Abstract
Background
Previous studies have demonstrated that indomethacin lowers the incidence and decreases the severity of intraventricular hemorrhage, as well as improves the cognitive outcome, in prematurely born male infants.
Objective
The purpose of this work was to use functional magnetic resonance imaging to test the hypothesis that neonatal indomethacin treatment would differentially affect brain activation across genders in school-aged, prematurely born children during performance of a language task.
Methods
Forty-seven prematurely born children (600–1250-g birth weight) and 24 matched term control subjects were evaluated using a functional magnetic resonance imaging passive language task and neurodevelopmental assessments that included the Wechsler Intelligence Scale for Children-III and the Peabody Picture Vocabulary Test-Revised. Neural activity was assessed during both phonologic and semantic processing in the functional magnetic resonance imaging protocol.
Results
Neurodevelopmental assessments demonstrated significant differences in full-scale, verbal, and performance intelligence quotient, as well as Peabody Picture Vocabulary Test scores, between the preterm and term control subjects. Rates of perinatal complications did not differ significantly across preterm treatment groups, but male preterm subjects randomly assigned to saline tended to have lower Peabody Picture Vocabulary Test-Revised scores than did all of the other preterm groups. During phonological processing, a significant treatment-by-gender effect was demonstrated in 3 brain regions: the left inferior parietal lobule, the left inferior frontal gyrus (Broca's area), and the right dorsolateral prefrontal cortex.
Conclusions
These data demonstrate a differential effect of indomethacin administration early in postnatal life on the subsequent development of neural systems that subserve language functioning in these male and female preterm infants.
Keywords: functional magnetic resonance imaging, premature, indomethacin, neurodevelopmental outcome
Indomethacin, a well-known inhibitor of cyclooxygenase, both lowers the incidence and decreases the severity of intraventricular hemorrhage (IVH) in very low birth-weight (VLBW) preterm infants.1–4 In addition, indomethacin closes the patent ductus arteriosus, and it tends to increase survival in these infants, who often are critically ill. Because indomethacin has been shown in animal models and in preterm neonates both to decrease cerebral blood flow and to modulate vascular reactivity,5–8 the neurodevelopmental outcome of infants randomly assigned to early low-dose indomethacin has been studied intensively, although with inconclusive results.1,9,10 Our recent analysis of the children participating in the Multicenter Randomized Indomethacin IVH Prevention Trial demonstrated that indomethacin significantly decreased the incidence of IVH, prevented parenchymal hemorrhage, and was associated with higher verbal test scores at ages 3 to 8 years, although this protective effect of indomethacin on cognitive outcome seemed to be specific only to male subjects.10
Emerging experience with perinatal interventions suggests that follow-up data are needed for the thorough assessment of the safety of these treatments, and this is particularly true for the low birth-weight preterm infant population.11–13 In recent years, MRI has permitted a better understanding of the effects of preterm birth on the developing central nervous system (CNS), and abnormalities in regional brain volumes and in measures of white matter integrity have correlated significantly with measures of neurodevelopmental outcome.14–20 Thus, MRI strategies are particularly suited to examine the impact of injury and repair in the developing CNS.
In contrast, although functional MRI (fMRI) has been used to explore differences in cerebral functioning between term and preterm children at school age,21,22 the use of functional imaging strategies to assess the effects of therapy in the pediatric population is relatively new. Als et al23 used diffusion tensor imaging to document the effect of the Newborn Individualized Developmental Care and Assessment Program, a neonatal intervention for preterm infants beginning shortly after birth and continuing to 2 weeks after the obstetric due date, in VLBW preterm infants. Similarly, Shaywitz et al24 documented the long-term influence of a reading intervention on neural systems subserving this skill in school-aged children. Study children were randomly assigned to a specially designed reading intervention, community remediation, or no intervention and scanned immediately before random assignment at the completion of the intervention/no intervention period and 1 year thereafter to document the long-lasting effect of this reading program.
We used an fMRI-based task of passive language comprehension to test the hypothesis that indomethacin would differentially affect neural processes that subserve language function in male and female prematurely born children. Based on the pattern of cognitive test scores in our study cohort, which demonstrated a protective effect for boys randomly assigned to early indomethacin,25 we hypothesized that patterns of brain activation in these preterm boys randomly assigned to indomethacin would be more similar to those of term control boys than to patterns of brain activity in the preterm saline group. Because IVH has been shown to be associated with prolonged decreases in cerebral blood flow, increased rates of periventricular leukomalacia, and more frequent low-pressure ventriculomegaly26–28 and because our finding of improved test scores in boys randomly assigned to indomethacin was independent of IVH status,10 we tested our a priori hypothesis only in subjects who had no evidence of IVH in the newborn period.
Methods
The clinical studies were conducted at Women and Infants' Hospital (Providence, RI) and Yale New Haven Hospital (New Haven, CT). The protocols and procedures described below were reviewed and approved by the institutional review boards of the participating institutions. Parent(s) provided written consent, and written assent was obtained from all of the study children. All of the MRI scans were obtained at Yale University School of Medicine.
Subjects and Their Assessment
The preterm cohort consisted of children enrolled in the follow-up component of the Multicenter Randomized Indomethacin IVH Prevention Trial at Yale University School of Medicine and Brown University, based on geographic proximity to New Haven, CT. All of the subjects were examined with cranial echoencephalography between 5 and 11 hours of age. As shown in Table 1, those 334 with no evidence of IVH were admitted to the trial to test the primary hypothesis that indomethacin would prevent IVH in this patient population; 279 (84%) had no IVH at postnatal day 5.2 Of these 279, 249 survived (89%), 209 (84%) of the 249 survivors were evaluated at 8 years old corrected age, and 194 lived within 200 miles of the Yale MRI Research Facility.
