Abstract
The prefrontal cortex (PFC) is relatively smaller, and the corpus callosum (CC) larger, in adults with Tourette syndrome (TS). The authors explored the possible roles of the PFC and the CC in mediating interhemispheric interference and coordination in TS adults. They measured performance on M. Kinsbourne and J. Cook's (1971) verbal–manual interference task and on the bimanual Purdue Pegboard in 38 adults with TS and 34 healthy adults. Compared with controls, TS subjects were impaired on the bimanual Purdue Pegboard. On the dual task, right-hand performance did not differ between groups, but the normally expected left-hand advantage (opposite hemisphere condition) was absent in TS subjects. In the control group only, better left-hand performance accompanied larger PFC volumes but not CC cross-sectional area. PFC dysfunction might have precluded executive control of interference in the TS group.
Keywords: Tourette syndrome, dual task, interference, prefrontal cortex, imaging
Tourette syndrome (TS) is defined by the presence of chronic, semi-involuntary motor and vocal tics. Largely on the basis of analogy with other movement disorders, early neurobiological studies of TS focused on the structure and function of basal ganglia nuclei (Hyde et al., 1995; Peterson et al., 1993, 1998; Singer et al., 1993). Subsequently, however, neuroimaging studies detected abnormal cortical volumes and differences in cross-sectional areas of the corpus callosum (CC; Peterson et al., 1993). Thus, conceptualization of the pathophysiology of TS has expanded to include disturbances in both prefrontal control processes and interhemispheric connectivity (Fredericksen et al., 2002; Gerard & Peterson, 2003; Peterson et al., 1998, 2001, 2003; Plessen et al., 2004).
Dual tasks provide behavioral measures for examining prefrontal control processes and interhemispheric connectivity. In simpler dual tasks, a limited-capacity response-selection processor is thought to control performance (for a review, see Pashler, 1994). Whether cross-talk between neural systems contributes to the interference observed in more complex dual tasks is unknown (Pashler, 1994). If cross-talk does control interference, then as tasks become more similar, interference should increase, and as tasks become less similar, interference should decrease. Studies of simpler dual tasks support the view that cross-talk does not underlie interference effects (Pashler, 1994). However, more complex dual tasks that address laterality effects, such as Kinsbourne and Cook's (1971) dual task, demonstrate that, even on disparate tasks, interference effects are quite evident when the additional cognitive task is lateralized in the same hemisphere as the baseline task, thus providing evidence of cross-talk.
Neuroimaging studies shed further light on what mechanisms underlie dual-task interference. Dual-task performance is thought to be under prefrontal control (D'Esposito et al., 1995; Herath, Klingberg, Young, Amunts, & Roland, 2001; Jiang, 2004; Szameitat, Schubert, Muller, & von Cramon, 2002) and to involve overlapping cerebral territories (Adcock, Constable, Gore, & Goldman-Rakic, 2000; Kinsbourne & Hicks, 1978; Klingberg & Roland, 1997; Roland & Zilles, 1998). The dual-task paradigms that have been validated thus far in functional neuroimaging studies do not incorporate a laterality component. Thus, whether overlapping cerebral territories and cross-talk contribute to interference is still unresolved.
Kinsbourne and Cook (1971) introduced a dual-task laterality paradigm that varies the potential for response interference. It compares performance on a manual motor task at baseline with performance of the same task concurrently with a hemispherically lateralized cognitive task. Among the many subsequent studies of this interhemispheric interference effect (Kinsbourne & Hiscock, 1983), the rate of finger tapping, comparing the right and left index fingers, has been a frequently used manual motor variable. A concurrent verbal task selectively involves the left hemisphere (in right-handers). Healthy right-handed individuals perform the manual task faster with the right hand. However, when the verbal task is simultaneously imposed, the tapping rate of the right hand declines, whereas that of the left remains unchanged or is far less affected. Researchers have explained this functional asymmetry in healthy individuals by the disparate degree of neural connectivity (functional distance) between motor and language systems, depending on whether the left hand or the right hand is used (for a review, see Kinsbourne & Hiscock, 1983). In right-handers, the concurrent language task activates primarily the left cerebral hemisphere, as does the right-hand motor task. The functional and anatomical distance between language and motor systems is therefore short during right-handed performance. The left-hand motor task, in contrast, activates the right cerebral hemisphere and therefore is represented further in functional distance from the hemisphere activated by the language task. Left-hand performance is thus less vulnerable than right-hand performance to interfering neural cross-talk between the motor and language systems. The effects of carotid sodium amytal testing on performance during this dual-task paradigm support the model of interfering cross-talk between the neural systems that subserve the component lateralizing tasks (Kosaka, Hiscock, Strauss, Wada, & Purves, 1993). Researchers presume that the hypothesized cross-talk is mediated by the CC.
