Abstract
Purpose
To review the principles of neural plasticity and make recommendations for research on the neural bases for rehabilitation of neurogenic speech disorders.
Method
A working group in speech motor control and disorders developed this report, which examines the potential relevance of basic research on the brain mechanisms involved in neural plasticity and discusses possible similarities and differences for application to speech motor control disorders. The possible involvement of neural plasticity in changes in speech production in normalcy, development, aging, and neurological diseases and disorders was considered. This report focuses on the appropriate use of functional and structural neuroimaging and the design of feasibility studies aimed at understanding how brain mechanisms are altered by environmental manipulations such as training and stimulation and how these changes might enhance the future development of rehabilitative methods for persons with speech motor control disorders.
Conclusions
Increased collaboration with neuroscientists working in clinical research centers addressing human communication disorders might foster research in this area. It is hoped that this paper will encourage future research on speech motor control disorders to address the principles of neural plasticity and their application for rehabilitation.
The purpose of this paper is to disseminate the outcome of discussions of a working group formed to consider the principles of neural plasticity that might relate to speech motor control disorders. The working group consisted of specialists in speech motor control who accepted the invitation of the Brain Rehabilitation Research Center, a Veterans Administration Rehabilitation Research and Development Center of Excellence, to convene to address the issues of neural plasticity and rehabilitation of speech disorders. The agenda was to identify potential directions for translational research on how environmental manipulations, and training in particular, could enhance neuroplasticity and recovery of function in neurological diseases and disorders. The group identified potential opportunities for the translation of principles from basic neuroscience into clinical research on the rehabilitation of neurogenic speech motor control disorders. Such disorders include the various forms of dysarthria and apraxia of speech secondary to stroke, nerve injury, neurodegenerative disease, brain tumors, or trauma (Duffy, 2005). Idiopathic disorders such as spasmodic dysphonia, oral-mandibular dystonia and essential tremor affecting the head and neck were also discussed. Subsequent to the meeting a manuscript was drafted and underwent considerable revision as additional information was incorporated over the next two years. Some of the concepts of neural plasticity that are described in greater detail in an accompanying manuscript (Kleim & Jones, in press), may or may not apply to speech motor control. Suggestions are provided to stimulate the consideration of translational research on the role of neural change in rehabilitation and recovery of speech motor control disorders.
I. Definition of Neural Plasticity
Neural plasticity is the ability of the central nervous system (CNS) to change and adapt in response to environmental cues, experience, behavior, injury or disease. Neural plasticity can result from a change in function within a particular neural substrate in the CNS through alterations in synaptic strength, neuronal excitability, neurogenesis or cell death (Brosh & Barkai, 2004). Changes in the function of a neural substrate can then alter behavior secondary to environmental influences such as experience, learning, development, aging, change in use, injury or response to injury such as unmasking due to the loss of surround inhibition with reduced afferent input (Tinazzi et al., 1998; Urasaki, Genmoto, Wada, Yokota, & Akamatsu, 2002; Ziemann, Hallett, & Cohen, 1998). Behavioral changes can also result from compensation, when residual neural substrate(s) are used to perform impaired functions, as may occur at some point during recovery from aphasia (Saur et al., 2006). Neural plasticity may also alter the function of the original neural substrate used to produce a behavior through neuronal sprouting and dendritic growth (Bellemare, et al., 1973). Although plasticity can be observed across multiple elements of the nervous system including the cerebrovasculature and glia (Magistretti, 2006; Yiu & He, 2006), the focus of this paper is on the role of experience dependent change in neural function at the level of the synapse as proposed by Hebb in the 1940s (Hebb, 1949).
II. Principles of Neural Plasticity
Several principles of neural plasticity have been proposed on the basis of animal research showing changes in synaptic processing in the cortex. Research in rats, for example, has shown that motor training will alter neural signaling pathways by up-regulating early immediate gene expression, such as c fos expression, which in turn can alter protein translation (Kleim, Lussnig, Schwarz, Comery, & Greenough, 1996). Changes in neuronal activity can produce changes in neurotransmission and synaptic strength. Synaptic plasticity produces changes in intracortical microcircuitry altering the topography of cortical maps. These changes can provide the basis for long term changes in motor performance, see Figure 5 in Monfils, 2005 (Monfils, Plautz, & Kleim, 2005). Changes in synaptic function can induce activity in previously silent latent connections (unmasking) or dendritic sprouting in animals (Bellemare, Woods, Johansson, & Bigland-Ritchie, 1973; Brosh & Barkai, 2004). That such changes are also occurring in the human cortex can only be hypothesized; indirect support comes from observed alterations in cortical physiology (Cohen et al., 1998). The relevance of alterations in neuronal function to speech motor control has yet to be examined. The principles reviewed by Kleim and Jones (in press) are discussed with particular reference to speech and voice functioning following brain injury or in neurodegenerative disease. It is recommended that some of these principles will need to be addressed by carefully designed studies with appropriate controls to assess the degree of plasticity possible in the neural substrates involved in human speech and voice production.
i. The Effect of Use on Neural Substrates
The first principle of neuroplasticity is that if a neural substrate is not biologically active, it will degrade in function. Merzenich and colleagues in the 1980s demonstrated that following the loss of sensory input from the hand to the cortex in adult owl and squirrel monkeys; cortical somatosensory representation for that body part became reduced (Kaas, Merzenich, & Killackey, 1983; Merzenich et al., 1983). Conversely, the same research group demonstrated that by increasing environmental input, cortical representation can be altered or enhanced (Nudo, Jenkins, & Merzenich, 1990). Following brain injury, further cortical loss may occur in the absence of retraining if functions formerly represented in the lesioned zone do not reappear spontaneously in adjacent cortical regions (Friel, Heddings, & Nudo, 2000; Nudo & Milliken, 1996). Although this is unstudied in speech motor control following brain injury, it may be relevant to rehabilitation strategies and important for motor retraining. For example, to determine the degree to which disuse affects speech following head injury, two groups of patients might be compared. The first group might be encouraged to speak aloud to a group at least two hours a day and the other group allowed to use computer projection of written expression with nonverbal facial or oral expression also in a group setting for at least two hours a day. In this way both groups have similar involvement in language formulation, covert and interpersonal non-speech communication while only one group is using speech production. By combining clinical studies with functional and structural neuroimaging, the effects of use or disuse on the cerebral activity for speech can be determined.
ii. Usage Improves Function
This principle, an extension of the first, states that with increased biological activity, future functioning can be enhanced. Over the last decade an emerging literature has demonstrated that training can lead to an enhancement of both function and structure of the neural mechanisms involved in that behavior (Carr & Shephard, 1999; Cohen et al., 1998; Nudo, 2003; Rioult-Pedotti, Friedman, & Donoghue, 2000; Rioult-Pedotti, Friedman, Hess, & Donoghue, 1998).
Most of this research involves training reaching behaviors in rats (Rioult-Pedotti, Friedman, & Donoghue, 2000; Rioult-Pedotti, Friedman, Hess, & Donoghue, 1998) or relatively simple movements in humans (Morgen, Kadom, Sawaki, Tessitore, Ohayon, McFarland et al., 2004). It is unknown whether or not the potential for recovery with retraining of reaching movements is the same as that for complex, over-learned, relatively automatic motor behaviors such as speech. Several differences are apparent between limb and speech movements: speech movements are learned throughout childhood, are used for several hours on a daily basis throughout a lifetime, and speech gestures require precision to achieve auditory targets. Only a few limb movements are equivalent such as writing, typing and piano playing that are used daily only in certain careers. Although some studies have shown neural plasticity of brain function for language following intensive training (Louis et al., 2001) or surgery (Voets et al., 2006), the potential for neural plasticity in the speech motor system with rehabilitation is not well known. In a case study of spontaneous recovery from cortical dysarthria post stroke without retraining, functional magnetic resonance imaging (fMRI) showed a selective shift of the cortical representation for speech motor control to the right Rolandic cortex and the left cerebellum (Riecker, Wildgruber, Grodd, & Ackermann, 2002). This differs from recent findings in limb control and aphasia where recovery was greatest when neural control returned to the original, involved hemisphere such as the contralateral hemisphere for an affected limb (Serrien, Strens, Cassidy, Thompson, & Brown, 2004) or to the left hemisphere for language recovery in aphasia (Saur et al., 2006).
It is important to determine if motor retraining alters brain function. To determine the effects of training on the recovery of brain function will require a non-treated control group to account for spontaneous recovery, which occurs without training and is expected to be greatest in the first three months post stroke or trauma. For speech production, it needs to be established whether retraining induces a return of function to the original neural substrates in the left primary orofacial cortex or whether alternate substrates such as in the somatosensory region are invoked (Jang et al., 2005). We need to establish which types of intervention will enhance the return of speech production following brain injury or in disease. By studying the results of different methods of rehabilitation we can identify the most effective strategies for recovery and which strategies are maladaptive.
Finally, speech production may differ from other movements in the effect of practice. In one study of short term learning in persons with cerebellar atrophy, demonstrated a difference in the effects of learning between speech and non-speech movements within groups of healthy volunteers and persons with cerebellar pathology. Although there were no differences between groups on either speech or non-speech movements, there was a difference in the effects of learning between speech and non-speech movements within both groups. Speech movements improved with practice while non-speech movements did not improve with practice in either group (Schulz, Dingwall, & Ludlow, 1999). This suggests that speech movements may have greater potential for retraining than non-speech movements in both patients with neurological disorders and healthy volunteers. Perhaps there are corresponding differences in the degree of change in cortical physiology in response to training for speech and non-speech tasks. It cannot be assumed that the type of pattern of cortical or behavior adaptations are equivalent for speech and non-speech tasks and speaks to the importance of this research in speech motor control.
iii. Plasticity is Experience Specific
This principle suggests that changes in neural function with practice may be limited to the specific function being trained. This is relevant to speech rehabilitation and suggests that training on lip strength, for example, may only benefit the neural control for lip movement and force but may not spontaneously “transfer” to speech production. This principle suggests that changes may occur only in the neural substrates involved in the particular behavior being trained (Kleim et al., 2002). This principle is distinct from the concept of cross-transfer when an untrained limb improves in performance to the same degree as the trained limb on the opposite side. In cross-transfer motor training on one side facilitates motor neuron firing in the contralateral muscle group (Nagel & Rice, 2001). Cross-transfer is likely due to alterations in spinal cord pathways, rather than changes in cortical control for the untrained limb.