TABLE 1. Origin of the Preterm Study Population.
| Available Subjects at Brown and Yale | n |
|---|---|
| Children with no IVH at 6 postnatal h | 334 |
| Children with no IVH at 5 postnatal d | 279 |
| Children with no IVH at 5 postnatal d who survived | 249 |
| Children with no IVH at 5 postnatal d who survived and were evaluated at 8 y old | 209 |
| No. of children seen on-site | 194 |
| No of children with any MRI | 102 |
| No of children who had fMRI scans for this study | 73 |
| No of children with usable fMRI data | 47 |
All of the children who did not have contraindications for MRI (usually orthodontia) were invited to participate in the imaging studies at the time of their 8-year-old cognitive assessments. Those who agreed were randomly assigned to volumetric, diffusion tensor, and/or functional imaging. From this potential pool of 194 subjects, 102 children (52%) took part in our MRI studies, and 92 of them underwent fMRI examinations. Scans from the first 19 preterm subjects without IVH were performed with MRI software incompatible with that for the current study and could not be included in this study. Data for these 19 subjects have been reported previously.22 Of the 73 remaining non-IVH preterm subjects, 17 were excluded because of excess motion artifact, and 9 were excluded because of the presence of overall activation levels >2 SD from the mean, which we assumed indicated excess task-related motion artifact. All of the subjects with usable fMRI data are included in this report, and the remaining 47 preterm subjects provided the basis for this study. Comparison of these 47 with the 162 children with no evidence of IVH and no fMRI data revealed no differences in birth weight, number of male subjects, number receiving special services in school, and years of maternal education, although preterm children with fMRI data had significantly higher verbal IQ scores than children without fMRI data (P = .005).
The 24 term control children, aged 7 to 9 years, were recruited from the local communities of the study children as described previously.29 Controls were additionally group-matched with the preterm subjects by age (±1 year), gender (male/female), maternal education (less than high school/high school graduate), and minority status (yes/no).
Neurodevelopmental Assessments
Assessments were performed at 8 years of age by testers blinded to the child's randomization status in the IVH prevention study. As described previously, the following tests were administered: (1) the Wechsler Intelligence Scale for Children, Third Edition, is an individually administered norm-referenced instrument for assessing the intellectual function of children ages 6 years 10 months through 16 years 11 months30; and (2) the Peabody Picture Vocabulary Test (PPVT)-Revised is a multiple-choice test that measures receptive vocabulary development and listening vocabulary in a format that requires no verbal response.31 The Wechsler Intelligence Scale for Children, Third Edition, generates full-scale intelligence quotient ([IQ] FSIQ), verbal IQ (VIQ), and performance IQ (PIQ) scores. The reading recognition, reading comprehension, and mathematics subtests of the Peabody Individualized Achievement Test-Revised were also administered to all of the study and term control subjects.32
Demographic data obtained from the parent or caregiver included information on household composition; languages spoken in the home; educational level of the biological, adoptive, or foster parent; school placement of the child; resource use; and need for medications. A standard neurologic examination was performed by a pediatric neurologist or developmental pediatrician at each site and included determinations of height, weight, occipitofrontal head circumference, visual field testing, pupillary function, eye movements, and facial strength. Tone, strength, reflexes, and cerebellar function were also assessed. A determination of normal, suspect, or abnormal neurologic status was made. Subjects with abnormal or suspect assessments were examined for the presence of microcephaly, spastic diplegia, hemiplegia, or quadriplegia.
fMRI Paradigms
Cognitive-behavioral tasks used in the fMRI subtraction paradigms were selected to help identify brain regions involved in the phonologic or semantic processing of spoken language. Because of the young age of the children and their wide range of cognitive functioning, we selected an age-appropriate, relatively simple cognitive task to perform in the scanner, a passive auditory listening task in which the children listened to 3 varying stimulus presentations of a pleasant children's story.
Stimulus 1
This was an audiotape of a young woman reading The Ugly Duckling in a pleasant voice. The story was presented in its entirety during the scanning session composed of 6 segments, each 35 seconds long.
Stimulus 2
This was the same story but with all phonemes of the story randomized in time. Phonemic randomization was intended to destroy the linguistic structure that made semantic comprehension possible. The story was read by the same woman, and the randomization was intended to contain the same acoustic spectral frequencies, phonemes, prosody, and duration as the original story. Comparing images acquired during stimulus 1 with those acquired during stimulus 2 was used to identify the differential brain activity needed to process the semantic components of the story.
Stimulus 3
A recording of the story was then low-passed filtered so that phonemes could not be discerned. Retaining the same prosody and duration as stimulus 1 and stimulus 2, it was devoid of phonemic content. Comparing imaging acquired as the children listened to stimulus 3 with images acquired as they listed to stimulus 2 was used to identify the differential brain activity associated with phonologic processing of the story. A staff member attended each child during the scan to ensure that the child remained alert, attended to the task, and held still.
Stimulus Presentation
Audiotaped stimuli were delivered through headphones. Portions of the 3 stimuli were presented sequentially in an alternating sequence. Each was presented as 2 separate segments in each of 3 experiment runs as described previously.22
Children were instructed to listen to the story closely and to try to understand and remember what they were hearing. They were told that they would not be able to understand some portions of what they heard. To assess their comprehension of the intelligible portions of the story, they were asked 10 multiple-choice questions about the content of the story after the scan.