In right-handed healthy adults, the size of the CC was previously reported to correlate inversely with the magnitude of dual-task interference (Yazgan, Wexler, Kinsbourne, Peterson, & Leckman, 1996). In particular, subjects with relatively large callosal cross-sectional areas exhibited less between-hemispheres interference, although within-hemisphere interference did not change. One determinant of left-hand performance of individuals with TS might then be the size of the CC.
The frontal cortex may contribute to the left-hand performance advantage in healthy subjects by minimizing interference effects. The prefrontal cortex may contribute to performance on the dual-task laterality paradigm, as it underlies performance that overcomes cognitive interference (Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2003; Dreher & Grafman, 2003; Herath et al., 2001; Kensiger, Clark, & Corkin, 2003; Kondo, Morishita, et al., 2004; Kondo, Osaka, & Osaka, 2004; Marcantoni, Lepage, Beaudoin, Bourgouin, & Richer, 2004; Peterson et al., 1999, 2002; Schubert & Szameitat, 2003). Moreover, the prefrontal cortex is smaller in adult TS subjects than in control subjects (Peterson et al., 2001; Spessot, Plessen, & Peterson, 2004), and if this morphological abnormality has corresponding functional implications, it could contribute to impaired performance on the dual task.
A complementary way to assess interhemispheric communication is by requiring coordination between the hands. One can presume that the CC, which connects homotopic locations in the two hemispheres, would mediate interhemispheric communication in the service of coordination. Agenesis of the CC is reported to result in motor slowing, particularly on bimanual tasks (Lassonde & Jeeves, 1994; Sauerwein & Lassonde, 1994). Performance on a measure of interhemispheric communication has predicted differences between complex and simple bimanual coordination (Fagard, Hardy-Leger, Kervella, & Marks, 2001).
The measures used in the present study were selected to assess functional interhemispheric connectivity among a group of adults with TS. In particular, we assessed performance on the dual task (Kinsbourne & Hiscock, 1983) and on the bimanual coordination portion of the Purdue Pegboard (Tiffin, 1968). Additionally, we examined functional lateralization through laterality tests to detect any confounding by disturbances in functional asymmetries within the central nervous systems of persons with TS. Because recent imaging studies of several hundred subjects have shown that brain structures are not abnormally lateralized in TS children or adults (Peterson et al., 2001, 2003), we did not expect to find abnormal functional asymmetries in TS subjects.
On the basis of our prior findings that TS adults have enlarged CC cross-sectional areas (Plessen et al., 2004), our a priori hypothesis was that adults with TS would exhibit impaired performance on the dual task and on the bimanual portion of the Purdue Pegboard test. Because the prefrontal cortex is also deviant in size in adults with TS, we also anticipated a relation between its size and dual-task performance. On the basis of our prior work, the groups should not differ on measures of functional lateralization. We tested our hypotheses on performance measures from right-handed individuals only. We also performed preliminary analyses of a small sample of left-handed subjects with TS and left-handed controls.
Method
Subjects and Characterization
Thirty-eight subjects with TS were recruited from the Tic Disorder Specialty Clinic at the Yale Child Study Center. Thirty-four healthy control subjects were recruited from local advertisements and from a list of 10,000 names, purchased from a telemarketing company, of people from the same neighborhood as the TS subjects, on the basis of ZIP code. (For more details about the recruitment and description of subjects, see Peterson et al., 2001, 2003.) All subjects gave consent in writing and were paid to participate.
Subjects ranged in age from 18 to 63 years. For inclusion in the study, the TS group was required to meet the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) criteria for the disorder. Exclusion criteria for the TS group included the presence of another movement disorder or another psychiatric disturbance, other than attention-deficit/hyperactivity disorder (ADHD) or obsessive–compulsive disorder (OCD), that antedated the onset of tics in childhood. Exclusionary criteria for the control subjects were as follows: any history of tic disorder, OCD, ADHD, or current DSM–IV Axis I disorder. Additional exclusionary criteria for both groups included the following: history of seizures; head injury with associated loss of consciousness; substance abuse or dependence; and a full-scale IQ of less than 80, as measured by the Wechsler Adult Intelligence Scale—Revised (Wechsler, 1981).
