Abstract
OBJECTIVE
Within a community-based, cluster-randomized study of the effects of 4.0% chlorhexidine on omphalitis and mortality risk, we aimed to describe the distribution of times to separation and the impact of topical chlorhexidine treatment on cord-separation times.
METHODS
Between November 2002 and March 2005, 15 123 infants were assigned randomly within communities in southern Nepal to receive 1 of the following 3 cord-care regimens: cleansing with 4.0% chlorhexidine, cleansing with soap and water, or dry cord care. In intervention clusters, field workers cleansed the cord in the home on days 1, 2, 3, 4, 6, 8, and 10 after birth. Newborns were monitored throughout the newborn period for signs of omphalitis, and the time to cord separation was noted. Separation times were compared across treatment groups. Cord infection risk and a range of infant and household characteristics were assessed for their relationships to separation time.
RESULTS
The mean separation time was shorter in dry cord care (4.24 days) and soap/water (4.25 days) clusters than in chlorhexidine clusters (5.32 days; mean difference: 1.08 days). Cords of infants who received chlorhexidine were 3.6 times more likely to separate after 7 days. Separation time was not associated with omphalitis. Home-delivered topical antiseptics, facility-based birth, and birth attendant hand-washing were associated with greater likelihoods of cord separation after 7 days of age.
CONCLUSIONS
In this setting, the umbilical cord separated more rapidly than observed in hospital-based studies, and the impact of chlorhexidine cleansing on separation times was negligible. Increased cord-separation time attributable to topical chlorhexidine treatment should not be considered a factor in decision-making in settings where the baseline risk of omphalitis is high and chlorhexidine might reduce infection and mortality risks significantly.
Keywords: umbilical cord, omphalitis, cord separation, chlorhexidine, antisepsis, antiseptic, Nepal
UMBILICAL CORD INFECTION contributes to neonatal mortality and morbidity risk in developing countries. Reliable data from low-resource settings have been largely lacking but are necessary to guide appropriately recommendations for optimal umbilical cord-care practices for newborns. Current World Health Organization recommendations1 for cord care in low-resource settings are based largely on incomplete information from developed countries,2,3 where the risk of infection and exposure of the umbilical cord stump to infectious pathogens differ substantially.
Topical antiseptic regimens for umbilical cord care were implemented widely in past decades, despite a lack of conclusive evidence that these regimens can reduce infection rates.2 Most studies were conducted in settings where overall infection risk was low, and the main focuses of randomized trials comparing antiseptics and nonantiseptics were secondary outcomes such as cord-separation time. It has been well established in many studies4–9 that application of a variety of topical antiseptics (as with any action that delays colonization of the stump and leukocyte infiltration of the site10) increases the time to separation of the cord, relative to protocols promoting dry cord care or nonantiseptic treatment. Combined with ambiguous results from inadequately powered studies, this consistent characteristic of cord antisepsis, which may contribute to maternal dissatisfaction and economic concerns, has prompted a movement toward dry cord care.11–15 This trend is reflected in current World Health Organization recommendations for developing countries,1 which promote dry cord care under routine circumstances but acknowledge that antiseptics may be helpful when harmful, unhygienic, traditional practices place newborns at increased risk for omphalitis.
We reported recently that topical antisepsis with 4.0% chlorhexidine reduced infection risk markedly among neonates in rural Nepal.16 Furthermore, if the cleansing intervention was initiated in the first 1 day of life, then the risk of death among neonates was reduced significantly. These results, given the importance of these outcomes relative to secondary outcomes emphasized more commonly in developed countries, are likely to renew debate regarding the use of topical antiseptics in developing countries.
Because cord-separation time has evolved as an important justification for recommending against the use of topical antiseptics, it is important to evaluate the effect of antiseptics on this outcome in settings where the risk of infection is high and the promotion of chlorhexidine may have a substantial public health impact. In such settings, many infants are born in the home under unhygienic conditions and bacterial colonization of the cord occurs rapidly, which suggests that baseline separation times may be comparatively short. Traditional practices, which often include applying substances such as chalk, herbs, ash, or household oils,17–19 may affect separation times but have not been studied in a prospective manner. Our randomized trial of the impact of 4.0% chlorhexidine cleansing of the cord on omphalitis and mortality rates16 in rural Nepal provided us with an opportunity to (1) evaluate the role of topical antiseptics on time to umbilical cord separation, (2) examine infant characteristics and behavioral practices related to separation time, and (3) assess the relationship between cord-separation time and umbilical cord infection.