Image Acquisition
Head positioning in the magnet was standardized using the canthomeatal line. A T1-weighted sagittal localizing scan was used to position the axial images. In all of the subjects, 10 oblique axial slices were acquired to correspond with 10 axial sections of the Talairach coordinate system oriented parallel to the anterior commissure-posterior commissure line. The slices were positioned with 2 slices below, 7 slices above, and 1 slice containing the anterior commissure-posterior commissure line. Slice thickness was a constant 7 mm, and the skip between slices varied between 0.5 and 2 mm to maintain a strict correspondence with the Talairach coordinate system across the axial slices.
Images were acquired on a GE Signa 1.5-T scanner equipped with echoplanar imaging hardware (GE LX 1.5 Tesla System, Waukesha, WI). The functional images were obtained with a gradient echo, echo planar imaging pulse sequence having a repetition time of 2060 milliseconds, echo time of 45 milliseconds, flip angle of 60°, 1 excitation per image, 20- × 40-cm field of view, and 64 × 128 matrix, providing a 3.1- × 3.1-mm in-plane resolution. During each run, 102 echoplanar images were acquired in each slice, providing 306 images per experiment and 102 images for each stimulus type. High-resolution, T1-weighted anatomic scans for volumetric measurements were acquired using a sagittal spoiled gradient recall sequence (repetition time of 24 milliseconds, echo time of 5 milliseconds, 45° flip, frequency encoding superior/inferior, no wrap, 256 × 192 matrix, field of view of 30 cm, 2 excitations, slice thickness of 1.2 mm, and 124 contiguous slices).
Image Processing
Images were motion corrected using SPM99 (www.fil.ion.ucl.ac.uk/spm/) for 6 translation and rotation directions. Subjects with motion trajectories >0.5 pixels were eliminated. Percentage of signal change for both contrasts of interest were calculated for each subject using a general linear model with a canonical hemodynamic response function time locked to the onset of the story conditions. Each subject's three-dimensional high-resolution volume scan was registered to a common reference brain space, and the regression coefficients from the general linear model were transformed to this common space for between-subject and between-group analyses.33
Statistical Analyses
The preterm subjects were divided into 4 groups for analyses: indomethacin boys, indomethacin girls, saline boys, and saline girls. Comparisons were also made between the total preterm group and the term controls.
Demographic, neurologic, and cognitive data were analyzed using standard χ2 analyses for categorical data and nonparametric Wilcoxon rank sum tests for continuous-valued data. To maintain control over false-positives that may arise from simultaneous testing of multiple factors across all brain voxels, whole-brain voxel-wise statistical testing was conducted first, and regions identified from the whole-brain analysis were submitted to more detailed mixed-effects modeling in SAS (SAS Institute, Cary, NC), as described below. To generate maps of average signal change across all of the subjects in each task, percentage of signal change values from all of the subjects were entered into a voxel-wise 1-sample t test. Monte-Carlo simulations were conducted to estimate a cluster-size threshold adequate for ensuring that the likelihood of a false-positive activation under the null hypothesis remained below P < .05.34 A voxel-wise threshold of P < .01 and a minimum cluster size of 71 voxels met this criterion.
The primary analysis, a voxel-wise analysis of variance program (part of AFNI software, http://afni.nimh.nih.gov/afni/) was used to identify regions of the brain exhibiting a significant gender × treatment interaction in each task (semantic and phonologic). For each region identified by this analysis, the percentage of signal changes in each task were averaged across the voxels comprising the activation cluster in each subject. Average values for each significant region, task, and subject were then submitted to a mixed-effects model in SAS for the purposes of adjusting for gender and behavioral and demographic covariates. We conducted significance tests and estimated adjusted means for each subject group to characterize the nature of the interaction in each region. Because the semantic and phonologic task subtractions are not directly comparable in a single model, they were analyzed separately.
Results
Subjects
The neonatal characteristics and neurocognitive data for the preterm subjects by gender and treatment assignment are found in Table 2. Rates of perinatal complications did not differ significantly across treatment groups. In addition, although treatment groups did not differ significantly in FSIQ, VIQ, or PIQ scores, male preterm subjects randomly assigned to saline tended to have both lower PPVT scores and lower Peabody Individualized Achievement Test-Revised reading recognition and reading comprehension scores than did the 3 other preterm groups (P = .05, .10, and .08, respectively), consistent with findings in the larger sample from which this group was drawn.22 Demographic, neurologic, and cognitive data for the 47 preterm subjects and 24 term controls subjects are shown in Table 3 and demonstrate significant differences in FSIQ, VIQ, PIQ, and PPVT scores between the preterm and term subjects, as expected based on the cognitive findings reported previously in the larger sample from which the preterm children were drawn.25 Fifty-three percent of the preterm subjects were receiving special services in school compared with 17% of the matched term control group (P = .005).
TABLE 2. Perinatal, Neurologic, and Cognitive Data for the Preterm Children.