We assessed socioeconomic status (SES) at the time of testing and at time of birth using the Hollingshead Index of Social Status (Hollingshead, 1975). We used SES at birth in analyses because it more accurately reflects SES of the TS subjects during the most influential periods of central nervous system development and because the SES of many adults with TS is lowered artificially by the occupational disability associated with their tic disorder. We assessed handedness using the Edinburgh Handedness Inventory (Oldfield, 1971). We determined neuropsychiatric diagnoses in all subjects through administration of the Schedule for Tourette's Syndrome and Other Behavioral Disorders. This structured interview includes the Schedule for Affective Disorders and Schizophrenia and more detailed sections on TS, OCD, and ADHD. We determined neuropsychiatric diagnoses through a best-estimate consensus procedure. A clinician (Bradley S. Peterson) evaluated the severity of tic and OCD symptoms using the Yale Global Tic Severity Scale (Leckman et al., 1989) and the Yale–Brown Obsessive–Compulsive Scale (Goodman et al., 1989). We assessed the severity of ADHD symptoms using the DuPaul–Barkeley ADHD rating scale (DuPaul, 1991).
In the TS group, 2 individuals (7%) had combined-type ADHD, 14 (50%) had OCD, and 2 (7%) had both comorbidities in their lifetime. At the time of the MRI scanning, 12 individuals (43%) had comorbid OCD, 1 had comorbid ADHD (3.6%), and none had both disorders. At the time of scanning, 17 subjects (60.7%) in the TS group were taking medication: traditional neuroleptics (n = 2, 7.1%), atypical neuroleptics (n = 1, 3.6%), alpha agonists (n = 2, 7.1%), selective serotonin reuptake inhibitors (n = 7, 25%), or tricyclics (n = 1, 3.6%). Control subjects were not taking any of these medications.
Neuropsychological Measures
We administered five measures of neuropsychological functioning. These were measures of interhemispheric connectivity (dual task, Purdue Pegboard) or cerebral lateralization (dichotic listening, line bisection, finger tapping).
Dual task
Dual tasks have been described in detail in earlier publications (for a review, see Kinsbourne & Hiscock, 1983). Subjects first completed a baseline motor-only task and then completed an interference task, in which they completed the same baseline task concurrently with a language task. Part B of the Trail Making Test (Reitan & Wolfson, 1987) served as the baseline task, and conjugating aurally presented irregular verbs into the past tense served as the concurrent language task in the interference condition (e.g., Yazgan et al., 1996). The time interval between verbs was 2–3 seconds. Subjects were instructed to try their best while performing the two tasks simultaneously and not to focus solely on one task. We recorded time to completion (measured in seconds). Subjects completed each task with each hand independently, which yielded four scores for each subject. We computed interference scores by subtracting the baseline score from the interference score for each hand and then dividing by the baseline score to yield right- and left-hand interference scores. Interference scores therefore controlled for individual variation in baseline performance. We also recorded errors in motor and language performance.
Purdue Pegboard
We asked subjects to place pegs in a board as quickly as possible and recorded the number of pegs correctly placed in 30 seconds. Subjects completed three conditions using first their dominant hand, then their nondominant hand, and finally both hands together. In each condition, subjects picked up pegs with the appropriate hand from a cup on the right or left side of the board and placed the pegs in the respective right or left column of holes in the board. The bimanual task required individuals to pick up a pin simultaneously from each cup and place it in the corresponding column of holes. Subjects performed the entire procedure twice, which yielded two scores for each condition. We computed scores for statistical analyses by averaging performance in each condition.
Fused word dichotic listening
Subjects listened to two words simultaneously (one presented in each ear) and indicated the word heard by circling one of four words written on a response sheet. The words differed only in their initial consonants. We recorded the number of words heard correctly in each ear as well as the number of errors. We computed scores reflecting lateralization in two ways. We first assessed the direction of cerebral lateralization for language in each subject, using the MISER algorithm (Halwes, 1990). The second algorithm quantified the degree of asymmetry by computation of a right-ear advantage (REA) score, calculated as the difference in number of words heard by each ear divided by the total number of words heard.
Line bisection
In the first portion of this task, subjects were presented with a horizontal line and asked to divide it precisely in half by drawing a vertical line through its midpoint. Five trials (each with a line of different length) were presented for completion with each hand, yielding 10 scores for each subject. We computed scores reflecting the degree of lateralization by subtracting the actual length of half the line from the length marked by the subject, dividing by the actual length of half the line, and multiplying by 100 to create a percentage deviation score. Negative scores indicated leftward deviation of the bisecting vertical line. In the second portion of the task, the examiner moved a pencil along the length of a horizontal line, and the subject indicated when the pencil reached midline, which thus allowed a comparison of performance with and without contributions from the motor system. During three trials, movement of the pencil began from the left side of the line; during three other trials, movement began from the right side. We computed percentage deviation scores on these trials as described above.