A long-standing debate within the speech community is to whether or not training on non-speech oral behaviors will enhance speech production (Clark, 2003; Weismer, 2006). For example, myofunctional therapy for the lingual musculature has been used (Ray, 2003) under the assumption that there will be transfer of increased function to speech production. One report found that training involving non-speech oral motor behaviors was helpful in a series of cases (Dworkin & Hartman, 1979), although no control group was included for comparison. On the other hand, others reported that non-speech oral movements were unrelated to residual speech in persons with dysarthria (McAuliffe, Ward, Murdoch, & Farrell, 2005; Solomon, Robin, & Luschei, 2000). Further, diadochokinetic syllable repetition skills and speech production rate and accuracy are often unrelated in adults with speech motor control disorders (McAuliffe, Ward, Murdoch, & Farrell, 2005), suggesting that training on diadochokinetic movements may not spontaneously improve speech. One reason for this difference may be that diadochokinetic syllable repetition does not require formulation of a new utterance for speech expression/communication. The neural substrates involved in speech repetition seem to be restricted to the left anterior insula, a localized region in the lateral premotor cortex, and the posterior pallidum (Wise, Greene, Buchel, & Scott, 1999) while speech expression likely involves a broader network of brain regions (Donnan, Carey, & Saling, 1999).
These issues can be examined using functional neural imaging to determine if the brain substrates involved in speech and non-speech behaviors are the same or different. A study of healthy speakers showed silent tongue movements produced symmetric brain activation in the right and left primary motor regions while phonation or phonation combined with tongue movements produced clusters of activation primarily in the left hemisphere (Terumitsu, Fujii, Suzuki, Kwee, & Nakada, 2006). In another study, syllable production activated regions in the left inferior frontal gyrus, left middle frontal gyrus, the caudate nuclei and the thalamus, whereas non-speech oral movements activated areas in the primary motor cortex.(Bonilha, Moser, Rorden, Baylis, & Fridriksson, 2006). Although the repetition of isolated syllables is not equivalent to speech production, this study suggests that even at the syllable production level there are both commonalities and differences in the neural substrates that are involved in speech-like and non-speech oral behaviors. Studies comparing changes in CNS function following training are required to determine if activity in similar or different neural substrates are enhanced during training using speech versus non-speech tasks.
Also related to the speech versus non-speech debate is the relevance of strength training to the rehabilitation of dynamic rapid movements that are needed for speech production. In general muscle forces used for speech are between 10 to 20 % of maximum for the lips (Barlow & Abbs, 1986) and activation of the laryngeal muscles for speech is between 10 and 20 % of maximum (Ludlow & Lou, 1996). Overall, the maximum force that can be produced is likely to be of much less consequence for speech production than the precision of low levels of force control (Barlow & Netsell, 1986). Some basic research in the rat has shown that motor skill training induces synaptogenesis and motor map reorganization while strength training does not (Remple, Bruneau, VandenBerg, Goertzen, & Kleim, 2001). One study found that strength gains in the early phase of an arm muscle training regimen were associated with an increase cortical excitability (Griffin & Cafarelli, 2006) while another in humans compared arm skill training with strength training and found increases in cortical excitability only occurred with skill training (Jensen, Marstrand, & Nielsen, 2005). Exercise alone, as opposed to skill training, may not alter motor map organization although it induces angiogenesis in the rat (Kleim, Cooper, & VandenBerg, 2002). Different adaptive changes may be evoked with strength training than those that occur with skill training (Jensen, Marstrand, & Nielsen, 2005). Also the relative benefits of strength and skill training should take into account the diverse neural substrates affected in different neuromotor disorders. For example, persons with diseases that affect motor neurons or the strength of synaptic inputs to excite motor neurons may benefit more from strength training than adults with a motor programming disorder, such as apraxia. These issues need to be examined systematically using functional neural imaging to compare brain changes during strength retraining versus training emphasizing voice and speech production skills in groups of adults with different neuromotor speech disorders. Transcranial magnetic stimulation (TMS) may be one technique for examining the effects of skill or strength training on motor map re-organization, however, the accuracy of mapping cranial muscles using TMS is less reliable than for limb muscles (Ludlow et al., 1994)
iv. Repetition of Training
This principle states that changes in neural substrates will occur only as a result of extensive and prolonged practice and that neural changes may not become consolidated until later in the training process (Kleim et al., 2004). Stimulation induced synaptic strength also requires a sufficient number of stimuli to induce long term potentiation (LTP) in animals (Lisman & Spruston, 2005). The number of repetitions per session and the number of sessions required for a behavior to become consolidated needs to be established for speech and voice motor control rehabilitation.
Although the importance of repetition/practice in consolidating a motor skill is well supported, which type of practice should be used is less clear. There may be species and population differences in the mechanisms involved in learning. Some of the human motor learning literature suggests that massed repetition for training complex skills may not be as effective as inter-leaving recall trials or withdrawal of knowledge of results during motor learning in humans (Wulf, Lee, & Schmidt, 1994; Wulf, Schmidt, & Deubel, 1993). However, motor learning for speech in brain injured adults may differ both from animal models and from learning in healthy adults. Differences occurred between children and adults when learning a non-speech oral motor task (Clark, Robin, McCullagh, & Schmidt, 2001) and also when learning novel non-words (Walsh, Smith, & Weber-Fox, 2006). Age may be an important consideration when designing training protocols in addition to the motor task (connected speech) and populations involved (different neurological diseases and disorders).
v. Intensity of Training
The principle that training must be continuous over long periods to induce neural change in animals (Fisher & Sullivan, 2001) is currently employed in neurorehabilitation programs (Teasell & Kalra, 2005). In animal models, long term potentiation of synaptic strength requires a sufficient level of stimulus intensity (Lisman & Spruston, 2005). However, several additional factors need to be considered for speech rehabilitation. If a participant is easily fatigued, for example, intensive retaining may not be appropriate, particularly in persons with motor neuron disease. A person’s medical status and other factors should be considered before assuming that intensive training can produce behavioral changes and neural plasticity. Maladaptive responses to intense motor treatment programs can include fatigue and muscle damage with variable responses dependent on the etiology of the disorders being treated (Gabriel, Kamen, & Frost, 2006). Before the appropriate intensity for speech rehabilitation training can be determined we need to identify factors that support or contradict high-intensity training in particular neuromuscular disorders.
vi. The Time of Training Onset
This principle states that different forms of neural plasticity may occur at various times in response to treatment. For example, during motor skill training in rats, changes in neuronal activity precede synaptic formation (Kleim, Lussnig, Schwarz, Comery, & Greenough, 1996), which are then followed by motor map reorganization (Kleim et al., 2004). Further, change in neuronal function is more likely to occur during the early spontaneous recovery period following brain injury, both in animals (Kleim et al., 2003; Plautz et al., 2003) and humans (Lendrem & Lincoln, 1985). Carefully designed studies need to examine possible interactions between time post injury or disease onset and the timing of treatment regimens. This need was also identified in evidence-based reviews of therapies for adults with dysarthria (Deane, Whurr, Playford, Ben-Shlomo, & Clarke, 2001a, 2001b). Functional brain imaging may be helpful for determining how the timing of training initiation and training duration might influence the ability to induce changes in brain function for speech.
vii. Salience of Training
The principle that training must be sufficiently salient to induce plasticity may be of considerable importance to speech. That is, simple repetitive movements or strength training may not enhance skilled movement and may have less potential for inducing changes in neural function underlying voice and speech production for communication. Neural plasticity may be enhanced when the movement is purposeful and related to the behavior being trained (Morgen, Kadom, Sawaki, Tessitore, Ohayon, Frank et al., 2004; Plautz, Milliken, & Nudo, 2000; Remple, Bruneau, VandenBerg, Goertzen, & Kleim, 2001). Reorganization within the auditory cortex requires that the tone be salient to the animal and engage attentional brain mechanisms (Kilgard & Merzenich, 1998). Similarly, training in voice and speech may need to involve meaningful communication. Functional brain imaging could address the degree to which meaningful speech communication may activate a different brain network than that used for syllable repetition, for example.
viii. Age Effects on Training
Although neural plasticity can occur over the entire lifespan, it is well recognized that training and environmentally induced plasticity occur more readily in younger than in older nervous systems (Kramer, Bherer, Colcombe, Dong, & Greenough, 2004; Sawaki, Yaseen, Kopylev, & Cohen, 2003). Differences in human non-speech motor learning have been found with age (Clark, Robin, McCullagh, & Schmidt, 2001). It is unknown whether learning some aspects of speech production, such as consonant articulation, may be more affected by aging than others. The degree to which speech can be retrained and whether changes in neural function can occur with retraining could provide information on the limits for rehabilitation of different speech attributes in different age groups.
ix. Transference
The principle of transference states that plasticity following training in one function may enhance related behaviors and has been studied both in animals and human rehabilitation (Chu & Jones, 2000; Frost, Barbay, Friel, Plautz, & Nudo, 2003; Jones, Chu, Grande, & Gregory, 1999; Spengler et al., 1997). This principal appears inconsistent with the principal of training specificity (iii) mentioned earlier. Possibly transference may be more likely to occur following some therapies than others. For example, training using “loud speech” enhanced swallowing in a group of persons with Parkinson disease (PD) (Sharkawi et al., 2002) suggesting transference. However, a controlled trial is needed to compare these effects with another therapy on swallowing. A comparison therapy group is needed to determine if a particular therapy is responsible for the enhancement of another behavior or whether transference occurs regardless of the type of therapy.
x. Interference
The interference principle is that plasticity can cause changes in neural function, which may interfere with behaviors or skills. For example, dystonic-like limb postures can develop following repetitive strain injuries with prolonged training in monkeys (Byl et al., 1997; Byl, Merzenich, & Jenkins, 1996). In another application of this principle, reducing input to, or restricting the use of the unaffected limb, can enhance training effects in the affected limb after stroke (Kopp et al., 1999). Thus retained functions may interfere with the recovery of lost functions after injury (Bury et al., 2000; Bury & Jones, 2002, 2004). Perhaps enhancing some speech or voice skills such as articulation might interfere with other aspects of speech production such as prosody or rate. Such questions can be addressed in small carefully designed feasibility studies as has been done for limb movement (Kopp et al., 1999). Neurophysiological recordings can quantify the change in neural function associated with retraining of various behaviors.