METHODS
Study Population and Design
The data on umbilical cord cleansing, infection, and time to separation were collected within a community-based trial of the impact of umbilical cord cleansing with chlorhexidine on neonatal mortality and morbidity risk in Sarlahi District, Nepal. The study site was divided into 413 clusters, based on the population that 1 local female worker could monitor (• 40−50 households). The population, recruitment and randomization procedures, cord treatment regimens and cleansing procedures, and follow-up activities were reported previously.16 Briefly, a computer-generated randomization sequence assigned all infants within clusters to 1 of 3 cord-care regimens (umbilical stump cleansing with 4.0% chlorhexidine, cleansing with soap and water, or dry cord care only). Investigators, field workers, and participants were masked with respect to the chlorhexidine and soap/ water treatment groups. Pregnant women were identified in the community by local female project workers, the study was explained, and oral informed consent was obtained. All women were provided with 400 mg of albendazole, iron-folate supplementation (90 days), a locally manufactured clean delivery kit, tetanus toxoid immunization, and basic educational messages regarding hygienic umbilical cord care during delivery and the postnatal period.
Data Collection
Infants who were born between November 18, 2002, and March 8, 2005, and were alive at • 1 home visit before 10 days of age were eligible for enrollment. Enrolled newborns were visited up to 11 times during the neonatal period (days 1−4, 6, 8, 10, 12, 14, 21, and 28) for evaluation of the umbilical cord for signs of infection (pus, redness, or swelling). This schedule for home visits was designed to maximize both the coverage of the intervention and the detection of cases of omphalitis. In intervention clusters, workers cleansed the cord stump gently with the assigned solution (4.0% chlorhexidine or soap and water) during home visits before day 12. On the basis of a validation exercise described previously,20 cord infection was defined as the presence of pus with redness of the skin around the cord stump or severe redness (extending • 2 cm) with or without pus.
Cord-Separation Time
At each visit, the worker assessed the cord-separation status and recorded the date and time of the visit. The cord was defined as fully separated if all visible signs of the necrotized cord had fallen from the stump. For infants who were assessed • 1 time both before and after the cord had separated (92.1%), the cord-separation time was defined as the age of the infant at the midpoint between the last visit before and the first visit after cord separation was noted. For infants whose visits all occurred after the cord had fallen off (6.4%), the cord-separation time was defined as one half the age of the infant at the first visit. Infants who were met only before the cord separated (1.6%) were excluded from the primary analyses, but the time under observation contributed to the time-to-event analysis.
Sample Size
These data were collected within a trial with primary outcomes of infection and death; therefore, the sample size was predicated on the number of participants in that trial. The final sample size of 14 887 infants provided • 99% power to detect a mean difference between treatment groups in the age at the time of cord separation of • 5 hours.
Analyses
Baseline infant, maternal, and household characteristics of enrolled infants were compared across the 3 treatment groups. Linear regression models were used to examine the difference in time to cord separation between any 2 cord regimen groups and included adjustment for variables that did not achieve balance across treatment groups. Associations between infant and behavioral characteristics were assessed with bivariate and multivariate regression models, with adjustment for treatment group. A Poisson regression model with robust variance estimation21 was constructed to assess the relationship between the incidence of umbilical cord infection and time to separation, with adjustment for cord care received. All models examining associations across treatment allocation were estimated by using generalized estimating equations to account for the cluster-randomized design of the trial.22 The impact of excluding right-censored infants (n • 236 [1.6%]) was assessed by estimating the Kaplan-Meier time-to-event function according to treatment group and comparing the area under the curve (mean separation time) with the means estimated from the restricted data set. Analyses followed an intention-to-treat approach.
All analyses were conducted with Stata 9.0 (Stata Corp, College Station, TX). The Nepal Health Research Council (Kathmandu, Nepal) and the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) approved the protocol. This trial is registered at ClinicalTrials.gov (study NCT00109616).