Finger tapping
Subjects used the index finger of their fisted hand to tap a key on a computer keyboard as many times as they could during a 10-second interval, for 10 trials, 5 with each hand. We computed scores by averaging performance with each hand over the 5 trials.
Anatomical Measures
We performed morphometric analyses on Sun Ultra 10 work stations using ANALYZE 7.5 software (Rochester, MN) while blind to subject characteristics and hemisphere (images were randomly flipped in the transverse plane prior to region definition). A second operator confirmed the accuracy of all procedures. Large-scale variations in image intensity were removed before the images were reformatted (Clarke et al., 1995; Peterson et al., 2000). We corrected head flexion and extension, rotation, and tilt prior to region definition using the anterior commissure–posterior commissure (AC-PC) commissure and standard midline landmarks. We used an isointensity contour function in conjunction with manual editing to isolate the cerebrum. Regions of interest were defined as follows.
CC
The slice containing the midline sagittal image was magnified eightfold in each in-plane dimension and filtered via anisotropic diffusion (κ = 10; iterations = 15). This slice was thresholded via an isointensity contour function and then edited manually to isolate the CC in sagittal cross-section.
Prefrontal cortical volumes
We divided the cerebral hemispheres using a curvilinear cubic splines plane (Peterson et al., 2001). We then subdivided each hemisphere using two-dimensional planes—one axial plane placed through the AC-PC line, and another placed tangent to the genu of the CC—to define the dorsal anterior prefrontal cortex (DAPFC) within each hemisphere as the tissue anterior to the genu of the CC and superior to the plane containing the AC-PC line. We examined this DAPFC brain region precisely because we were more interested in the cognitive and executive aspects of the dual-task performance (situated more anteriorly) than in the motor-planning aspects of the task (situated more posteriorly in premotor cortices). The validity of related parcellation schemes has previously been documented (Caviness, Meyer, Makris, & Kennedy, 1996; Filipek, Richelme, Kennedy, & Caviness, 1994; Jernigan, Press, & Hesselink, 1990; Jernigan & Tallal, 1990; Kennedy et al., 1998). Whole brain volume (WBV), used as a covariate in all statistical analyses to control for general scaling effects in the brain, was defined as all gray and white matter, together with CSF (cerebrospinal fluid) of the cortical sulci, ventricles, and cisterns. We included CSF spaces to minimize the effects of cerebral atrophy in the brain of older subjects.
Data Analysis
We evaluated performance on measures of interhemispheric connectivity, the dual task and Purdue Pegboard, for each test separately with repeated measures analysis of variance (ANOVA). The repeated measure was hand use (either right, left, or bimanual when appropriate). The between-subjects factor was diagnostic group, and this had two levels, right-handed controls (RNCs) and right-handed TS subjects (RTSs). We further evaluated the bimanual portion of the Purdue Pegboard test through ANOVA, with dominant-hand performance on finger tapping as a covariate to control for differences in simple motor speed. We inspected correlations of dependent measures with demographic variables (age, SES, IQ, and sex) to determine which demographic variables were appropriate for inclusion as covariates. Those variables that correlated significantly with the dependent measures were included simultaneously in statistical models for hypothesis testing. Additionally, we examined diagnoses of ADHD and OCD (either current or lifetime) as covariates to control for the effects of these comorbid conditions. We evaluated possible medication effects by including the dichotomously coded variable of medication versus no medication as a covariate in all final analyses. Final analyses included as covariates only those variables that were significant. We conducted identical analyses separately for subjects without diagnoses of ADHD or OCD and for subjects who were not taking medication.
We also evaluated separately performance on each lateralizing task. For the fused-word dichotic listening task, a one-way ANOVA evaluated differences among the diagnostic groups in REA and MISER scores. For the line bisection task, a repeated measures ANOVA with condition (right, left, or visual only) as the within-subject measure evaluated differences between the diagnostic groups in scores for percentage of deviation from midline. Finally, multiple regression analysis examined the degree to which performance on fused-word and line bisection tasks contributed to predicting the variance in dual-task interference difference scores.