III. Potential Role of Neural Progenitors and Growth Factors to Enhance Recovery
Recent animal studies have idenitified two adult mammalian brain regions that contain endogenous neural progenitor cells capable of producing new neurons. Those in the subventricular zone produce neuroblasts that migrate to the olfactory bulb, while others are in the dentate gyrus of the hippocampus (Lichtenwalner & Parent, 2006). Hippocampal progenitors release new neurons with learning while growth and neurotrophic factors can enhance adult neurogenesis in rodents (Nakatomi et al., 2002). Several neurotrophic factors such as brain-derived neurotrophic factor (BDNF) can enhance neurogenesis (Kruger & Morrison, 2002; Lichtenwalner & Parent, 2006). Of particular relevance is the evidence that many types of brain injuries, including ischemia, can enhance the generation of neurons by progenitors in the adult mammalian brain with neuronal migration to the area of injury (Nakatomi et al., 2002). The intraventricular infusion of exogenous growth factors has potential to enhance this process, although the long-term survival and functionality of such neurons remains an unexamined issue in the adult human brain post stroke (Lichtenwalner & Parent, 2006). These mechanisms of progenitor enhancement hold great promise but may be reduced in the stroke population because of reduced effects of endogenous growth factors with age (Hattiangady, Rao, Shetty, & Shetty, 2005). However, if infusion methods were developed which could be applied in humans these might be combined with behavioral and environmental therapies to enhance functional recovery post brain injury.
IV. Application of these Principles to Speech Motor Control Recovery and Rehabilitation
Studies are needed to determine if the principles described above can be applied to the study of neural mechanisms involved in motor speech functioning and rehabilitation in a systematic fashion. For effective training methods already identified, the study of how such techniques alter neural function involved in speech production could increase understanding of the mechanisms involved in speech recovery and guide the development of new therapies. For example, it would be important to know whether emphasis should be placed on invoking alternate brain mechanisms for speech recovery or if the return of function in the original substrates is needed.
To date only a few well controlled treatment trials in speech motor control disorders have been published which demonstrate effective treatments for a few speech/voice disorders (Deane, Whurr, Playford, Ben-Shlomo, & Clarke, 2001a, 2001b; Ramig & Verdolini, 1998; Sellars, Hughes, & Langhorne, 2001, 2002, 2005; Yorkston, 1996; Yorkston & Spencer, 2003). Lee Silverman Voice Therapy (LSVT) had greater benefit than a placebo treatment (i.e. respiratory training where the participants passively breathed out) in aiding persons with PD (Ramig, Countryman, O’Brien, Hoehn, & Thompson, 1996; Ramig et al., 2001; Ramig, Sapir, Fox, & Countryman, 2001).. Also in PD, prosody treatment with visual feedback was found more effective than prosody treatment without visual feedback (Scott & Caird, 1983). Although only well controlled studies can identify which types of treatments can induce significant and long-lasting improvement in persons with speech motor control disorders, exploratory small trials could identify potential new treatment approaches for specific populations at different levels of severity and time post onset (Deane, Whurr, Playford, Ben-Shlomo, & Clarke, 2001a, 2001b). Those found to have potential could then be evaluated along with functional brain imaging to then determine how the return of speech function is re-established in the brain.
Some of the issues that are of specific importance to speech rehabilitation include: whether oromotor strength training will have transference to aid the return of speech production skills; whether training paradigms developed for spinal systems pertain to craniofacial bulbar systems and, whether speech production skills, which are normally automatic and precise by adulthood (Smith & Zelaznik, 2004), can be relearned in post adolescent and aging brain-injured adults. There are limits to neural plasticity following adult brain injury, and these limits need to be determined for speech communication. Small, well-controlled experimental feasibility studies on the rehabilitation of motor speech and voice disorders would be the first step.
V. Approaches to Translational Research
Translational research is an interactive process between basic research, translation studies and feasibility studies. Basic studies, in this context, necessitate both (1) animal studies of neural plasticity processes and the effects of disease on cell loss or synaptic function and (2) human studies aimed at measuring behavior and brain function using functional neuroimaging such as positron emission tomography (PET) and fMRI, electrophysiological recordings such as magnetoencephalography (MEG) and electroencephalography (EEG), and testing techniques such as transcranial magnetic stimulation (TMS). Translational studies involve either animals or humans to examine how changes in neural functioning (neural plasticity) due to training are modified by aging, developmental or disease processes. These translational studies then serve as the bases for designing feasibility studies, which are small group or pilot studies with well defined hypotheses, experimental controls and specific adult populations. Feasibility studies are designed to determine if training, stimulation or constraints can alter both behavior and neural functioning in persons with motor speech disorders. A constant interaction between concepts from basic research, translational studies, and feasibility studies is necessary as scientists and clinicians explore new concepts for modifying neural function and behavioral performance.
i. Basic Research in Neuroplasticity
The purpose of basic research is to identify the particular neural mechanisms underlying change in CNS function during development, aging, disease and injury. Next, it can be determined how these processes can be modified by environmental manipulations such as training or sensory stimulation.
Animal models of disease can be developed using neurotoxins to induce specific cell death or to emulate a neurodegenerative process. For example, retrograde transport of a neurotoxin within efferent axons could induce motor neuron cell death to provide a model of amyotrophic lateral sclerosis. Similarly, administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) will produce marked lesions in the nigrostriatal pathway as a model for PD. Neuronal activation and synaptogenesis can then be examined with and without environmental manipulation or training in animal models. Immunohistochemistry can detect and quantify c fos expression (an immediate early gene expressed during neuron firing) and quantitative electron microscopy can be used to measure synaptic density on neurons in experimental and control animals (Kleim, Lussnig, Schwarz, Comery, & Greenough, 1996). Repetitive skills training can be used to determine whether such manipulations might put additional strain on motor neuron physiology in motor neuron disease causing increased rates of cell death. Middle cerebral artery occlusion, can be used to produce animal models for stroke and allow for the study of the neural effects of repetitive skills training on neuronal firing and synaptogenesis in regions both inside and outside the infarct area.
ii. Using Brain Imaging to Identify the Neural Substrates Involved in Speech Motor Control
Research in humans is needed to determine the neuronal substrates involved in healthy speech production and their potential for plasticity (Guenther, Ghosh, & Tourville, 2005; Ingham, Ingham, Finn, & Fox, 2003). Because speech is unique to humans there cannot be an adequate animal model for speech. However, some relevant elements can be studied. For example, vocal learning is extensive in song birds, although the avian CNS is not as close to the human system as the CNS of non-human primates (Gil-da-Costa et al., 2006). The range of vocal behavior that can be learned in the non-human primate, however, is limited when compared to the human (Jurgens, 2002). The CNS control for voice and speech, therefore, is best determined using human brain imaging technology to identify the neural substrates involved (Huang, Carr, & Cao, 2002). Methods such as fMRI and PET can be used to determine how these neural substrates can be modified through learning, development, aging and following disease.
fMRI is a non-invasive tool which reflects changes in neuronal firing within neural substrates by quantifying blood oxygenation level dependent (BOLD) changes. Brain activity coincident with speech production can be quantified using event-related or sparse sampling paradigms. Delayed sampling of the hemodynamic response which reaches its peak approximately 6 seconds after speech is produced, avoids movement artifacts induced during speaking (Birn, Bandettini, Cox, & Shaker, 1999). A recent fMRI study of paced syllable repetitions (Riecker et al., 2005) provides evidence for two levels of speech motor control, one which is apparently related to motor preparation and the other to execution processes. The same study gave insight into the types of abnormal speaking rates that occur in PD and cerebellar disorders.
PET can also be used to study brain activation for speech and/or voice production. PET has less temporal and spatial resolution than fMRI although more recent developments have increased its spatial resolution. PET scanning measures the uptake of radio-labeled isotopes such as oxygen (O 15) over a one minute period to reflect the aggregate of neuronal activity occurring during speech or voice production (Schulz, Varga, Jeffires, Ludlow, & Braun, 2005). PET is most useful for examining particular neurotransmitter functions in the brain using radiolabeled ligands for selected transporters or receptors that may reflect disease abnormalities (Kugaya et al., 2003). New radiolabeled ligands include serotonin transporters, serotonin 5-HT-1A receptors (Fu et al., 2002), dopamine D1 receptor, dopamine D2 receptor antagonists, and D2 receptor agonists (van Dyck et al., 1996), to mention just a few. PET with fluorodopa can detect the early loss of dopaminergic neurons in pre-symptomatic PD by quantifying reduced dopamine turnover in the nigrostriatal pathway in participants (Brooks et al., 2003; Ravina et al., 2005). Such techniques can measure the effects of intervention on the disease process itself, that is, neuronal cell death in the nigrostriatal pathway.
