Abstract
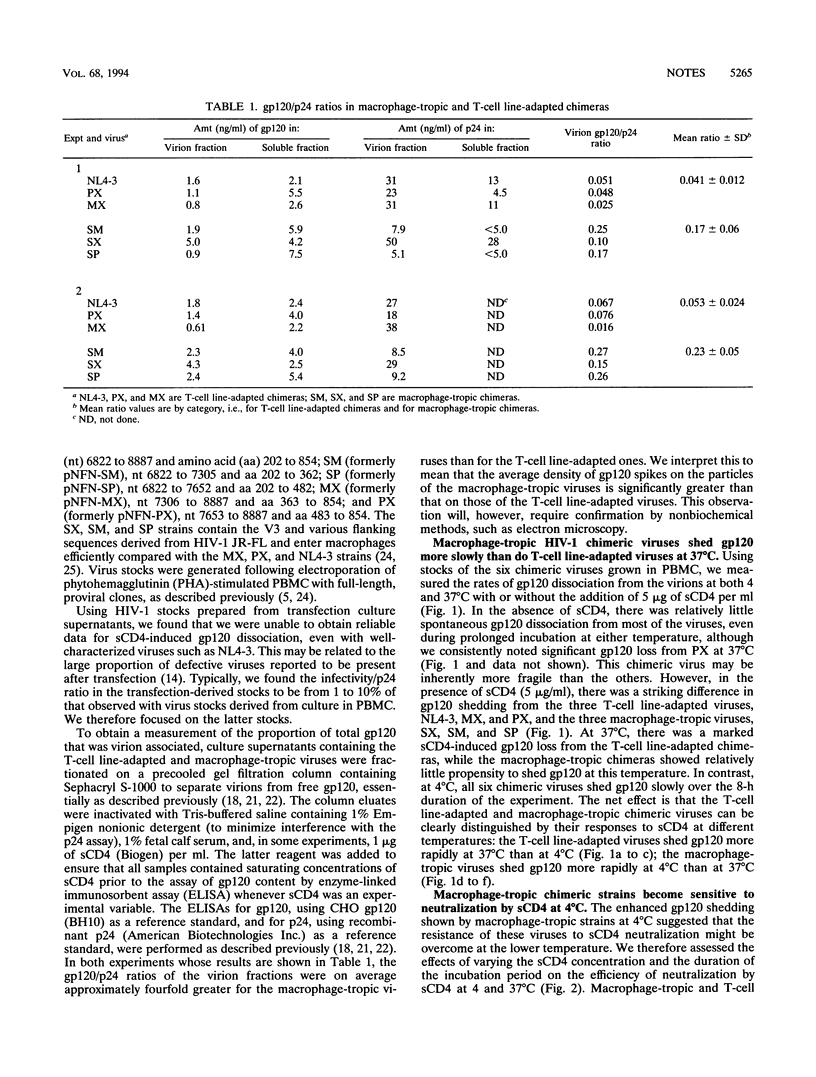
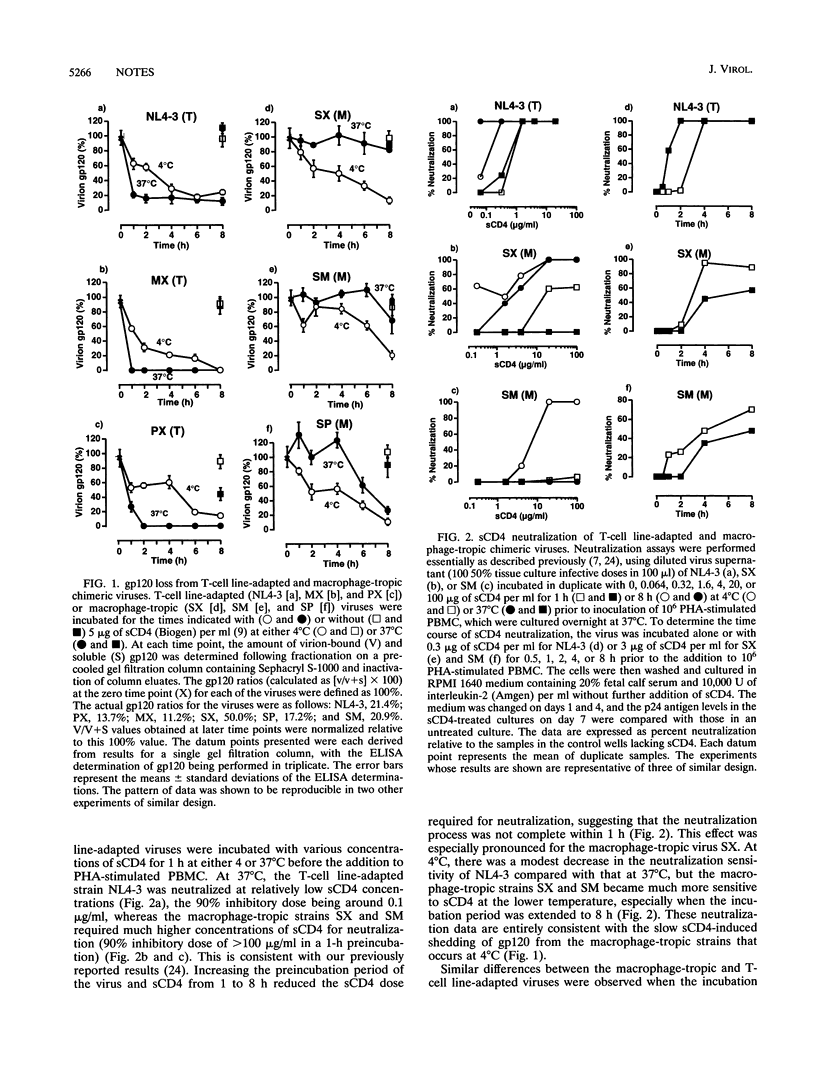
Molecular clones of three macrophage-tropic and three T-cell line-adapted strains of human immunodeficiency virus type 1 (HIV-1) were used to explore the mechanism of HIV-1 resistance to neutralization by soluble CD4 (sCD4). The three macrophage-tropic viruses, each possessing the V3 and flanking regions of JR-FL, were all resistant to sCD4 neutralization under the standard conditions of a short preincubation of the virus and sCD4 at 37 degrees C prior to inoculation of peripheral blood mononuclear cells. In contrast, the three T-cell line-adapted viruses, NL4-3 and two chimeras possessing the V3 and flanking regions of NL4-3 in the envelope background of JR-FL, were all sCD4 sensitive under these conditions. Sensitivity to sCD4 neutralization at 37 degrees C corresponded with rapid, sCD4-induced gp120 shedding from the viruses. However, when the incubation temperature of the sCD4 and virus was reduced to 4 degrees C, the three macrophage-tropic viruses shed gp120 and became more sensitive to sCD4 neutralization. In contrast, the rates of sCD4-induced gp120 shedding and virus neutralization were reduced for the three T-cell line-adapted viruses at 4 degrees C. Thus, HIV resistance to sCD4 is a conditional phenomenon; macrophage-tropic and T-cell line-adapted strains can be distinguished by the temperature dependencies of their neutralization by sCD4. The average density of gp120 molecules on the macrophage-tropic viruses exceeded by about fourfold that on the T-cell line-adapted viruses, suggesting that HIV growth in T-cell lines may select for a destabilized envelope glycoprotein complex. Further studies of early events in HIV-1 infection should focus on primary virus strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold E., Arnold G. F. Human immunodeficiency virus structure: implications for antiviral design. Adv Virus Res. 1991;39:1–87. doi: 10.1016/s0065-3527(08)60792-7. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Smith D. H., Marsters S. A., Riddle L., Gregory T. J., Ho D. D., Capon D. J. Resistance of primary isolates of human immunodeficiency virus type 1 to soluble CD4 is independent of CD4-rgp120 binding affinity. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7056–7060. doi: 10.1073/pnas.88.16.