| Variable | Indomethacin
|
Saline
|
Control for Treatment
p |
||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| No. | 17 | 8 | 8 | 14 | .03 |
| Perinatal data | |||||
| GA | 28.0 ± 1.5 | 27.5 ± 2.4 | 28.0 ± 2.4 | 28.1 ± 1.7 | .58 |
| BW | 989.8 ± 131 | 948.6 ± 200 | 942.5 ± 240 | 961 ± 183 | .60 |
| AN steroids | 7 (41%) | 1 (13%) | 5 (63%) | 6 (43%) | .11 |
| RDS | 17 (100%) | 7 (88%) | 7 (88%) | 10 (71%) | .16 |
| BPD | 7 (41%) | 3 (38%) | 4 (50%) | 3 (21%) | .30 |
| NEC | 0 | 0 | 1 (7%) | 1 (13%) | .68 |
| PVL | 0 | 0 | 0 | 1 (7%) | .45 |
| VM | 0 | 0 | 0 | 0 | |
| Neurocognitive data | |||||
| FSIQ | 97.2 ± 12.5 | 96.5 ± 17.9 | 88.1 ± 20.2 | 102.1 ± 11.7 | .11 |
| VIQ | 101.6 ± 13.4 | 99.8 ± 17.3 | 92.4 ± 17.3 | 105.9 ± 13.9 | .10 |
| PIQ | 93.6 ± 14.1 | 94.4 ± 16.3 | 86.1 ± 25.7 | 98.3 ± 11.6 | .26 |
| PPVT | 99.5 ± 10.8 | 94.9 ± 24.2 | 79.4 ± 34.6 | 99.9 ± 16.4 | .051 |
| Read reca | 97.9 ± 17.7 | 99.6 ± 20.3 | 80.3 ± 16.7 | 101.3 ± 20.1 | .10 |
| Read coma | 98.9 ± 17.1 | 100.9 ± 22.8 | 80.4 ± 20.6 | 103.7 ± 19.8 | .08 |
| Matha | 92.0 ± 17.3 | 91.3 ± 19.9 | 81.3 ± 18.4 | 95.3 ± 19.8 | .21 |
| CP | 1 (5.9%) | 0 | 0 | 0 | .55 |
| Spec services | 10 (59%) | 4 (50%) | 4 (50%) | 7 (50%) | .77 |
| Mat ed < HS | 2 (12%) | 0 | 0 | 2 (14%) | .92 |
AN indicates antenatal; Mat ed, maternal education; HS, high school; Spec, special; GA, gestational age; BW, body weight. Data are n, n (%), or mean ± SD.
Reading recognition, reading comprehension, and mathematics of the Peabody Individualized Achievement Test-Revised.
TABLE 3. Demographic, Neurologic, and Cognitive Data for the Study Children.
| Variable | Preterm | Term | P |
|---|---|---|---|
| No. | 47 | 24 | |
| Males | 25 (53%) | 11 (46%) | .62 |
| Right-handed | 33 of 38 (87%) | 11 of 12 (92%) | .65 |
| Minority status | 12 (26%) | 5 (21%) | .45 |
| FSIQ | 97.0 ± 15.0 | 109.8 ± 13.6 | .003 |
| VIQ | 101.0 ± 15.2 | 111.5 ± 11.4 | .006 |
| PIQ | 93.9 ± 16.3 | 106.0 ± 15.3 | .012 |
| PPVT | 95.4 ± 21.1 | 113.4 ± 17.8 | <.001 |
| Reading recognitiona | 96.2 ± 19.6 | 107.3 ± 15.7 | .019 |
| Reading comprehensiona | 97.5 ± 20.6 | 109.3 ± 16.6 | .018 |
| Matha | 91.0 ± 18.7 | 108.4 ± 18.6 | .004 |
| Special services | 25 (53%) | 4 of 23 (17%) | .005 |
| Age at scan, y | 9.26 ± 0.7 | 8.70 ± 0.6 | .001 |
| Maternal education < high school | 4 (8.5%) | 1 (4.2%) | .65 |
Data are n, n (%), or mean ± SD.
Peabody Individualized Achievement Test-Revised.
Group Average Activation Maps
Maps representing the voxel-wise group average signal changes for semantic and phonologic processing are shown in Fig 1. Semantic processing (Fig 1A) was characterized by both positive and negative signal changes. Negative signal changes were seen in bilateral parietal (Brodmann's area [BA] 40) and insular cortex, largely overlapping with the positive activation seen in the phonologic contrast. This implies that signal in these regions was highest while listening to scrambled speech, compared with both low-pass filtered speech and with the original, unaltered narrative. Additional negative changes were detected in the left inferior frontal gyrus (BA 47). Positive activations were detected in the semantic contrast in regions presumably reflecting meaningful linguistic processing, including the posterior superior and middle temporal gyri (BA 22, Wernicke's area) and a more anterior section of the superior temporal gyrus (BA 12).
FIGURE 1.
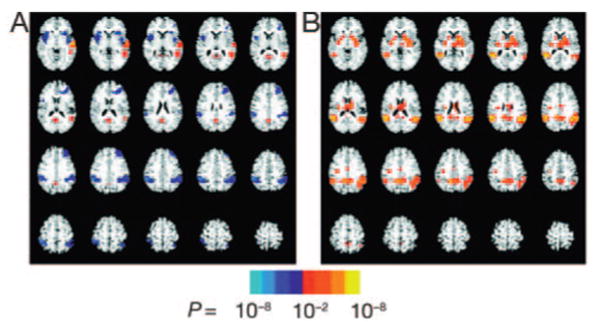
Semantic (A) and phonologic (B) contrast group average activation maps. All 71 subjects are included. The voxel-wise threshold is P < .01, and the significance level for the regions is indicated by the color bar. The semantic task is characterized by both positive and negative signal changes. Negative signal changes, shown in blue and aqua, are seen in the bilateral parietal (BA 40) and insular cortex and in the left IFG (BA 47). This implies that the signal in these regions was highest while listening to scrambled speech compared with both low-pass filtered speech and with the original, unaltered speech. Positive activations in the semantic contrast, shown in red and yellow, reflect meaningful linguistic processing and are seen in the posterior superior and middle temporal gyri (BA 22, Wernicke's area) and a more anterior section of the superior temporal gyrus (BA 12).