Two studies have used PET scans to measure cerebral blood flow during speech tasks pre-and post- treatment in persons with PD. In one, increased activation of motor and premotor cortex (M1-mouth, supplementary motor cortex, and inferior lateral premotor cortex and primary motor cortex) was reported during speech in adults with PD before LSVT (Liotti et al., 2003). These abnormal activations were shown to significantly reduce after LSVT. On the other hand, Pinto and colleagues examined persons with PD who had been implanted with deep brain stimulators (DBS) in the subthalamic nucleus and scanned them with the stimulator turned on and off without medication (Pinto et al., 2004). With the stimulators turned off PET scans showed speech related activity was abnormally reduced in the primary motor, premotor and right supplementary regions. With stimulation, activation increased in the same regions and was similar to the healthy controls. Differences in these two studies may relate to the presence or absence of medication; in the Liotti (2003) study persons with PD were on medication while in the Pinto (2004) study participants were un-medicated for 12 hours prior to scanning. Further study is needed with appropriate control groups to determine what changes occur in brain activity with and without intervention and medication during speech in adults with PD.
One of the issues with using functional neuroimaging with speech motor control disorders is that affected adults often find speech more effortful than the controls and may have heightened brain activity as a result. This difference in effort renders the results difficult to interpret. It is not known if the heightened cortical activity is simply a reflection of the affected adults’ difficulty with the task or if it reflects the pathophysiology underlying the speech disorder. Here comparisons between the affected adults and controls on an unaffected task such as listening to speech might provide another measure of pathophysiology, although consideration has to be given to whether patients may also have auditory signal processing abnormalities as evidenced by delayed or reduced brain stem evoked potentials (Gawel, Das, Vincent, & Rose, 1981).
PET fluorodopa can also be used in a controlled study to determine if a particular therapy can slow the disease processes in persons with PD. Future PET studies with different neurotransmitter ligands could address the role of various neurotransmitters in the speech production network in normalcy and in disease. In addition, PET technology could identify the neural substrates that might be the target for neuropharmacological manipulation for combined therapies including both speech rehabilitation and medication.
MEG and EEG both have high temporal resolution needed to examine rapid changes in neuronal firing prior to motor tasks. Because jaw muscle activation for speech interferes with recording small electrical or magnetic potentials, neither of these technologies can easily be used for the study of speech production. Nevertheless, brain activity during speech preparation can be studied with MEG or EEG by examining the change in dynamic interplay between onsets and/or peak changes in neuronal activity in different brain regions prior to speech execution (Salmelin, Schnitzler, Schmitz, & Freund, 2000). The intervals between activation in two neural substrates prior to speech may be disorganized following injury and recovery of the normal pattern might relate to intervention benefits in persons with speech motor control disorders.
TMS has been used extensively to map cortical regions controlling muscles for hand and limb control (Cohen, Hallett, & Lelli, 1990) and to assess changes in cortical excitability before and after training in normalcy and disease (Classen, Liepert, Wise, Hallett, & Cohen, 1998; Ziemann, Chen, Cohen, & Hallett, 1998). This technique has not been applied frequently to facial or laryngeal muscles because 1) the motor cortex for these regions is deeper and less accessible and 2) the magnet is closer to cranial nerves in the periphery resulting in peripheral responses which can confound central responses (Benecke, Meyer, Schonle, & Conrad, 1988; Cruccu, Beradelli, Inghilleri, & Manfredi, 1990). Recent technical changes such as coil orientation and size may improve the validity and reliability of this technique for studying the cranial musculature (Desiato, Bernardi, Hagi, Boffa, & Caramia, 2002; Guggisberg, Dubach, Hess, Wuthrich, & Mathis, 2001). TMS may be useful for measuring corticobulbar transmission and changes in cortical excitability before and after training (Cohen et al., 1998), which could offer important insights into speech motor control.
Several caveats and challenges underlie the use of functional neuroimaging to study neuroplasticity. First as behavioral performance changes in an individual, the brain substrates activated during that behavior are likely to change not necessarily due to changes in synaptic physiology (Poldrack, 2000). As a person become more skilled on a motor task their mode of behavior and brain activation may change. For example, during procedural learning when declarative knowledge emerges additional brain regions are likely to be activated (Willingham, Salidis, & Gabrieli, 2002). Associated changes in the pattern of brain activation likely result from alterations in performance strategies rather than changes in synaptic physiology. Other performance changes, such as more rapid response times, may also alter measures of brain activation particularly on BOLD fMRI. If a subject initially takes several seconds to perform a task the hemodynamic response will be prolonged. This will change when the individual becomes more skilled and performs the gesture within a second resulting in a shorter hemodynamic response that may reduce the measured BOLD response. Therefore, great caution must be used when interpreting changes in functional neuroimaging during recovery of function. As has been pointed out, limited information is available on “the biophysical effects of plastic neural changes on functional imaging signals” (Poldrack, 2000)p. 1. Therefore relating changes in functional neuroimaging measures requires caution and awaits further basic research.
Another difficulty with interpretation of functional neuroimaging results is that increased blood flow or blood oxygenation may occur as a result of synaptic activity that is either inhibitory or excitatory, complicating interpretation of both PET and fMRI results. fMRI measures of BOLD contrast percent oxygenation change between two states. Therefore, brain activity in one state can only be measured relative to another state, often a resting state. With PET, blood flow can be measured both at rest and during an activated state, which provides an added benefit if there are alterations in resting brain activity due to disease or a brain lesion.
Functional neuroimaging has inherent issues regarding group analyses as these require locating corresponding neuroanatomical locations across brains. Several approaches have been used such as fitting brains to a standard space either based on an atlas of one brain or several brains from the Montreal Neurological Institute, although none are satisfactory given the inherent variability in gyri and sulci and well as cytoarchitecture between brains (Devlin & Poldrack, 2007). The preferred approach would be to locate the anatomical structures on individual brains (Fadiga, 2007), although this is extremely labor intensive and is seldom used. To study change in brain function within individuals, however, the individual approach to data analysis and for aligning functional change to neuroanatomy may be required.
Structural neuroimaging is now demonstrating significant alterations in both grey matter volume, using voxel based morphometry (Ashburner & Friston, 2000), and alignment within white matter tracts, using diffusion tensor imaging to measure the degree of fractional anisotropy of water molecules aligned along white matter bundles(Buchel et al., 2004; S. M. Smith et al., 2006). Recent studies have shown transient anatomical changes in grey matter as a result of motor skill training (Draganski et al., 2004) and more long term as a result of extensive musical training (Gaser & Schlaug, 2003). Further differences in white matter have been shown as a result of piano practicing (Bengtsson et al., 2005) and handedness (Buchel et al., 2004).
Fractional anisotropy also supports tractography, the reconstruction of white matter tracts between voxels in two regions, a seed region and a target region. This technique has already demonstrated impressive left-right differences in the arcuate fasiculus related to language laterality (Nucifora, Verma, Melhem, Gur, & Gur, 2005). Several techniques are currently being used for tractography that have not yet been standardized. Some of the current difficulties are not being able to distinguish between adjacent tracts producing errors in “jumping” across tracts; difficulties in following tracts that make sharp turns requiring multiple regions of interest being used to track the fibers at multiple points in their trajectories; and difficulties in resolving when fiber tracts cross each other such as between the corona radiata and the superior longitudinal fasiculus (Mukherjee, 2005). These problems are compounded in stroke although use of tractography in subacute and chronic stroke has revealed changes in white matter tracts over time when patients are followed longitudinally and may be useful in predicting patient outcome (Mukherjee, 2005). On the other hand, the degree of secondary Wallerian degeneration three months post stroke may also alter results (Liang et al., 2007). The potential of this application for relating the integrity of tracts to recovery is exciting (Moller et al., 2007) but caution is needed regarding technical issues.
Finally the combination of using both functional neuroimaging connectivity analysis and tractography holds great promise for the future in examining changes in brain anatomy and function post brain injury (Cherubini et al., 2007; Guye et al., 2003). The use of both anatomical and functional neuroimaging will allow examination of how behavioral intervention can alter brain structure and function in both normalcy and different disease states (Bozzali & Cherubini, 2007).
iii. Translational Studies
Translational studies can determine the degree of neural plasticity induced within the neuronal substrates of the motor control system by training within animals, healthy humans and following disease or injury. Determining the degree of possible plasticity in the speech motor control system is crucial because speech is thought to have automaticity once development is complete following adolescence (A. Smith & Zelaznik, 2004). Neurophysiological studies of training effects at different points in the lifespan are needed to determine the extent to which the nervous system for speech can be altered.