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighty D. W., Rosenberg M., Chen I. S., Ivey-Hoyle M. Envelope proteins from clinical isolates of human immunodeficiency virus type 1 that are refractory to neutralization by soluble CD4 possess high affinity for the CD4 receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7802–7805. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Jitters jeopardize AIDS vaccine trials. Science. 1993 Nov 12;262(5136):980–981. doi: 10.1126/science.8235635. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Li X. L., Moudgil T., Ho D. D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Fujita K., Silver J., Peden K. Changes in both gp120 and gp41 can account for increased growth potential and expanded host range of human immunodeficiency virus type 1. J Virol. 1992 Jul;66(7):4445–4451. doi: 10.1128/jvi.66.7.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of envelope V3 loop as the major determinant of CD4 neutralization sensitivity of HIV-1. Science. 1992 Jul 24;257(5069):535–537. doi: 10.1126/science.1636088. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Kabat D., Kozak S. L., Wehrly K., Chesebro B. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol. 1994 Apr;68(4):2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpton J., Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992 Apr;66(4):2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Mascola J. R., Louwagie J., McCutchan F. E., Fischer C. L., Hegerich P. A., Wagner K. F., Fowler A. K., McNeil J. G., Burke D. S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994 Jan;169(1):48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Yarchoan R., Broder S. Molecular targets for AIDS therapy. Science. 1990 Sep 28;249(4976):1533–1544. doi: 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Burkly L. C., Connor R. I., Cao Y., Tizard R., Ho D. D., Fisher R. A. Adaptation of two primary human immunodeficiency virus type 1 isolates to growth in transformed T cell lines correlates with alterations in the responses of their envelope glycoproteins to soluble CD4. AIDS Res Hum Retroviruses. 1993 Jun;9(6):529–539. doi: 10.1089/aid.1993.9.529. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Ho D. D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993 Feb;67(2):863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Huang Y. X., Ashkenazi A., Ho D. D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992 Jan;66(1):235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Moore J., Ho D. HIV tropism. Nature. 