In contrast, phonologic processing (Fig 1B) engaged large portions of the parietal lobe bilaterally, centered in the inferior parietal lobe (BA 40). Additional activation was detected in the posterior cingulate and the bilateral insular cortex. No negative signal changes were detected in the phonologic contrast, which is to say that the response of the brain to meaningless phonemically scrambled speech was greater than the response to unintelligible low-pass filtered speech in all regions showing a significant change.
Hypothesis Testing
A whole-brain voxel-wise analysis of variance was used to identify brain regions exhibiting a significant gender-by-treatment interaction effect, testing the preliminary hypothesis of a differential effect of indomethacin treatment between boys and girls. Average signal change levels extracted from regions so identified were submitted to a mixed-effects model, controlling for both VIQ and age at the time of MRI scan. Pairwise comparisons were used to evaluate the hypothesis that male preterm subjects randomly assigned to receive indomethacin would be more likely to have patterns of brain activation similar to term control boys than male preterm subjects randomly assigned to saline and that such an effect would not be present in girls. Statistical modeling was performed separately for phonologic and semantic tasks.
On the basis of the voxel-wise whole-brain analysis of variance analysis, no significant gender-by-treatment interactions were noted in fMRI analyses of the semantic contrast. In contrast, 3 regions were identified showing a significant treatment-by-gender effect in the phonologic contrast. These include the left inferior parietal lobule ([IPL] BA 40), the left inferior frontal gyrus (IFG), or Broca's area (BA 44) and the right dorsolateral prefrontal cortex ([DLPF] BA 46; peak voxel P = .0002, .0011, and .0002, respectively). These regions are shown in Fig 2.
FIGURE 2.
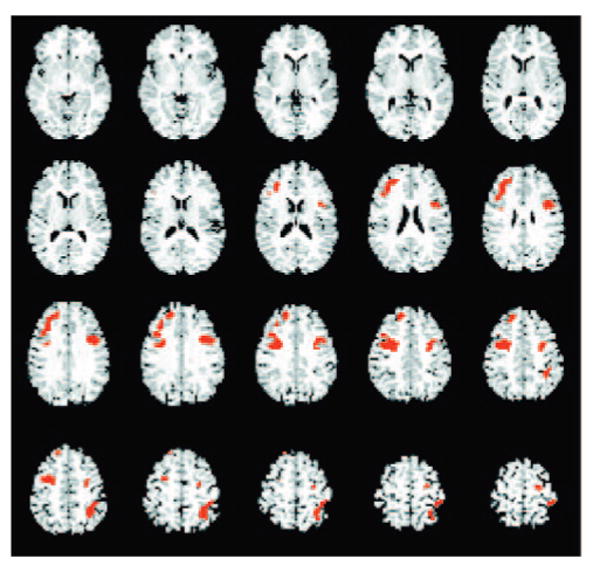
Map of areas exhibiting a treatment-by-gender interaction effect on the phonologic task, in a preliminary whole-brain voxel-wise analysis of variance, include the left IPL (BA 40), the left IFG or Broca's area (BA 44), and the right DLPF (BA 46). For this treatment-by-gender map, all significant regions are shown in red, the voxel-wise threshold is P < .01, and the cluster-size corrected is P < .05.
Adjusted percentage of signal change data for the study groups are shown in Table 4 and Fig 3. Analyses of the left IPL (BA 40) demonstrated that activations for preterm boys randomly assigned to indomethacin were significantly greater than those for preterm boys randomly assigned to saline (P = .013) but not significantly different from control boys (P = .77; Fig 3A). In contrast, activations for preterm boys randomly assigned to saline were significantly less than those for the term control boys (P = .045). For girls, activation in the left IPL differed across all 3 of the comparisons (indomethacin preterm versus saline preterm subjects, P = .0003; indomethacin girls versus term control girls, P = .058; and saline girls versus term controls, P = .043). These data confirm our primary hypothesis that preterm boys randomly assigned to indomethacin will demonstrate activation patterns more similar to term control subjects than preterm boys randomly assigned to saline, whereas girls did not exhibit this effect of early indomethacin.
TABLE 4. Percentage of Signal Changes for the Phonologic Processing Task.
| Treatment | Gender | % Signal Change | SE |
|---|---|---|---|
| Left IPL | |||
| Indomethacin | Females | −0.087 | 0.058 |
| Males | 0.056 | 0.040 | |
| Saline | Females | 0.195 | 0.044 |
| Males | −0.127 | 0.060 | |
| Term controls | Females | 0.060 | 0.048 |
| Males | 0.037 | 0.050 | |
| Left IFG | |||
| Indomethacin | Females | −0.045 | 0.048 |
| Males | 0.062 | 0.033 | |
| Saline | Females | 0.110 | 0.037 |
| Males | −0.074 | 0.050 | |
| Term controls | Females | 0.030 | 0.039 |
| Males | 0.056 | 0.041 | |
| Right DLPF | |||
| Indomethacin | Females | −0.006 | 0.045 |
| Males | 0.046 | 0.032 | |
| Saline | Females | 0.099 | 0.035 |
| Males | −0.110 | 0.047 | |
| Term controls | Females | 0.008 | 0.037 |
| Males | 0.104 | 0.039 |
Adjusted for age at scan and VIQ.
FIGURE 3.