Studies are needed to determine the degree to which the neural substrates for speech production can be altered following neurological diseases or disorders. Recovery of speech may not be possible if injury involves white matter tracts between particular brain regions (Naeser, Palumbo, Helm-Estabrooks, Stiassny-Eder, & Albert, 1989). On the other hand, if certain white matter tracts are spared then improved functioning within the speech neural control system may be possible (Riecker, Wildgruber, Grodd, & Ackermann, 2002). Careful studies addressing this hypothesis may improve our understanding of why speech recovery is limited in some persons. For example, cortical grey matter volume becomes increased with intensive long term training in musicians (Gaser & Schlaug, 2003). We need to know if changes are possible with intensive speech training in brain-injured adults.
iv. Characteristics of Feasibility Studies
The purpose of feasibility studies is to determine how neural plasticity can be modified to bring about lasting change in performance after nervous system injury or in neurological disease. Such studies are ongoing in limb control following stroke, where both performance and the physiological function of the neural substrates involved are examined during training (Stinear & Byblow, 2004). Outcome measures of speech communication or motor control could assess how speech performance has changed while neurophysiological methods can be used to study the brain mechanisms underlying that change. As mentioned previously, neurophysiological methods for quantifying change in neural functioning include: TMS to assess corticobulbar connectivity; fMRI or MEG to examine network connectivity; and PET to measure changes in neurotransmission such as dopamine release. By understanding how the CNS responds to training, training methods that can produce long term changes in brain function can be identified.
The natural process of a disease must be well-known before determining if intervention has altered that process. There will be individual differences in both the pattern of cerebral dysfunction and the recovery process. However, careful study of the overall pattern of change in brain dysfunction after injury will serve as a basis for developing interventions aimed at enhancing recovery through training. The purpose of the interventions is to alter the natural history over time. Figure 1a provides an example of comparing two interventions while attempting to alter the natural process following a stroke. The natural process involves the initial period of injury, the onset of the spontaneous recovery period and then a long period of limited change. By examining the effects of interventions at different times during the recovery process, the interaction between intervention and the time post brain injury can be examined. Here the natural process of recovery for both behavior and brain function are contrasted during two different interventions.
Figure 1.
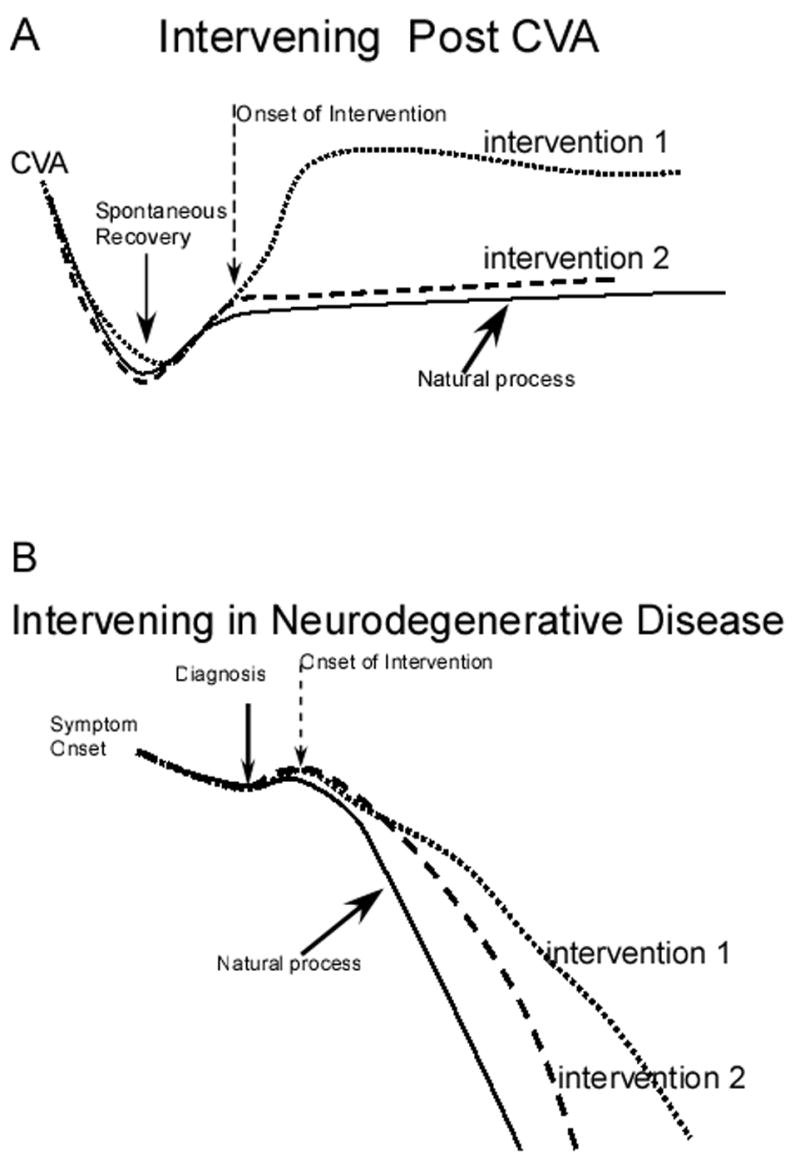
Schematic diagrams of the design of feasibility studies to determine the neural mechanisms involved in the natural process and intervention for speech motor control disorders during recovery from brain injury following a stroke or brain trauma (Figure 1A), and in neurodegenerative disease (Figure 1B).
One could argue that it might be better to first conduct controlled treatment trials to identify which treatments are most effective and then to determine how those treatments modify brain function. Controlled treatment trials are expensive and take many years to complete. Alternatively, small exploratory studies could identify those treatment approaches that can most readily produce rapid and long term changes in brain function for re-establishing speech motor control. In this way, treatments with the greatest potential could be identified before conducting controlled clinical trials. Some of the clinical neurophysiological techniques discussed, such as TMS, EEG and MEG, are non-invasive, relatively inexpensive and increasingly available in many medical centers. These techniques could be applied in small feasibility studies aimed at identifying training approaches that can induce behavioral recovery and long-term improvement in brain function for future use in large scale controlled clinical trials.
The natural history of various neurodegenerative diseases differs (Figure 1b). Here the aim of intervention will be to reduce the rate of behavioral impairment and loss of neural function. After diagnosis, there may be some recovery as the person adapts to the disease, then with intervention some reduction in the rate of increasing behavioral impairment and reduced neurophysiological function may occur depending upon treatment effectiveness.
An example of a feasibility study in a neurodegenerative disease process such as PD (Figure 1b) might include fluorodopa PET to determine the extent of the disease in each participant. Then, the interaction between individual differences in disease extent and the behavioral and neural consequences of intervention can be studied. MEG measures of neural functioning such as the interval between beta desynchronization and speech initiation could be used (Muthukumaraswamy, Johnson, Gaetz, & Cheyne, 2006). Comparisons could be made between the behavioral improvements and neurophysiological processing changes with different interventions. If participants are randomly assigned to treatment groups identification of which treatment has potential for altering both brain functioning and behavior could occur
VI. Models of Feasibility Studies in Speech Motor Control Disorders
Examples of feasibility studies for the study of neural plasticity during intervention in speech motor control disorders are presented to illustrate how such designs might test hypotheses regarding the relationship between changes in speech production behavior and the neural mechanisms involved. These examples certainly could be elaborated on and are provided only for illustrative purposes. Most are treatment comparisons with one intervention being the experimental intervention and the other being the control. Comparisons between two conditions are needed to determine if the changes in the experimental condition are specific to that condition, and not a placebo effect present when any treatment is provided.
i. Speech Mechanisms Involved in Recovery Post Bilateral Internal Capsule Lesions Following Stroke
One example is to study the outcome of the speech disturbance due to bilateral lesions involving the internal capsule post stroke. Bilateral internal capsule lesions could affect both corticobulbar and corticospinal axonal pathways interfering with cortical control of the motor neurons for both cranial and spinal systems. For that reason such lesions could produce significant deficits in speech motor control (Naeser, Palumbo, Helm-Estabrooks, Stiassny-Eder, & Albert, 1989). The purpose of the proposed study would be to determine if intervention can alter speech production and brain functioning in affected adults. Two interventions could be compared, one addressing specific speech motor control deficits versus a control intervention. Examples of possible outcome measures might include measures of speech intelligibility, acceptability and speech rate. Methods for the study of brain mechanisms could include: MEG to examine the presence/timing of beta desynchronization over M1 for speech; MRI diffusion tensor imaging to quantify deficits in white matter tracts using fractional anisotropy (Smith et al., 2006); tractography to compare the integrity of the corticobulbar and corticospinal white matter (Moller et al., 2007), and TMS to quantify changes in corticospinal and interhemispheric functional connectivity (Chouinard, Leonard, & Paus, 2006);. Speech changes over time in the experimental and control therapy groups could be compared and the relationships between changes in speech function and brain function could be examined within each group.
ii. Genotype/Phenotype Relationships in Spinocerebellar Disease
This example would address questions related to the role of the cerebellum in speech and brain mechanisms. There are several genetic forms of spinocerebellar disease which can cause degeneration in specific regions of the cerebellum (Day, Schut, Moseley, Durand, & Ranum, 2000; Mariotti & Di Donato, 2001). By studying the natural history of disease and whether or not intervention can alter that history, it could be learned (a) what speech impairments occur with neurodegeneration of specific regions of the cerebellum, and (b) whether intervention can alter the cerebellar dysfunction for speech. Particular interventions might address the speech rhythm and rate deficits often associated with ataxic dysarthria compared with a more general approach to speech rehabilitation. Outcome measures could include those for speech acceptability and intelligibility and those could be related to cerebellar and cortical activation during speech on fMRI. Event-related BOLD could measure activity changes for speech in contrast with rest with limited movement artifacts in controls (Loucks, Poletto, Simonyan, Reynolds, & Ludlow, 2007) as well as in patients. Voxel based morphometry could be used to measure white matter and grey matter volumes in the cerebellum in particular (Daniels et al., 2006). The purpose would be to determine the effects of cerebellar disease on cortical functioning for speech early in the disease process and if it could be modified by intervention.
iii. Mechanisms Involved in Mutism Recovery after Surgery for Posterior Fossa Tumor in Childhood
Another illustrative example is to identify the brain mechanisms involved in mutism and how such mechanisms are altered during the natural recovery process from posterior fossa tumors in children. Although recovery is frequent, it is not clear whether or not intervention alters the course of recovery (Arslantas, Erhan, Emre, & Esref, 2002; Ozgur, Berberian, Aryan, Meltzer, & Levy, 2006; Steinbok, Cochrane, Perrin, & Price, 2003). For this study, participants would be randomized between experimental and control groups to determine if intervention alters the natural recovery process. The interventions could be singing along with videos (Ozgur, Berberian, Aryan, Meltzer, & Levy, 2006), versus a sham intervention and outcome measures would include the rate of recovery of vocalized speech for communication. Measures of brain mechanisms would include fMRI of the cortical and subcortical networks involved in voice production and diffusion tensor imaging of white matter tracts with fractional anisotropy (Smith et al., 2006).