RESULTS
Participants
Infants eligible for this analysis included a subset of those enrolled in the main trial of the effects of chlorhexidine cleansing on cord infection and mortality risk. A total of 15 123 infants were enrolled in that trial; analyses here were restricted to 14 887 (98.4%) of those infants, for whom specific information on time to cord separation was collected. There were 4853, 5037, and 4999 infants in the 4% chlorhexidine, soap and water, and dry cord care clusters, respectively (Fig 1). Among enrolled infants, only 29 mothers (0.2%) refused • 1 household visit during the intervention period; most of those mothers (n • 23 [79%]) were in the nonchlorhexidine clusters (relative risk [RR]: 1.81; 95% confidence interval [CI]: 0.70 to 4.70). No adverse events in response to the intervention were reported for any of the groups.
FIGURE 1.
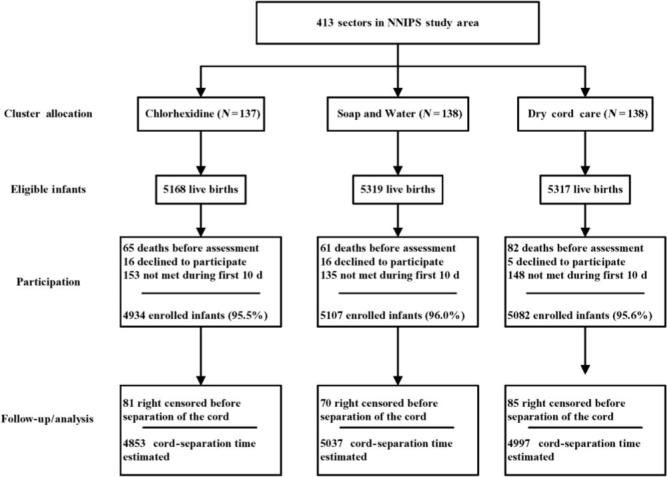
Study participants flow diagram. NNIPS indicates Nepal Nutrition Intervention Project, Sarlahi.
Baseline Comparison
Infant, maternal, and newborn care behaviors were generally well balanced across the groups (Table 1). In the intervention groups, maternal literacy was slightly higher and more infants were born to families originating from the hills (Pahadi), compared with the plains region of Nepal (Madeshi). The most commonly reported nonstudy application to the cord was mustard oil; this was applied more frequently in the dry cord care group. A range of other substances (such as mud, ash, herbs, spices, or breast milk; 4.6%) and nonstudy antiseptics (7.5%) were applied less commonly, and there was less exposure to nonstudy antiseptics in the chlorhexidine group. The overall prevalence of low birth weight (• 2500 g) was 29.9%, and rates were equal across the treatment groups.
TABLE 1.
Baseline Characteristics According to Treatment Group
| Variable | Chlorhexidine | Soap/Water | Dry Cord Care |
|---|---|---|---|
| No. of clusters (%) | 137 (100.0) | 138 (100.0) | 138 (100.0) |
| No. of infants per cluster, mean | 35.4 | 36.5 | 36.2 |
| Total No. of infants (%) | 4853 (100.0) | 5037 (100.0) | 4999 (100.0) |
| No. of home visits per infant, mean • SD | 9.5 • 1.8 | 9.5 • 1.8 | 9.5 • 1.8 |
| Gender, n (%) | |||
| Female | 2342 (48.3) | 2497 (49.6) | 2433 (48.7) |
| Male | 2511 (51.7) | 2540 (50.4) | 2564 (51.3) |
| Birth weight, n (%) | |||
| • 2500 g | 1376 (28.7) | 1487 (30.0) | 1482 (30.0) |
| • 2500 g | 3419 (71.3) | 3462 (70.0) | 3450 (70.0) |
| Ethnic group, n (%) | |||
| Plains (Madeshi) | 3160 (66.4) | 3453 (70.2) | 3655 (74.5) |
| Hills (Pahadi) | 1618 (33.6) | 1486 (29.8) | 1264 (25.5) |
| Maternal literacy, n (%) | |||
| No | 3540 (73.0) | 3746 (74.4) | 3732 (74.7) |
| Yes | 1309 (27.0) | 1288 (25.6) | 1263 (25.3) |
| Delivery place, n (%) | |||
| Nonfacility | 4314 (92.0) | 4423 (90.5) | 4479 (92.1) |
| Facility | 376 (8.0) | 462 (9.5) | 383 (7.9) |
| Birth assistant washed hands, n (%) | |||
| No | 1715 (38.4) | 1805 (38.7) | 1786 (38.4) |
| Yes | 2751 (61.6) | 2854 (61.3) | 2866 (61.6) |
| Mother/infant skin-to-skin care, n (%) | |||
| No | 4198 (95.6) | 4284 (95.0) | 4320 (96.2) |
| Yes | 193 (4.4) | 225 (5.0) | 173 (3.1) |
| Home applications to cord, n (%) | |||
| Mustard oil | |||
| No | 2510 (51.7) | 2664 (52.9) | 2316 (46.4) |