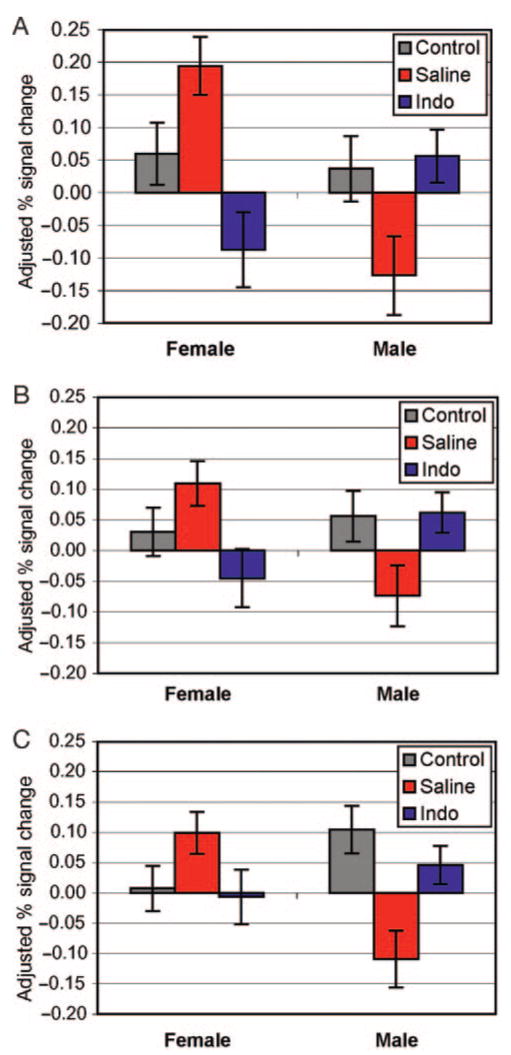
Least-squares mean ± SEM for selected fixed effects. These graphs represent regions in which the fixed-effects analyses demonstrate treatment-by-gender effects for the phonologic processing task and show percent signal change adjusted for age at scan and VIQ. A, Left IPL (P = .0002); B, Left IFG (P = .0011); and C, Right DLPF (P = .0002).
Similarly, as shown in Fig 3B, regression analysis for the left IFG (BA 44), or Broca's region, demonstrated that activations for preterm boys randomly assigned to indomethacin were significantly different from those for preterm boys randomly assigned to saline (P = .025) but not different from control boys (P = .914). Furthermore, the left IFG activations for preterm boys randomly assigned to saline differed from those for the term control boys at the P = .053 level. In contrast, although the activation patterns in the left IFG were statistically different between those female preterm subjects randomly assigned to indomethacin when compared with preterm girls randomly assigned to saline (P = .012), neither group of preterm subjects differed significantly from the term controls (P = .23 and .15, respectively). These data again suggest that preterm boys randomly assigned to indomethacin demonstrate phonologic activation patterns similar to term control boys, but girls have no such differential effect.
Finally, for the right DLPF, a similar pattern of actions was found: preterm boys randomly assigned to indomethacin differed significantly from preterm boys randomly assigned to saline (P = .007) but not from term control boys (P = .26), as shown in Fig 3C. As expected to confirm our hypothesis testing, preterm boys randomly assigned to saline were significantly different from term control boys (P = .001). Furthermore, there were no significant differences for any of the comparisons among the female groups in the right DLPF (preterm indomethacin girls versus preterm saline girls, P = .07; preterm indomethacin girls versus term control girls, P = .82; preterm girls randomly assigned to saline versus term control girls, P = .082).
Discussion
While using fMRI strategies for the processing of spoken language, we demonstrated a differential effect of early indomethacin on developing brain in this cohort of male and female preterm subjects. Patterns of fMRI activation during phonologic processing in prematurely born boys who were randomly assigned to early indomethacin were not statistically different from term control boys. However, we detected significant differences between patterns found in boys randomly assigned to indomethacin compared with boys randomly assigned to saline, as well as between boys randomly assigned to saline and term control boys. These findings are consistent with our previous reports of improvement in cognitive test scores for boys randomly assigned to indomethacin in the significantly larger study of the entire cohort at 8 years old.10
The regions of differential effect, the left IPL, IFG, and right DLPF, have all been implicated in the phonologic processing of language. The IPL is the classical locus of phonological encoding, as evidenced by a vast number of lesion and human imaging studies (reviewed by Eckert35 and Heim36). Often less appreciated, however, is that BA 44 in the left IFG, or Broca's region, although widely regarded as the locus of expressive language functioning, also participates in phonological processing. For auditory tasks, activation of this region has been attributed to the recoding of acoustic phonetic information into its corresponding articulatory gestures, and Burton37 has shown that the region of peak activation during auditory phonologic tasks tends to be situated in the superior and posterior aspects of Broca's area (BA 44), thus providing a plausible explanation for this finding. More recent studies by Burton et al38 have shown stronger increases in activation of BA 44 in response to pseudowords, such as those found in stimulus 2 of our paradigm, when compared with either words or sequences. In addition, BA 44 has been shown to coactivate with the left IPL (BA 40) near the supramarginal gyrus during controlled use of phonologic processing.39 Moreover, in addition to BA 44, phonologic tasks have been shown to activate several other portions of the right frontal cortex, including DLPF (BA 46), particularly during pseudoword tasks such as the 1 used to engage phonological processing systems here. These prefrontal regions are believed to subserve the general working memory functions needed for decoding processes within other brain regions.40,41
In contrast, although randomization to early indomethacin was associated with negative blood oxygen level-dependent signal in the left IPL and left IFG, we noted no consistent treatment effect for the female preterm study children. These data are consistent with the cognitive and language testing results, which show no significant differences between preterm girls randomly assigned to indomethacin and preterm girls randomly assigned to saline placebo (data not shown).