VII. Collaborative Research Consortiums
The benefits of collaboration within a community of specialists in speech motor control disorders became apparent amongst the workgroup members when discussing examples of feasibility studies. Adults with specific disorders are usually not available in adequate numbers in single centers and multiple center collaborations are likely to be needed for research on neural plasticity and speech motor control. Further, by working as a community, speech disorders specialists could develop consensus on diagnostic, assessment and intervention methods for use in feasibility studies.
i. Clinical Trials Consortium
Feasibility studies could be fostered by collaborations between speech specialists with expertise in speech intervention and outcome measures and neuroscientists with expertise in neural imaging and clinical neurophysiology, and neurosurgery. A consortium of such groups would help to develop consensus on: speech outcomes; measures of neural substrates involved in speech motor control; designs for feasibility studies; as well as allow interaction with other disciplines for the study of neural plasticity in relation with speech motor control.
ii. Collaborative Efforts
An example of a high priority study that could be conducted by such a consortium would be a study on the effects of deep brain stimulation on speech and voice motor control. Current PD rating scales do not assess speech, voice and swallowing in detail. For example, The Unified Parkinson Disease Rating Scale collapses all three into one rating category (Fahn, Elton, & Committee, 1987). Deep brain stimulation in the subthalamic nucleus (STN) in persons with PD can be beneficial to limb motor functions and improvements are related to the restoration of higher levels of brain activity in the presupplementary motor area, and premotor cortices (Sestini et al., 2005). DBS in PD may cause some persons to deteriorate in voice, speech and swallowing, while others improve (Dromey, Kumar, Lang, & Lozano, 2000; Gentil, Garcia-Ruiz, Pollak, & Benabid, 2000; Pinto et al., 2005; Rascol et al., 2003; Rousseaux et al., 2004; Schulz, Peterson, Sapienza, Greer, & Friedman, 1999). Side effects often occur in these functions as the intensity or frequency of stimulation is increased. Other surgical techniques have had detrimental effects on these functions in PD; for example, bilateral pallidotomy with ablation was detrimental to speech in some persons (Schulz, Peterson, Sapienza, Greer, & Friedman, 1999). Several authors have concluded that speech is often not benefited to the same extent as limb control and may be unrelated to limb control following bilateral pallidotomy, thalamotomy, thalamic stimulation and in some cases of stimulation in the subthalamic nucleus (Dromey, Kumar, Lang, & Lozano, 2000; Gentil, Garcia-Ruiz, Pollak, & Benabid, 2000; Schulz, Peterson, Sapienza, Greer, & Friedman, 1999). The disparity between limb control benefits and speech/voice and swallowing following surgical treatment of movement disorders (Dromey, Kumar, Lang, & Lozano, 2000; Rascol et al., 2003) makes the mapping of speech motor control within the basal ganglia of both clinical and basic importance.
The purpose of a controlled feasibility study would be to determine which factors predict adverse events or improvements in speech, voice and swallowing with deep brain stimulation (DBS) in the STN in a wide range of operated and unoperated persons with PD. Factors that could be examined include: persons’ speech, voice and swallowing functioning and the brain activation abnormalities prior to implantation; lead location as judged from recording/stimulation during placement including proximity to the internal capsule and/or location within the STN; the active stimulator contacts, type of stimulator, unipolar versus bipolar, intensity, pulse width, and rate of DBS; the extent of PD disease progression; and, the effect of DBS on the axial symptoms of gait and balance and speech, voice and swallowing in comparison with a control group treated with conventional therapy over the same time period.
Although some studies of these issues have been initiated at a few institutions, it is estimated that several high volume centers would be needed to test each of the factors independent of a particular neurosurgical team. Intake profiles might include fluorodopa PET scanning, neurophysiological studies of brain activation for voice, speech and swallowing and multiple baseline assessments using common methods for voice, speech and swallowing functioning across centers. Such a study could have an immediate benefit in aiding future persons with PD by avoiding those factors found to predict adverse outcomes in speech, voice and swallowing with DBS.
iii. Education and Dissemination
To increase research attention given to the role of brain mechanisms and neural plasticity for developing interventions in speech motor control disorders, new investigators will be needed. A consortium of collaborative teams on neural plasticity and recovery and rehabilitation of speech disorders could enhance research in this area by: inviting speakers from basic neuroscience and clinical neurophysiology to present at meetings on speech motor control and disorders; encouraging the involvement of neuroscientists in doctoral education programs in human communication sciences and disorders (CSD); providing continuing education seminars and workshops between neuroscience and CSD; assisting investigators with developing collaborative teams between neuroscientists and CSD in their own institutions; encouraging new CSD Ph.D.’s to take postdoctoral training in neuroscience; and assisting new faculty who are seeking consultants in neuroscience for advice during the development of their research program.
Acknowledgments
This manuscript resulted from a “Workshop on Plasticity/NeuroRehabilitation Research” held at the University of Florida, Gainesville, Florida (April 10–13, 2005) and sponsored by the Brain Rehabilitation Research Center, a Veterans Administration Rehabilitation Research and Development Center of Excellence. Particular thanks are to Leslie Gonzalez-Rothi the organizer of the Workshop along with Jay Rosenbek, Nan Musson and Christine Sapienza, a co-author on this report. The authors appreciate comments received from Jeffrey Kleim on the content of the manuscript. Drs. Anne Smith and Elaine Stathopoulos participated in the discussions that led to this manuscript as well as the following doctoral students from the University of Florida: Chris Carmichael, Neila Donovan, Amber Hollingsworth, and Harrison Jones. Preparation of this report was supported in part by the Intramural Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Contributor Information
Christy L. Ludlow, National Institute of Neurological Disorders and Stroke, Bethesda, MD
Jeannette Hoit, University of Arizona, Tucson, AZ.
Raymond Kent, University of Wisconsin, Madison, WI.
Lorraine O. Ramig, University of Colorado, Boulder, CO
Rahul Shrivastav, University of Florida, Gainesville, FL.
Edythe Strand, Mayo Clinic, Rochester, MI.
Kathryn Yorkston, University of Washington, Seattle, WA.
Christine Sapienza, University of Florida, Gainesville, FL.