| Yes | 2343 (48.3) | 2373 (47.1) | 2681 (53.6) |
| Home antiseptics | |||
| No | 4527 (93.3) | 4638 (92.1) | 4606 (92.2) |
| Yes | 326 (6.7) | 399 (7.9) | 391 (7.8) |
| Other (mud, ash, herbs, or spices) | |||
| No | 4626 (95.3) | 4826 (95.8) | 4755 (95.2) |
| Yes | 227 (4.7) | 211 (4.2) | 242 (4.8) |
Time to Cord Separation
The overall mean • SD and median times to cord separation were 4.60 • 2.0 days and 4.33 days (interquartile range: 3.29−5.13 days), respectively. If infants with estimated cord-separation times (6.4%) were excluded, then the mean time to infection was only 0.1 day (2.4 hours) greater; therefore, the entire sample was used. The umbilical cord separated fully within the first 1 week of life for 13 658 infants (91.7%). Cord-separation times varied significantly across the treatment groups. Among infants who received chlorhexidine cleansing, the mean time to separation was 5.32 • 2.4 days, whereas the mean ages at cord separation for infants in the soap/ water and dry cord care groups were 4.25 • 1.6 days and 4.24 • 1.6 days, respectively. The cord-separation time was significantly longer (1.08 days; 95% CI: 0.99 to 1.16 days) in the chlorhexidine clusters, compared with the nonchlorhexidine groups combined. This estimate did not change substantially after adjustment for ethnic group, maternal literacy, and home-delivered applications to the cord (1.05 days; 95% CI: 0.96 to 1.14 days). Approximately 65% (n • 802) of the 1229 infants whose cord separation was delayed past 7 days were in the chlorhexidine group (RR: 3.64; 95% CI: 3.09 to 4.28).
The Kaplan-Meier time-to-event functions according to treatment group, including the 236 right-censored infants, are shown in Fig 2. The restricted mean and exponentially extended mean for each of these curves were identical within treatment groups and were not different from the mean separation times estimated when the censored observations were excluded.
FIGURE 2.
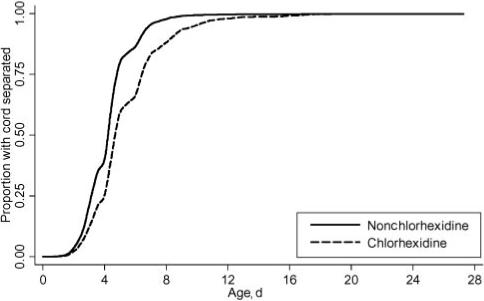
Kaplan-Meier curves for times to cord separation for chlorhexidine versus nonchlorhexidine groups. The soap/water and dry cord care groups were combined.
Factors Associated With Cord-Separation Time
The mean cord-separation time and risk of delayed (• 7 days) separation, stratified according to various factors and adjusted for cord care treatment group, are shown in Table 2. Given the large sample size, even small differences in the mean time to separation were statistically significant. The stratum-specific time to separation differed by • 0.25 days (• 6 hours) only for home-delivered applications of antiseptics, ethnic group, maternal literacy, and place of birth. Furthermore, in a multivariate model of separation time, including factors listed in Table 2, the stratum-specific adjusted difference in time to separation exceeded 0.25 days only for ethnic group (0.41 days; 95% CI: 0.32 to 0.50 days). A range of characteristics were associated with delayed (• 7 days) separation time. In a multivariate model, the most important factors included male gender (RR: 1.15; 95% CI: 1.03 to 1.28), birth weight of • 2500 g (RR: 1.15; 95% CI: 1.01 to 1.31), Pahadi ethnic group (RR: 1.56; 95% CI: 1.36 to 1.80), facility-based birth (RR: 1.26; 95% CI: 1.01 to 1.57), and home-delivered antiseptics (RR: 1.34; 95% CI: 1.09 to 1.66).