1993 Jan 28;361(6410):309–310. doi: 10.1038/361309b0. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Chen I. S., Ho D. D., Daar E. S. Mapping genetic determinants for human immunodeficiency virus type 1 resistance to soluble CD4. J Virol. 1992 May;66(5):3125–3130. doi: 10.1128/jvi.66.5.3125-3130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Orloff S. L., Kennedy M. S., Belperron A. A., Maddon P. J., McDougal J. S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993 Mar;67(3):1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Stamatatos L., Cheng-Mayer C. Evidence that the structural conformation of envelope gp120 affects human immunodeficiency virus type 1 infectivity, host range, and syncytium-forming ability. J Virol. 1993 Sep;67(9):5635–5639. doi: 10.1128/jvi.67.9.5635-5639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Furman C., Helseth E., Repke H., Sodroski J. Lack of correlation between soluble CD4-induced shedding of the human immunodeficiency virus type 1 exterior envelope glycoprotein and subsequent membrane fusion events. J Virol. 1992 Sep;66(9):5516–5524. doi: 10.1128/jvi.66.9.5516-5524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S., Tizard R., DeMarinis J., Pepinsky R. B., Zullo J., Schooley R., Fisher R. Resistance of primary isolates of human immunodeficiency virus type 1 to neutralization by soluble CD4 is not due to lower affinity with the viral envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1335–1339. doi: 10.1073/pnas.89.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Vartanian J. P., Henry M., Chenciner N., Cheynier R., Delassus S., Martins L. P., Sala M., Nugeyre M. T., Guétard D. LAV revisited: origins of the early HIV-1 isolates from Institut Pasteur. Science. 1991 May 17;252(5008):961–965. doi: 10.1126/science.2035026. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Martin M. A., Peden K. W. Increase in soluble CD4 binding to and CD4-induced dissociation of gp120 from virions correlates with infectivity of human immunodeficiency virus type 1. J Virol. 1994 Feb;68(2):1029–1039. doi: 10.1128/jvi.68.2.1029-1039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Ross E. K., Buckler-White A. J., Theodore T. S., Martin M. A. Functional interaction of constant and variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3595–3600. doi: 10.1128/jvi.63.9.3595-3600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