The spectrum of actions of indomethacin in the developing brain remains largely unknown. Whereas earlier studies emphasized the effects of this agent on cerebral blood flow and vascular reactivity,5,6 more recent investigations have demonstrated the ability of indomethacin to suppress mediators of CNS inflammation, including prostaglandin E2 and interleukin 1β; inhibit activation of the transcription factor nuclear factor-κB; and act as a direct ligand of the peroxisome proliferator-activated receptor-γ (for review see Townsend and Pratico42).
Furthermore, increasing evidence suggests the presence of inborn gender differences in the response to injury of the developing CNS,13 and numerous studies have shown that these differences are attributable to intrinsic properties of individual cells.43–47 Male and female neurons exhibit differential gene expression even in the absence of hormonal influences, and there seems to be an innate gender-based proclivity of the response to cytotoxicity and apoptotic pathways in both neurons and developing oligodendroglial cells.43–47 It is noteworthy, therefore, that both white matter injury and IVH are more common in preterm boys than in preterm girls.48
As with other studies of functional imaging, limitations of our study include sample size and selection bias within the study group. All of the study children were invited to participate, and our sample size is consistent with or greater than sample sizes of other fMRI studies of children at this age.24,49 None of the children with usable fMRI data were excluded from our analyses, and we do not wish to imply that the subjects studied reflect the full cohort of children without IVH at the Brown and Yale sites of our clinical trial. Finally, using data from the Trial of Indomethacin Prophylaxis in the Preterms Study cohort, Ohlsson et al13 have reported recently both a strong negative effect of indomethacin in female preterm infants, as well as a weaker positive one in male subjects. The Trial of Indomethacin Prophylaxis in the Preterms Study differed significantly from our study, however. Because early ultrasounds were not required for study admission, both subjects with early onset IVH (within the first 6 postnatal hours) and those with no IVH were enrolled; furthermore, indomethacin was administered within the first 6 postnatal hours, a time interval during which indomethacin may have significant impact on renal blood flow and mesenteric vascular stability. First ultrasounds were done at 5 to 8 days of life.50
The present data use functional imaging strategies to suggest a possible long-term influence of a neonatal intervention on the developing CNS in this population of children. We have previously reported indomethacin-by-gender interactions influencing the rates of IVH and cognitive performance in school-aged children. The current study extends this work by identifying language processes that indomethacin may uniquely affect and by demonstrating brain regions that may serve as targets for the effects of indomethacin in the brains of prematurely born boys. The biological mechanisms of indomethacin and its effect on corticogenesis remain to be explored.51–55
Acknowledgments
This work was supported in part by National Institutes of Health grants NS 27116, NS 35476, MO1-RR06022, MO1-RR00125, NIMH K02-74677, NIDA DA017820, NS38467, and EB00473.
We thank Dr Deborah Hirtz for her scientific expertise, Marjorene Ainley for follow-up coordination, Lisa Perry and Victoria Watson for neurodevelopmental testing, and Hedy Sarofin and Terry Hicky for their technical assistance.
Abbreviations
- IVH
intraventricular hemorrhage
- VLBW
very low birth weight
- CNS
central nervous system
- fMRI
functional magnetic resonance imaging
- PPVT
Peabody Picture Vocabulary Test
- IQ
intelligence quotient
- FSIQ
full-scale intelligence quotient
- VIQ
verbal intelligence quotient
- PIQ
performance intelligence quotient
- IPL
inferior parietal lobule
- IFG
inferior frontal gyrus
- DLPF
dorsolateral prefrontal cortex
- BA
Brodmann's area
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Schmidt B, David P. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 2.Ment LR, Oh W, Ehrenkranz RA, Philip AGS, et al. Low dose indomethacin and prevention of intraventicular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–550. [PubMed] [Google Scholar]
- 3.Bada HS, Green RS, Pourcyrous M, et al. Indomethacin reduced the risk of severe intraventricular hemorrhage. J Pediatr. 1989;115:631–637. doi: 10.1016/s0022-3476(89)80300-2. [DOI] [PubMed] [Google Scholar]
- 4.Banstra ES, Montalvo BM, Goldberg RN, et al. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics. 1988;82:533–542. [PubMed] [Google Scholar]
- 5.Leffler CW, Busija DW, Fletcher AM, Beasley DG, Hessler JG, Green RS. Effects of indomethacin upon cerebral hemodynamics of newborn pigs. Pediatr Res. 1985;19:1160–1164. doi: 10.1203/00006450-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Leffler CW, Busija DW, Beasley DG, et al. Effects of Indomethacin on cardiac outcome distribution in normal and asphyxiated piglets. Prostaglandins. 1986;31:183–190. doi: 10.1016/0090-6980(86)90045-6. [DOI] [PubMed] [Google Scholar]
- 7.Hammerman C, Glaser J, Schimmel MS, Ferber B, Kaplan M, Eidelman AI. Continuous versus multiple rapid infusions of indomethacin effects of cerebral blood flow velocity. Pediatrics. 1995;95:244–248. [PubMed] [Google Scholar]
- 8.Volpe JJ. Brain injury caused by intraventicular hemorrhage: Is indomethacin the silver bullet for prevention? Pediatrics. 1994;93:673–677. [PubMed] [Google Scholar]
- 9.Couser RJ, Hoekstra RE, Ferrara TB, Wright GB, Cabalka AK, Connett JE. Neurodevelopmental follow-up at 36 months' corrected age of preterm infants treated with prophylactic indomethacin. Arch Pediatr Adolesc Med. 2000;154:598–602. doi: 10.1001/archpedi.154.6.598. [DOI] [PubMed] [Google Scholar]
- 10.Ment LR, Vohr B, Makuch RW, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145:832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 11.O'shea TM, Goldstein DJ. Follow-up data - their use in evidence-based decision making. Clin Perinatol. 2003;30:217–250. doi: 10.1016/s0095-5108(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 12.Gross SJ, Anbar RD, Mettelman BB. Follow-up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics. 2005;115:681–687. doi: 10.1542/peds.2004-0956. [DOI] [PubMed] [Google Scholar]
