increases and then decreases as concentration goes from 1% to 32% [30].
A recent study in the author’s laboratory used a PR operant licking task to determine if B6 mice are more attracted than are 129 mice to a 16% sucrose solution [29]. The mice had free access to food and water during the 22 h/day sessions. In addition, 16% sucrose was available either from a sipper tube attached to a bottle (“bottle test”) or from an operant sipper tube that delivered fixed sugar rewards (0.025 ml) after the required number of licks was emitted. In particular, on a “PR1-1” schedule, the mice were required to emit 20 licks for the first reward, 21 licks for the second reward, 23 licks for the third reward, etc. Other operant schedules and sweetener solutions were used as described in detail in [29]. The first experiment was conducted with mice with extensive prior experience with sucrose [28]. In the bottle test the 129 and B6 mice did not significantly differ in their 16% sucrose intake (16.7 vs 17.6 g/day) or total licks/day (12,258 vs. 14,074). As expected, when sucrose was available on the PR1-1 operant schedule, sugar intakes significantly declined while total licks/day greatly increased. Unexpectedly, however, 129 mice consumed more sucrose and licked substantially more than did B6 mice in the PR test (7.9 vs. 6.7 g/day, 67,695 vs. 47,561 licks/day).
The vigorous PR licking displayed by the B6 mice and especially the 129 mice in the first experiment may represent an enhanced sweet appetite that was conditioned by their extensive prior experience with sucrose. A second experiment therefore measured the PR licking response in sucrosenaive mice that had prior experience only with saccharin. When the mice were first tested with 0.4% saccharin on the PR1-1 schedule, saccharin intake and PR licking were significantly higher in the B6 than in the 129 mice (2.5 vs. 1.3 g/day, 8,538 vs. 3,824 licks/day). These results are consistent with the well established strain difference in saccharin preference and intake. When next tested with sucrose for the first time, the B6 and 129 mice did not differ in their PR response to 16% sucrose (4.2 vs. 4.5 g/day, 22,201 vs. 26,162 licks/day). The mice were then given bottle access to 16% sucrose for three days before being retested on the PR1-1 schedule. The 129 mice consumed slightly, but not significantly less 16% sucrose than did the B6 mice in the bottle test (12.0 vs. 13.7 g/day). Yet in the subsequent PR test, the 129 mice responded more vigorously than did the B6 mice for 16% sucrose (5.5 vs. 4.0 g/day, 33,825 vs. 19,077 licks/day). Note that PR responding in both strains was less pronounced than that observed in the first experiment in which the mice had considerably more exposure to sucrose solutions prior to the operant test.
Contrary to the prediction that B6 mice would lick more that 129 mice for 16% sucrose on a demanding PR schedule, the results of the two experiments revealed that 129 mice licked as much or considerably more than B6 mice. The increased PR response of 129 mice for 16% sucrose occurred only after the animals had consumed substantial amounts of sugar in bottle tests which suggests that it represents a conditioned increase in sugar appetite. The PR data suggest, therefore, that the post-oral actions of sucrose enhance sugar appetite more in 129 mice than in B6 mice, although this effect is apparent only under certain conditions, i.e., with concentrated sugar solutions and when the sugar is difficult to obtain. Irrespective of the strain differences, the PR tests revealed a remarkable avidity for sucrose in both the B6 and 129 mice. During the tests the mice had unlimited access to chow and water and thus had no physiological need for sugar. Yet the B6 and 129 mice consumed substantially more 16% sucrose than water in the PR1-1 tests despite the escalating cost of the sugar throughout the daily sessions.