TABLE 2.
Cord-Separation Times According to Infant, Maternal, and Household Characteristics
| Variable | n | Cord-Separation Time, Mean• SD, d | Difference (95% Cl), d | RR for Cord-Separation Time of• 7 d (95% Cl) |
|---|---|---|---|---|
| Gender | ||||
| Female | 7272 | 4.53• 1.9 | ||
| Male | 7615 | 4.66• 2.0 | 0.12 (0.06 to 0.19) | 1.17 (1.05 to 1.30) |
| Birth weight | ||||
| • 2500 g | 4345 | 4.49• 1.9 | ||
| • 2500 g | 10 331 | 4.65• 2.0 | 0.12 (0.05 to 0.19) | 1.21 (1.07 to 1.36) |
| Ethnic group | ||||
| Plains (Madeshi) | 10 406 | 4.42• 1.9 | ||
| Hills (Pahadi) | 4368 | 5.01• 2.1 | 0.53 (0.46 to 0.60) | 1.62 (1.45 to 1.82) |
| Maternal literacy | ||||
| No | 11 018 | 4.48• 1.9 | ||
| Yes | 3860 | 4.94• 2.2 | 0.41 (0.34 to 0.48) | 1.41 (1.26 to 1.58) |
| Delivery place | ||||
| Nonfacility | 13 216 | 4.57• 1.9 | ||
| Facility | 1221 | 5.21• 2.5 | 0.62 (0.51 to 0.74) | 1.89 (1.62 to 2.16) |
| Birth assistant washed hands | ||||
| No | 5306 | 4.52• 1.9 | ||
| Yes | 8471 | 4.65• 2.0 | 0.12 (0.06 to 0.19) | 1.16 (1.03 to 1.30) |
| Mother/infant skin-to-skin care | ||||
| No | 12 802 | 4.60• 2.0 | ||
| Yes | 591 | 4.48• 1.9 | • 0.06 (• 0.22 to 0.10) | 0.98 (0.75 to 1.29) |
| Home applications to cord | ||||
| Mustard oil | ||||
| No | 7490 | 4.50• 2.0 | ||
| Yes | 7397 | 4.69• 1.9 | 0.19 (0.13 to 0.25) | 1.09 (0.98 to 1.22) |
| Home antiseptics | ||||
| No | 13 771 | 4.55• 1.9 | ||
| Yes | 1116 | 5.17• 2.4 | 0.61 (0.50 to 0.73) | 1.86 (1.60 to 2.17) |
| Other (mud, ash, herbs, or spices) | ||||
| No | 14 207 | 4.60• 2.0 | ||
| Yes | 680 | 4.53• 2.0 | • 0.07 (• 0.22 to 0.08) | 0.94 (0.73 to 1.22) |
Cord-Separation Time and Infection
There were 742 incident cases of omphalitis in the cord cleansing trial, and the risk varied significantly according to treatment group.16 We collected cord-separation time information for 737 (99%) of these cases. The mean time to separation among cases (4.43 days) was slightly less than that among noncases (4.60 days) but, after adjustment for treatment received and ethnic group, this difference was not significant (• 0.01 days; 95% CI: • 0.15 to 0.13 days).