References
- Arslantas A, Erhan C, Emre E, Esref T. Transient cerebellar mutism after posterior fossa surgery. J Postgrad Med. 2002;48(2):158–159. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Abbs JH. Fine force and position control of select orofacial structures in the upper motor neuron syndrome. Exp Neurol. 1986;94:699–713. doi: 10.1016/0014-4886(86)90248-7. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Netsell R. Differential fine force control of the upper and lower lips. J Speech Hear Res. 1986;29:163–169. doi: 10.1044/jshr.2902.163. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor unit discharge rates in macimal voluntary contrations of three human muscles. J Neurophysiol. 1973;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Schonle P, Conrad B. Transcranial magnetic stimulation of the human brain: responses in muscles supplied by cranial nerves. Exp Brain Res. 1988;71:623–632. doi: 10.1007/BF00248756. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Human Brain Mapping. 1999;7(2):106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Moser D, Rorden C, Baylis GC, Fridriksson J. Speech apraxia without oral apraxia: can normal brain function explain the physiopathology? Neuroreport. 2006;17(10):1027–1031. doi: 10.1097/01.wnr.0000223388.28834.50. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Cherubini A. Diffusion tensor MRI to investigate dementias: a brief review. Magn Reson Imaging. 2007 doi: 10.1016/j.mri.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, et al. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Exp Neurol. 2003;184(Suppl 1):S68–79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Brosh I, Barkai E. Learning-induced long-term synaptic modifications in the olfactory cortex. Curr Neurovasc Res. 2004;1(4):389–395. doi: 10.2174/1567202043362090. [DOI] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14(9):945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Bury SD, Adkins DL, Ishida JT, Kotzer CM, Eichhorn AC, Jones TA. Denervation facilitates neuronal growth in the motor cortex of rats in the presence of behavioral demand. Neurosci Lett. 2000;287(2):85–88. doi: 10.1016/s0304-3940(00)01138-1. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22(19):8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Facilitation of motor skill learning by callosal denervation or forced forelimb use in adult rats. Behav Brain Res. 2004;150(1–2):43–53. doi: 10.1016/S0166-4328(03)00253-5. [DOI] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Cheung S, Bedenbaugh P, Nagarajan SS, Jenkins WM. A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys Ther. 1997;77(3):269–284. doi: 10.1093/ptj/77.3.269. [DOI] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Jenkins WM. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology. 1996;47(2):508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- Carr J, Shephard R. Neurological Rehabilitaiton: Optimizing Motor Performance. Wobrun, MA: Butterworth/Heinmann; 1999. [Google Scholar]
- Cherubini A, Luccichenti G, Peran P, Hagberg GE, Barba C, Formisano R, et al. Multimodal fMRI tractography in normal subjects and in clinically recovered traumatic brain injury patients. Neuroimage. 2007;34(4):1331–1341. doi: 10.1016/j.neuroimage.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. Changes in effective connectivity of the primary motor cortex in stroke patients after rehabilitative therapy. Exp Neurol. 2006;201(2):375–387. doi: 10.1016/j.expneurol.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Jones TA. Experience-dependent structural plasticity in cortex heterotopic to focal sensorimotor cortical damage. Exp Neurol. 2000;166(2):403–414. doi: 10.1006/exnr.2000.7509. [DOI] [PubMed] [Google Scholar]
- Clark HM. Neuromuscular treatments for speech and swallowing: a tutorial. Am J Speech Lang Pathol. 2003;12(4):400–415. doi: 10.1044/1058-0360(2003/086). [DOI] [PubMed] [Google Scholar]
- Clark HM, Robin DA, McCullagh G, Schmidt RA. Motor control in children and adults during a non-speech oral task. J Speech Lang Hear Res. 2001;44(5):1015–1025. doi: 10.1044/1092-4388(2001/080). [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79(2):1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Hallett M, Lelli S. Noninvasive mapping of human motor cortex with transcranial magnetic stimulation. In: Chokroverty S, editor. Magnetic stimulation in clinical neurophysiology. Stoneham,MA: Butterworths; 1990. pp. 113–120. [Google Scholar]
- Cohen LG, Ziemann U, Chen R, Classen J, Hallett M, Gerloff C, et al. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):305–324. doi: 10.1097/00004691-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Beradelli A, Inghilleri M, Manfredi M. Corticobulbar projections to upper and lower facial motorneurons. A study of magnetic transcranial stimulation in man. Neuroscience Letters. 1990;117:68–73. doi: 10.1016/0304-3940(90)90121-o. [DOI] [PubMed] [Google Scholar]
- Daniels C, Peller M, Wolff S, Alfke K, Witt K, Gaser C, et al. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology. 2006;67(8):1452–1456. doi: 10.1212/01.wnl.0000240130.94408.99. [DOI] [PubMed] [Google Scholar]
- Day JW, Schut LJ, Moseley ML, Durand AC, Ranum LP. Spinocerebellar ataxia type 8: clinical features in a large family. Neurology. 2000;55(5):649–657. doi: 10.1212/wnl.55.5.649. [DOI] [PubMed] [Google Scholar]
- Deane KH, Whurr R, Playford ED, Ben-Shlomo Y, Clarke CE. A comparison of speech and language therapy techniques for dysarthria in Parkinson’s disease. Cochrane Database Syst Rev. 2001a;(2):CD002814. doi: 10.1002/14651858.CD002814. [DOI] [PubMed] [Google Scholar]
- Deane KH, Whurr R, Playford ED, Ben-Shlomo Y, Clarke CE. Speech and language therapy for dysarthria in Parkinson’s disease. Cochrane Database Syst Rev. 2001b;(2):CD002812. doi: 10.1002/14651858.CD002812. [DOI] [PubMed] [Google Scholar]
- Desiato MT, Bernardi G, Hagi HA, Boffa L, Caramia MD. Transcranial magnetic stimulation of motor pathways directed to muscles supplied by cranial nerves in amyotrophic lateral sclerosis. Clin Neurophysiol. 2002;113(1):132–140. doi: 10.1016/s1388-2457(01)00724-6. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Poldrack RA. In praise of tedious anatomy. NeuroImage. 2007 doi: 10.1016/j.neuroimage.2006.09.055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan GA, Carey LM, Saling MM. More (or less) on Broca. Lancet. 1999;353(9158):1031–1032. doi: 10.1016/S0140-6736(99)90052-1. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord. 2000;15(6):1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis: Elsevier Mosby; 2005. [Google Scholar]
- Dworkin JP, Hartman DE. Progressive speech deterioration and dysphagia in amyotrophic lateral sclerosis: case report. Arch Phys Med Rehabil. 1979;60(9):423–425. [PubMed] [Google Scholar]
- Fadiga L. Functional magnetic resonance imaging: Measuring versus estimating. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R, Committee MotU. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s Disease. Vol. 2. New York: Raven PPress; 1987. pp. 153–164. [Google Scholar]
- Fisher BE, Sullivan KJ. Activity-dependent factors affecting poststroke functional outcomes. Top Stroke Rehabil. 2001;8(3):31–44. doi: 10.1310/B3JD-NML4-V1FB-5YHG. [DOI] [PubMed] [Google Scholar]
- Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14(3):187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89(6):3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Fu X, Tan PZ, Kula NS, Baldessarini R, Tamagnan G, Innis RB, et al. Synthesis, receptor potency, and selectivity of halogenated diphenylpiperidines as serotonin 5-HT2A ligands for PET or SPECT brain imaging. J Med Chem. 2002;45(11):2319–2324. doi: 10.1021/jm0200411. [DOI] [PubMed] [Google Scholar]
- Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Gray matter differences between musicians and nonmusicians. Ann N Y Acad Sci. 2003;999:514–517. doi: 10.1196/annals.1284.062. [DOI] [PubMed] [Google Scholar]
- Gawel MJ, Das P, Vincent S, Rose FC. Visual and auditory evoked responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1981;44(3):227–232. doi: 10.1136/jnnp.44.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL. Effect of bilateral deep-brain stimulation on oral control of patients with parkinsonism. Eur Neurol. 2000;44(3):147–152. doi: 10.1159/000008224. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Martin A, Lopes MA, Munoz M, Fritz JB, Braun AR. Species-specific calls activate homologs of Broca’s and Wernicke’s areas in the macaque. Nat Neurosci. 2006;9(8):1064–1070. doi: 10.1038/nn1741. [DOI] [PubMed] [Google Scholar]
- Griffin L, Cafarelli E. Transcranial magnetic stimulation during resistance training of the tibialis anterior muscle. J Electromyogr Kinesiol. 2006 doi: 10.1016/j.jelekin.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2005 doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Dubach P, Hess CW, Wuthrich C, Mathis J. Motor evoked potentials from masseter muscle induced by transcranial magnetic stimulation of the pyramidal tract: the importance of coil orientation. Clin Neurophysiol. 2001;112(12):2312–2319. doi: 10.1016/s1388-2457(01)00677-0. [DOI] [PubMed] [Google Scholar]
- Guye M, Parker GJ, Symms M, Boulby P, Wheeler-Kingshott CA, Salek-Haddadi A, et al. Combined functional MRI and tractography to demonstrate the connectivity of the human primary motor cortex in vivo. Neuroimage. 2003;19(4):1349–1360. doi: 10.1016/s1053-8119(03)00165-4. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: John Wiley; 1949. [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Human Brain Mapping. 2002;15(1):39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham RJ, Ingham JC, Finn P, Fox PT. Towards a functional neural systems model of developmental stuttering. J Fluency Disord. 2003;28(4):297–317. doi: 10.1016/j.jfludis.2003.07.004. quiz 317–298. [DOI] [PubMed] [Google Scholar]
- Jang SH, Ahn SH, Yang DS, Lee DK, Kim DK, Son SM. Cortical reorganization of hand motor function to primary sensory cortex in hemiparetic patients with a primary motor cortex infarct. Arch Phys Med Rehabil. 2005;86(8):1706–1708. doi: 10.1016/j.apmr.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol. 2005;99(4):1558–1568. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19(22):10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26(2):235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing animals. Annual Rev Neuroscience. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77(1):63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25(8):789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934(1):1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation after Brain Damage. Journal of Speech-Language-Hearing Research. doi: 10.1044/1092-4388(2008/018). in press. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16(14):4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp B, Kunkel A, Muhlnickel W, Villringer K, Taub E, Flor H. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport. 1999;10(4):807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Bherer L, Colcombe SJ, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. J Gerontol A Biol Sci Med Sci. 2004;59(9):M940–957. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- Kruger GM, Morrison SJ. Brain repair by endogenous progenitors. Cell. 2002;110(4):399–402. doi: 10.1016/s0092-8674(02)00899-1. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G, Verhoeff NP, Fujita M, Mason GF, Seneca NM, et al. Cerebral benzodiazepine receptors in depressed patients measured with [123I]iomazenil SPECT. Biol Psychiatry. 2003;54(8):792–799. doi: 10.1016/s0006-3223(02)01788-2. [DOI] [PubMed] [Google Scholar]
- Lendrem W, Lincoln NB. Spontaneous recovery of language in patients with aphasia between 4 and 34 weeks after stroke. J Neurol Neurosurg Psychiatry. 1985;48(8):743–748. doi: 10.1136/jnnp.48.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Zeng J, Liu S, Ling X, Xu A, Yu J, et al. A prospective study of secondary degeneration following subcortical infarction using diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2007;78(6):581–586. doi: 10.1136/jnnp.2006.099077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26(1):1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, et al. Hypophonia in Parkinson’s disease: neural correlates of voice treatment revealed by PET. Neurology. 2003;60(3):432–440. doi: 10.1212/wnl.60.3.432. [DOI] [PubMed] [Google Scholar]
- Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8(7):839–841. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. Neuroimage. 2007;36(1):131–143. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Espesser R, Rey V, Daffaure V, Di Cristo A, Habib M. Intensive training of phonological skills in progressive aphasia: a model of brain plasticity in neurodegenerative disease. Brain Cogn. 2001;46(1–2):197–201. doi: 10.1016/s0278-2626(01)80065-8. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Lou G. Observations on human laryngeal muscle control. In: Fletcher N, Davis P, editors. Controlling complexity and chaos: 9th Vocal Fold Physiology Symposium. Sandiego, CA: Singular Press; 1996. pp. 201–218. [Google Scholar]
- Ludlow CL, Yeh J, Cohen LG, Van Pelt F, Rhew K, Hallett M. Limitations of electromyography and magnetic stimulation for assessing laryngeal muscle control. Ann Otol Rhinol Laryngol. 1994;103(1):16–27. doi: 10.1177/000348949410300103. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Mariotti C, Di Donato S. Cerebellar/spinocerebellar syndromes. Neurol Sci. 2001;22(Suppl 2):S88–92. doi: 10.1007/s100720100042. [DOI] [PubMed] [Google Scholar]
- McAuliffe MJ, Ward EC, Murdoch BE, Farrell AM. A nonspeech investigation of tongue function in Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2005;60(5):667–674. doi: 10.1093/gerona/60.5.667. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10(3):639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Moller M, Frandsen J, Andersen G, Gjedde A, Vestergaard-Poulsen P, Ostergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J Neurol Neurosurg Psychiatry. 2007;78(6):587–592. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11(5):471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, Frank J, et al. Kinematic specificity of cortical reorganization associated with motor training. Neuroimage. 2004;21(3):1182–1187. doi: 10.1016/j.neuroimage.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, McFarland H, et al. Training-dependent plasticity in patients with multiple sclerosis. Brain. 2004;127(Pt 11):2506–2517. doi: 10.1093/brain/awh266. [DOI] [PubMed] [Google Scholar]
- Mukherjee P. Diffusion tensor imaging and fiber tractography in acute stroke. Neuroimaging Clin N Am. 2005;15(3):655–665. xii. doi: 10.1016/j.nic.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, Gaetz WC, Cheyne DO. Neural processing of observed oro-facial movements reflects multiple action encoding strategies in the human brain. Brain Res. 2006;1071(1):105–112. doi: 10.1016/j.brainres.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112(Pt 1):1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- Nagel MJ, Rice MS. Cross-transfer effects in the upper extremity during an occupationally embedded exercise. Am J Occup Ther. 2001;55(3):317–323. doi: 10.5014/ajot.55.3.317. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16(8):791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S57–76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens Mot Res. 1990;7(4):463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75(5):2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Ozgur BM, Berberian J, Aryan HE, Meltzer HS, Levy ML. The pathophysiologic mechanism of cerebellar mutism. Surg Neurol. 2006;66(1):18–25. doi: 10.1016/j.surneu.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pinto S, Gentil M, Krack P, Sauleau P, Fraix V, Benabid AL, et al. Changes induced by levodopa and subthalamic nucleus stimulation on parkinsonian speech. Mov Disord. 2005 doi: 10.1002/mds.20601. [DOI] [PubMed] [Google Scholar]
- Pinto S, Thobois S, Costes N, Le Bars D, Benabid AL, Broussolle E, et al. Subthalamic nucleus stimulation and dysarthria in Parkinson’s disease: a PET study. Brain. 2004;127(Pt 3):602–615. doi: 10.1093/brain/awh074. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25(8):801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74(1):27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Imaging brain plasticity: conceptual and methodological issues--a theoretical review. Neuroimage. 2000;12(1):1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Countryman S, O’Brien C, Hoehn M, Thompson L. Intensive speech treatment for patients with Parkinson’s disease: short-and long-term comparison of two techniques. Neurology. 1996;47(6):1496–1504. doi: 10.1212/wnl.47.6.1496. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, et al. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry. 2001;71(4):493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT) in individuals with Parkinson’s disease: a comparison with untreated patients and normal age-matched controls. Mov Disord. 2001;16(1):79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Verdolini K. Treatment efficacy: voice disorders. J Speech Lang Hear Res. 1998;41(1):S101–116. doi: 10.1044/jslhr.4101.s101. [DOI] [PubMed] [Google Scholar]
- Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson’s disease therapy. Ann Neurol. 2003;53(Suppl 3):S3–12. doi: 10.1002/ana.10513. discussion S12–15. [DOI] [PubMed] [Google Scholar]
- Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64(2):208–215. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- Ray J. Effects of orofacial myofunctional therapy on speech intelligibility in individuals with persistent articulatory impairments. Int J Orofacial Myology. 2003;29:5–14. [PubMed] [Google Scholar]
- Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res. 2001;123(2):133–141. doi: 10.1016/s0166-4328(01)00199-1. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, et al. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64(4):700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Grodd W, Ackermann H. Reorganization of speech production at the motor cortex and cerebellum following capsular infarction: a follow-up functional magnetic resonance imaging study. Neurocase. 2002;8(6):417–423. doi: 10.1076/neur.8.5.417.16181. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1(3):230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Krystkowiak P, Kozlowski O, Ozsancak C, Blond S, Destee A. Effects of subthalamic nucleus stimulation on parkinsonian dysarthria and speech intelligibility. J Neurol. 2004;251(3):327–334. doi: 10.1007/s00415-004-0327-1. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund H. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123(Pt 6):1184–1202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53(4):521–524. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Dingwall WO, Ludlow CL. Speech and oral motor learning in individuals with cerebellar atrophy. J Speech Lang Hear Res. 1999;42(5):1157–1175. doi: 10.1044/jslhr.4205.1157. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Peterson T, Sapienza CM, Greer M, Friedman W. Voice and speech characteristics of persons with Parkinson’s disease pre- and post-pallidotomy surgery: preliminary findings. J Speech Lang Hear Res. 1999;42(5):1176–1194. doi: 10.1044/jslhr.4205.1176. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cereb Cortex. 2005;15(12):1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- Scott S, Caird FI. Speech therapy for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46(2):140–144. doi: 10.1136/jnnp.46.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non-progressive brain damage. Cochrane Database Syst Rev. 2001;(2):CD002088. doi: 10.1002/14651858.CD002088. [DOI] [PubMed] [Google Scholar]
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to nonprogressive brain damage: a systematic Cochrane review. Clin Rehabil. 2002;16(1):61–68. doi: 10.1191/0269215502cr468oa. [DOI] [PubMed] [Google Scholar]
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non-progressive brain damage. Cochrane Database Syst Rev. 2005;(3):CD002088. doi: 10.1002/14651858.CD002088.pub2. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P. Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol. 2004;190(2):425–432. doi: 10.1016/j.expneurol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson’s disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med. 2005;46(9):1444–1454. [PubMed] [Google Scholar]
- Sharkawi AE, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, et al. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. J Neurol Neurosurg Psychiatry. 2002;72(1):31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Zelaznik HN. Development of functional synergies for speech motor coordination in childhood and adolescence. Dev Psychobiol. 2004;45(1):22–33. doi: 10.1002/dev.20009. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267. doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- Spengler F, Roberts TP, Poeppel D, Byl N, Wang X, Rowley HA, et al. Learning transfer and neuronal plasticity in humans trained in tactile discrimination. Neurosci Lett. 1997;232(3):151–154. doi: 10.1016/s0304-3940(97)00602-2. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39(4):179–183. doi: 10.1159/000072468. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol. 2004;21(2):124–131. doi: 10.1097/00004691-200403000-00008. [DOI] [PubMed] [Google Scholar]
- Teasell RW, Kalra L. What’s new in stroke rehabilitation: back to basics. Stroke. 2005;36(2):215–217. doi: 10.1161/01.STR.0000153061.02375.04. [DOI] [PubMed] [Google Scholar]
- Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T. Human primary motor cortex shows hemispheric specialization for speech. Neuroreport. 2006;17(11):1091–1095. doi: 10.1097/01.wnr.0000224778.97399.c4. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G, Volpato D, Testoni R, Bonato C, Manganotti P, et al. Neurophysiological evidence of neuroplasticity at multiple levels of the somatosensory system in patients with carpal tunnel syndrome. Brain. 1998;121(Pt 9):1785–1794. doi: 10.1093/brain/121.9.1785. [DOI] [PubMed] [Google Scholar]
- Urasaki E, Genmoto T, Wada S, Yokota A, Akamatsu N. Dynamic changes in area 1 somatosensory cortex during transient sensory deprivation: a preliminary study. J Clin Neurophysiol. 2002;19(3):219–231. doi: 10.1097/00004691-200206000-00005. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Seibyl JP, Stubbs JB, Zoghbi S, Wisniewski G, Baldwin RM, et al. Human biodistribution and dosimetry of the SPECT D2 dopamine receptor radioligand [123I]IBF. Nucl Med Biol. 1996;23(1):9–16. doi: 10.1016/0969-8051(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TE, Hart Y, Stacey R, et al. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain. 2006;129(Pt 3):754–766. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A, Weber-Fox C. Short-term plasticity in children’s speech motor systems. Developmental Psychobiology. 2006 doi: 10.1002/dev.20185. in press. [DOI] [PubMed] [Google Scholar]
- Weismer G. Philosophy of research in motor speech disorders. Clin Linguist Phon. 2006;20(5):315–349. doi: 10.1080/02699200400024806. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002;88(3):1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353(9158):1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Wulf G, Lee TD, Schmidt RA. Reducing Knowledge of Results About Relative Versus Absolute Timing: Differential Effects on Learning. J Mot Behav. 1994;26(4):362–369. doi: 10.1080/00222895.1994.9941692. [DOI] [PubMed] [Google Scholar]
- Wulf G, Schmidt RA, Deubel H. Reduced feedback frequency enhances generalized motor program learning but not parameterization learning. J Exp Psychol Learn Mem Cogn. 1993;19(5):1134–1150. doi: 10.1037//0278-7393.19.5.1134. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston KM. Treatment efficacy: dysarthria. J Speech Hear Res. 1996;39(5):S46–57. doi: 10.1044/jshr.3905.s46. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Spencer KA. Behavioral management of respiratory/phonatory dysfunction from dysarthria: A systematic review of the evidence. Journal of Medical Speech-Language Pathology. 2003;11(2):xiii–xxxviii. [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51(5):1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 1998;18(17):7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