6. Summary
Sweet taste begins with activation of T1R2/T1R3 receptors (and perhaps others) in the mouth but sweet appetite is a centrally mediated process that is influenced by a multitude of factors. Thus, variation of the T1R3 taste receptor, while it significantly affects sweetener preference at some concentrations, cannot completely account for strain differences in sweetener intake or motivation as noted by several investigators [2,7,9,15]. It is interesting to note that Fuller [8] originally hypothesized that the Sac locus gene modulated the central incentive evaluation of sweet taste rather than the peripheral sensitivity to sweeteners. While this was not confirmed in subsequent molecular studies, Fuller’s emphasis on the importance of central factors in sweetener appetite foreshadowed recent findings. These include results demonstrating the significant influence sugar experience and post-oral conditioning have on sweetener preference and intake. Most dramatically, prior exposure to sucrose solutions was found to increase sucrose preference in sweet sensitive B6 mice and even more so in sweet subsensitive 129 mice so that the strain difference in sucrose preference was eliminated [28]. Exposure to nonnutritive saccharin solutions also enhanced subsequent sweetener preference but not as profoundly as did sucrose [29]. The differential effect of sucrose and saccharin experience implicated the importance of post-oral nutritive factors in sweetener appetite, which was confirmed by the results of intragastric infusion studies. IG sucrose conditioned increased intake and preference for flavored sweet solutions in both B6 and 129 mice [32]. B6 mice were not more responsive to the post-oral reinforcing action of sucrose when intake was equated, but their enhanced sweet taste sensitivity, by promoting greater sugar intake, may expose them to stronger post-oral reinforcement. This, in turn, may further enhance their sugar intake via a positive feedback cycle. Experience with the oral and post-oral effects of sucrose also affects the avidity for sweeteners as measured by a progressive ratio operant task. Unexpectedly, sweet subsensitive 129 mice displayed more vigorous responding than did sweet sensitive B6 mice for 16% sucrose [29]. Continued studies of inbred and genetically modified mice strains may help to elucidate how central motivation systems integrate oral and post-oral stimulation by sugars to determine the appetite for sugars and other nutrients.
Acknowledgements
This paper is based on a presentation given at the Ascona Workshop on Peripheral - Central Interactions in the Control of Food Intake and Energy Balance, August 2005. The author’s research was supported by grants DK31135 and DK59360 from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant from the PSC-CUNY Award program. The author thanks Karen Ackroff for her helpful comments on this paper.
References
- 1.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Li X, Beauchamp GK. Genetics of sweet taste preferences. Pure Appl Chem. 2002;74:1135–1140. doi: 10.1351/pac200274071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capeless CG, Whitney G. The genetic basis of preference for sweet substances among inbred strains of mice: preference ratio phenotypes and the alleles of the Sac and dpa loci. Chem Senses. 1995;20:291–298. doi: 10.1093/chemse/20.3.291. [DOI] [PubMed] [Google Scholar]
- 5.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 6.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 7.Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in Sac ‘Taster’ and ‘Non-taster’ mice. Chem Senses. 2004;29:639–649. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- 8.Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- 9.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of Tas1R3 polymorphisms. Chem Senses. 2005;30:601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- 10.Harder DB, Maggio JC, Whitney G. Assessing gustatory detection capabilities using preference procedures. Chem Senses. 1989;14:547–564. [Google Scholar]
- 11.Hoshishima K, Yokoyama S, Seto K. Taste sensitivity in various strains of mice. Am J Physiol. 1962;202:1200–1204. doi: 10.1152/ajplegacy.1962.202.6.1200. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci. 2004;24:2296–2303. doi: 10.1523/JNEUROSCI.4439-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotlus BS, Blizard DA. Measuring gustatory variation in mice: A short-term fluid-intake test. Physiol Behav. 1998;64:37–47. doi: 10.1016/s0031-9384(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mammalian Genome. 2001;12:13–16. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 18.Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol. 2002;12:366–371. doi: 10.1016/s0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- 19.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 20.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res. 1984;322:83–92. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- 21.Pelz WE, Whitney G, Smith JC. Genetic influences on saccharin preference of mice. Physiol Behav. 1973;10:263–265. doi: 10.1016/0031-9384(73)90308-9. [DOI] [PubMed] [Google Scholar]
- 22.Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez I, Fuller JL. Genetic influence on water and sweetened water consumption in mice. Physiol Behav. 1976;16:163–168. doi: 10.1016/0031-9384(76)90300-0. [DOI] [PubMed] [Google Scholar]
- 24.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly S. Reinforcement value of gustatory stimuli determined by progressive ratio performance. Pharmacol Biochem Behav. 1999;63:301–311. doi: 10.1016/s0091-3057(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 26.Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses. 1994;19:425–431. doi: 10.1093/chemse/19.5.425. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.01.016. in press. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.01.017. in press. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 31.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 Mice: Oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 33.Stockton MD, Whitney G. Effects of genotype, sugar, and concentration on sugar preference of laboratory mice (Mus musculus) J Comp Physiol Psychol. 1974;86:62–68. doi: 10.1037/h0035929. [DOI] [PubMed] [Google Scholar]
- 34.Uebayashi H, Hatanaka T, Kanemura F, Tonosaki K. Acute anosmia in the mouse: behavioral discrimination among the four basic taste substances. Physiol Behav. 2001;72:291–296. doi: 10.1016/s0031-9384(00)00441-8. [DOI] [PubMed] [Google Scholar]
- 35.Yiin Y-M, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and unrestricted rats. Physiol Behav. 2005;84:217–231. doi: 10.1016/j.physbeh.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]