We restricted analysis of the association between separation time and infection to infants in the nonchlorhexidine groups, given the strong correlation between this treatment and both separation time and infection risk. In a Poisson regression model with the time to separation as a continuous independent variable and adjustment for ethnic group, there was a decreased risk of infection of 3% for each additional day the cord did not separate, although this was not statistically significant (RR: 0.97; 95% CI: 0.92 to 1.03). Among infants whose cord separated after the first 1 week of life, the incidence of infection was 4.5 cases per 100 neonatal periods, compared with 5.9 cases per 100 neonatal periods among infants whose cord separated before the seventh day (Fig 3). This difference was not statistically significant (RR: 0.73; 95% CI: 0.48 to 1.17), and the comparative risk estimate was diminished in magnitude after adjustment for variables potentially associated with infection status, such as ethnic group, birth assistant hand-washing, and home-delivered applications to the cord (RR: 0.83; 95% CI: 0.52 to 1.33).
FIGURE 3.
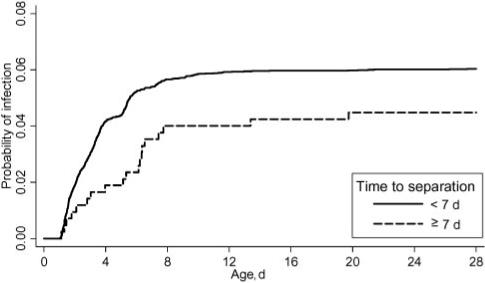
Time to cord infection according to cord-separation status (• 7 vs • 7 days). The analysis was restricted to infants in the nonchlorhexidine groups.
DISCUSSION
Many studies examining the time to separation of the umbilical cord have concluded that topical antiseptics delay separation of the cord. There are, however, no published reports from prospective studies conducted in communities in developing countries examining the time to cord separation, the impact of topical antiseptics on this separation time, or the relationship between separation time and risk of infection. This study of 15 000 infants in rural Nepal, where • 90% of infants are born in the home without trained assistance, contrasts sharply with data available from developed settings. These data demonstrate that the umbilical cord separates considerably earlier among rural newborn infants in Nepal than among infants in tertiary care institutions in developed2,23,24 or developing25–27 countries and that rapid separation time is a consistent characteristic, regardless of the use of topical antiseptics.
There is considerable variation in times to cord separation in developed countries, depending on the care provided.6,8,24,28 With or without topical antiseptics, the observed mean time to separation is generally • 10 days, and mean separation times of • 7 days have been reported only rarely.18,29,30 Monu and Okolo17 suggested that a traditional concoction of chalk and herbs with desiccating properties might lead to the cord stump falling off within 2 days after birth in communities of Nigeria, but no specific data were presented. The results from our study confirm this observation and suggest that cord separation is rapid in communities in developing countries where infants are exposed to unhygienic practices.
The time to cord separation was • 1 day longer among infants who received chlorhexidine, compared with those who received soap/water cleansings or dry cord care, which represents a 25% relative increase. Furthermore, the treated infants were also 3.6 times more likely to have delayed (• 7 days) separation. Relative mean increases of this magnitude and an increased likelihood of delayed separation have been observed in developed countries; in some cases, this decreased maternal satisfaction and raised economic concerns.11–13 In such settings, a recommendation against chlorhexidine or other topical antiseptics may be justified. In this setting, however, where the risk of serious infection is high, the benefits of chlorhexidine cleansing of the cord16 outweigh greatly any concerns related to increased separation time. The absolute difference in mean time to separation according to treatment was only 24 hours, and these data provide evidence that delayed cord separation is not associated with increased risk of infection. Newborn infants whose cord separated later (• 7 days) were at slightly less risk of infection, but there seems to be no causal relationship; the difference was not significant, and almost all infections in this setting occurred during the first 1 week of life (Fig 3).
In this setting, we did not attempt to measure directly maternal satisfaction with the protocol. The level of early withdrawal from the study, however, was very low (0.2%), which suggests that maternal dissatisfaction was also low. Because the mean increase in separation time was only 24 hours, it is possible that any slight delay in separation, relative to previous nonparticipating children, went unnoticed for many mothers.