- 13.Ohlsson A, Roberts RS, Schmidt B, et al. Male/female differences in indomethacin effects in preterm infants. J Pediatr. 2005;147:860–862. doi: 10.1016/j.jpeds.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neurorad. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 15.Inder TE, Warfield S, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 16.Huppi PS, Murphy B, Jaier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs E, Lucas A, Chong WK, et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713–720. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs EB, Edmonds C, Lucas A, Gadian DS. Calculation difficulties in children of very low birthweight: a neural correlate. Brain. 2001;124:60–66. doi: 10.1093/brain/124.9.1701. [DOI] [PubMed] [Google Scholar]
- 19.Nosarti C, Al-Asady MHS, Frangou S, Stewart A, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 20.Nosarti C, Rushe TM, Woodruff PWR, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- 21.Rushe TM, Temple CM, Rifkin L, et al. Lateralisation of language function in young adults born very preterm. Arch Dis Child Fetal Neonatal Ed. 2004;89:F112–F118. doi: 10.1136/adc.2001.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson BS, Vohr BR, Kane M, et al. A functional MRI study of language processing and cognitive outcome in prematurely born children. Pediatrics. 2002;110:1153–1162. doi: 10.1542/peds.110.6.1153. [DOI] [PubMed] [Google Scholar]
- 23.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;114:1738–1739. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 24.Shaywitz BA, Shaywitz SE, Blachman BA, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Vohr BR, Allan WA, Westerveld M, et al. School age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111(4) doi: 10.1542/peds.111.4.e340. Available at: www.pediatrics.org/cgi/content/full/111/4/e340. [DOI] [PubMed]
- 26.Volpe JJ, Herscovitch P, Perlman JM. Positron emission tomography in the newborn; Extensive impairment of regional cerebral blood flow with intraventricular hemorrhage and hemorrhagic intracerebral involvement. Pediatrics. 1983;72:589–601. [PubMed] [Google Scholar]
- 27.Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Shalak L, Perlman JM. Hemorrhagic-ischemic cerebral injury in the preterm infant. Current concepts. Clin Perinatol. 2002;29:745–763. doi: 10.1016/s0095-5108(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 29.Peterson BS, Vohr B, Cannistraci CJ, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Intelligence Scale for Children. 3rd. New York, NY: The Psychological Corporation Harcourt Brace Co; 1991. [Google Scholar]
- 31.Peabody. Peabody Picture Vocabulary Test-Revised. Circle Pines, NM: American Guidance Service; 1981. [Google Scholar]
- 32.Markwardt FCJ. Peabody Individual Achievement Test - Revised Manual. Circle Pines, MN: American Guidance Service; 1989. [Google Scholar]
- 33.Studholme C, Constable RT, Duncan JS. Accurate alignment of functional EPI data to anatomical MRI physics based distortion model. IEEE Trans Med Imag. 2001;19:1115–1127. doi: 10.1109/42.896788. [DOI] [PubMed] [Google Scholar]
- 34.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintum MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 35.Eckert M. Neuroanatomical markers for dyslexia: a review of dyslexia structural imaging studies. Neuroscientist. 2004;10:362–371. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- 36.Heim S. The structure and dynamics of normal language processing: insights from neuroimaging. Acta Neurobiol Exp (Wars) 2005;65:95–116. doi: 10.55782/ane-2005-1543. [DOI] [PubMed] [Google Scholar]
- 37.Burton MW. The role of inferior frontal cortex in phonological processing. Cogn Sci. 2001;25:679–690. [Google Scholar]
- 38.Burton MW, LoCasto PC, Krebs-Noble D, Gullapalli RP. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage. 2005;26:647–661. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonologic tasks. Neuron. 2002;35:808–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 40.Cabeza R, Nyberg L. Imaging cognition II: AN empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 41.Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam MM. Language network specializations: An analysis with parallel task designs and functional magnetic resonance imaging. Neuroimage. 2005;26:975–985. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Townsend KP, Pratico D. Novel therapeutic opportunities for Alzheimer's disease: Focus on nonsteroidal anti-inflammatory drugs. FASEB J. 2005;19:1592–1601. doi: 10.1096/fj.04-3620rev. [DOI] [PubMed] [Google Scholar]
- 43.Du L, Byis H, Lai Y, Zhang X, Kochanek PM, Watkins SC. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 44.Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- 45.Hagberg H, Wilson MA, Matshushita H, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 46.Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury. Does sex matter? Stroke. 2005;36:193–195. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- 47.Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26:245–254. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- 48.Edwards D. Brain protection for girls and boys. J Pediatr. 2004;145:223–224. doi: 10.1016/j.jpeds.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 49.Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children Brain. Lang. 2005 doi: 10.1016/j.bandl.2005.07.004. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guighard JP. The effects of prostaglandin synthesis inhibitors in the newborn rabbit. Semin Perinatol. 2002;26:398–405. doi: 10.1053/sper.2002.37310. [DOI] [PubMed] [Google Scholar]
- 51.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 52.Huttenlocher PR, de Courten C, Garey LJ, van der Loos H. Synaptogenesis in human visual cortex-evidence for synapse elimination during normal development. Neuroscience Lett. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 53.Huttenlocher PR, deCourten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- 54.Huttenlocher P, Dabholkar A. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 55.Reiss AL, Kesler SR, Vohr BR, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145:242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]