There are consistencies between data presented here and previous reports from hospital-based studies regarding factors associated with cord separation. Hygienic behaviors or procedures that reduce the overall exposure of the cord to bacterial colonization (such as cesarean section31) tend to increase cord-separation times. In this study, facility-based birth, hand-washing with soap by birth attendants, and the use of home-delivered antiseptics all were associated with increased likelihood of cord separation after 7 days. Separation time was 17% more likely to occur before 7 days for female subjects, compared with male subjects, as suggested previously,32 but this difference has not been observed consistently.25–27
None of these factors, however, or more distal factors (such as ethnic group or maternal literacy) that were associated statistically with differences in cord-separation time represents an increase of any practical significance. Furthermore, all had substantially less impact on separation time than was seen with cord-cleansing treatment. Among infants who were not treated with chlorhexidine, increased cord-separation time was not associated with increased infection risk. In communities of developing countries in which many infants are born at home and hygienic conditions during the perinatal period are difficult to achieve, the potential for delayed cord separation as a result of topical antiseptic treatment seems to be negligible. Where baseline risks of omphalitis are high, early topical cleansing of the cord with chlorhexidine should be promoted widely.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institute of Child Health and Human Development (grants HD44004 and HD38753) and the Bill and Melinda Gates Foundation (grant 810–2054) and cooperative agreements between the Johns Hopkins Bloomberg School of Public Health and the Office of Heath and Nutrition, US Agency for International Development (agreements HRN-A-00-97-00015-00 and GHS-A-00-03-000019-00).
Abbreviations
- CI
confidence interval
- RR
relative risk
Footnotes
The funding sources played no role in study design, data collection, data analysis, writing of the report, or the decision to submit the manuscript for publication.
REFERENCES
- 1.World Health Organization . Care of the Umbilical Cord. Switzerland: World Health Organization; Geneva: 1998. [Google Scholar]
- 2.Zupan J, Garner P, Omari AAA. Topical umbilical cord care at birth. Cochrane Database Syst Rev. 2004;(3):CD001057. doi: 10.1002/14651858.CD001057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullany LC, Darmstadt GL, Tielsch JM. Role of antimicrobial applications to the umbilical cord in neonates to prevent bacterial colonization and infection: a review of the evidence. Pediatr Infect Dis J. 2003;22:996–1002. doi: 10.1097/01.inf.0000095429.97172.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magowan M, Andrews A, Pinder B, Allen S. The effect of an antibiotic spray on umbilical cord separation times. Nurs Times. 1980;76:1841. [PubMed] [Google Scholar]
- 5.Smales O. A comparison of umbilical cord treatment in the control of superficial infection. N Z Med J. 1988;101:453–455. [PubMed] [Google Scholar]
- 6.Arad I, Eyal F, Fainmesser P. Umbilical care and cord separation. Arch Dis Child. 1981;56:887–888. doi: 10.1136/adc.56.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rais-Bharami K, Schulte EB, Naqvi M. Postnatal timing of spontaneous umbilical cord separation. Am J Perinatol. 1993;10:453–454. doi: 10.1055/s-2007-994630. [DOI] [PubMed] [Google Scholar]
- 8.Ronchera-Oms C, Hernandez C, Jimemez NV. Antiseptic cord care reduces bacterial colonization but delays cord detachment. Arch Dis Child Fetal Neonatal Ed. 1994;71:F70. doi: 10.1136/fn.71.1.f70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verber IG, Pagan FS. What cord care, if any? Arch Dis Child. 1993;68:594–596. doi: 10.1136/adc.68.5_spec_no.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ente G, Penzer PH. The umbilical cord: normal parameters. JR Soc Health. 1991;111:138–140. doi: 10.1177/146642409111100406. [DOI] [PubMed] [Google Scholar]
- 11.Medves JM, O'Brien BAC. Cleaning solutions and bacterial colonization in promoting healing and early separation of the umbilical cord in healthy newborns. Can J Public Health. 1997;88:380–382. doi: 10.1007/BF03403910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugford M, Somchiwong M, Waterhouse IL. Treatment of umbilical cords: a randomized trial to assess the effect of treatment methods on the work of midwives. Midwifery. 1986;2:177–186. doi: 10.1016/s0266-6138(86)80043-2. [DOI] [PubMed] [Google Scholar]
- 13.Dore S, Buchan D, Coulas S, et al. Alcohol versus natural drying for newborn cord care. J Obstet Gynecol Neonatal Nurs. 1998;27:621–627. doi: 10.1111/j.1552-6909.1998.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 14.Ford LA, Ritchie JA. Maternal perceptions of newborn umbilical cord treatments and healing. J Obstet Gynecol Neonatal Nurs. 1999;28:501–506. doi: 10.1111/j.1552-6909.1999.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 15.Barr J. The umbilical cord: to treat or not to treat? Midwives Chron. 1984;97:224–226. [PubMed] [Google Scholar]
- 16.Mullany LC, Darmstadt GL, Khatry SK, et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Lancet. 2006;367:910–918. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monu JU, Okolo AA. Neonatal necrotizing fasciitis: a complication of poor cord hygiene: report of three cases. Ann Trop Paediatr. 1990;10:299–303. doi: 10.1080/02724936.1990.11747446. [DOI] [PubMed] [Google Scholar]
- 18.Faridi MM, Rattan A, Ahmad SH. Omphalitis neonatorum. J Indian Med Assoc. 1993;91:283–285. [PubMed] [Google Scholar]
- 19.Mullany LC, Darmstadt GL, Khatry SK, Tielsch JM. Traditional massage of newborns in Nepal: implications for trials of improved practice. J Trop Pediatr. 2005;51:82–86. doi: 10.1093/tropej/fmh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullany LC, Darmstadt GL, Katz J, et al. Development of clinical sign-based algorithms for community-based assessment of omphalitis. Arch Dis Child Fetal Neonatal Ed. 2006;91:F99–F104. doi: 10.1136/adc.2005.080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.Evens K, George J, Angst D, Schweig L. Does umbilical cord care in preterm infants influence cord bacterial colonization or detachment? J Perinatol. 2004;24:100–104. doi: 10.1038/sj.jp.7211027. [DOI] [PubMed] [Google Scholar]
- 24.Wilson CB, Ochs HD, Almquist J, Dassel S, Mauseth R, Ochs UH. When is umbilical cord separation delayed? J Pediatr. 1985;107:292–294. doi: 10.1016/s0022-3476(85)80154-2. [DOI] [PubMed] [Google Scholar]
- 25.Chamnanvanakij S, Decharachakul K, Rasamimaree P, Vanprapar N. A randomized study of 3 umbilical cord care regimens at home in Thai neonates: comparison of time to umbilical cord separation, parental satisfaction and bacterial colonization. J Med Assoc Thai. 2005;88:967–972. [PubMed] [Google Scholar]
- 26.Aygun C, Subasi A, Kucukoduk S. Timing of umbilical cord separation and neonatal intensive care unit practices. Am J Perinatol. 2005;22:249–251. doi: 10.1055/s-2005-870661. [DOI] [PubMed] [Google Scholar]
- 27.Vural G, Kisa S. Umbilical cord care: a pilot study comparing topical human milk, povidone-iodine, and dry care. J Obstet Gynecol Neonatal Nurs. 2006;35:123–128. doi: 10.1111/j.1552-6909.2006.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladstone IM, Clapper L, Thorpe JW, Wright DI. Randomized study of six umbilical cord care regimens. Clin Pediatr (Phila) 1988;27:127–129. doi: 10.1177/000992288802700302. [DOI] [PubMed] [Google Scholar]
- 29.Naor N, Merlob P, Litwin A, Wielunksy E. Time of separation of the umbilical cord: a comparative study of treatment with alcohol and Rikospray. Eur J Obstet Gynecol Reprod Biol. 1989;32:89–93. doi: 10.1016/0028-2243(89)90188-3. [DOI] [PubMed] [Google Scholar]
- 30.Bhalla JN, Nafis N, Rohatgi P, Singh J. Some observations on separation of the umbilical stump in the newborn. Indian J Pediatr. 1975;42:329–334. doi: 10.1007/BF02829329. [DOI] [PubMed] [Google Scholar]
- 31.Novack AH, Mueller B, Ochs H. Umbilical cord separation in the normal newborn. Am J Dis Child. 1988;142:220–223. doi: 10.1001/archpedi.1988.02150020122046. [DOI] [PubMed] [Google Scholar]
- 32.Oudesluys-Murphy AM, Eilers GAM, de Groot CJ. The time of separation of the umbilical cord. Eur J Pediatr. 1987;146:387–389. doi: 10.1007/BF00444944. [DOI] [PubMed] [Google Scholar]