Our a priori prediction was that the RTSs would evidence anomalous performance on the dual task and the bimanual portion of the Purdue Pegboard test, compared with the performance of RNCs. Our secondary prediction was that performance on lateralizing tasks would not differ significantly between groups, nor would it significantly predict variance in dual-task interference scores. Because the Purdue Pegboard task requires subjects to integrate the motor effectors but not to overcome cognitive interference or time-sharing demands, we did not expect in our study to find an association of performance on this task with measures of working memory performance, executive functioning, or volume of the prefrontal cortex. Post hoc analyses examined whether hand dominance significantly predicted performance on the dual task or the Purdue Pegboard. Additionally, we examined the performance of left-handed controls (LNCs) and left-handed TS subjects (LTSs) on these measures.
We separately evaluated associations of anatomical measures (CC cross-sectional area, volumes of left and right prefrontal cortices) with behavioral measures. For RNCs and RTSs, we regressed the total cross-sectional area of the CC on the interaction between diagnostic group and the difference between right- and left-hand dual-task interference scores, with WBV included as a covariate to control for overall scaling effects within the brain. We regressed prefrontal volumes on the interaction of left-hand interference scores with diagnostic group. We included full-scale IQ and WBV as covariates. We predicted a significant interaction between interference difference scores and diagnostic group on CC area. We also predicted a significant interaction between left-hand interference scores and diagnostic group for prefrontal volumes in each hemisphere.
All statistical models were hierarchically well formulated (i.e., all possible lower order terms had to be included in the model, regardless of their statistical significance). Statistical analyses were performed in SPSS, Version 11.0, and all significance values reported were of the two-sided type. The magnitude of reported effects is estimated with eta-squared.
Results
Preliminary analyses included (a) a comparison of demographic variables for the two diagnostic groups (Tables 1 and 2) and (b) assessments of correlations between demographic variables considered for inclusion as covariates and neuropsychological test performance (see Table 3). No significant differences were noted between the groups; variables that significantly correlated with outcome measures were included as covariates in appropriate analyses.
Table 1. Frequencies of Lifetime Diagnosis of Comorbid Disorders, Handedness, and Gender.
| Right-handed | Left-handed | |||||
|---|---|---|---|---|---|---|
| Diagnosis | Male | Female | Total | Male | Female | Total |
| Control group | 16 | 14 | 30 | 4 | 0 | 4 |
| TS only | 5 | 5 | 10 | 1 | 2 | 3 |
| TS + ADHD | 1 | 1 | 2 | 1 | 1 | 2 |
| TS + OCD | 8 | 6 | 14 | 1 | 3 | 4 |
| TS + ADHD/OCD | 2 | 0 | 2 | 1 | 0 | 1 |
Note. TS = Tourette syndrome; ADHD = attention-deficit/hyperactivity disorder; OCD = obsessive–compulsive disorder.
Table 2. Demographic Characteristics of Right-Handed Individuals With Tourette Syndrome and the Control Group.
| RTS | RNC | ||||||
|---|---|---|---|---|---|---|---|
| Variable | % | M | SD | % | M | SD | Significance |
| Gender (male) | 57 | 53 | χ2(1, N = 58) = 0.085, p = .771 | ||||
| Age (years) | 35 | 11.6 | 31 | 11.7 | F(1, 57) = 2.0, p = .17 | ||
| SES | 42 | 11.7 | 48 | 13.3 | F(1, 52) = 2.7, p = .11 | ||
Note. RTS = right-handed people with Tourette syndrome; RNC = right-handed control group; SES = socioeconomic status.
Table 3. Correlations of Neuropsychological Test Performance With Demographic Variables.
| Behavioral measure | Gender | Age | IQ | SES |
|---|---|---|---|---|
| Dual interference right hand | −.045
(.718) |
.105
(.392) |
.029
(.818) |
−.156
(.214) |
| Dual interference left hand | .102
(.406) |
.047
(.704) |
−.091
(.465) |
−.103
(.412) |
| Purdue Pegboard dominant hand | −.345**
(.005) |
−.262*
(.034) |
−.037
(.771) |
.319*
(.011) |
| Purdue Pegboard nondominant hand | −.267*
(.030) |
−.184
(.139) |
−.027
(.830) |
.122
(.342) |
| Purdue Pegboard bimanual | −.244*
(.049) |
−.197
(.114) |
.006
(.964) |
.215
(.090) |
| Dichotic listening REA score | .006
(.961) |
−.057
(.663) |
−.048
(.717) |
.067
(.620) |
| Dichotic listening MISER score | −.084
(.518) |
−.107
(.413) |
−.120
(.360) |
.102
(.444) |
| Line bisection percentage deviation right hand score | .173
(.165) |
.179
(.150) |
−.116
(.357) |
−.250*
(.048) |
| Line bisection percentage deviation left hand score | −.027
(.829) |
.113
(.367) |
.058
(.646) |
−.144
.259 |
| Line bisection percentage deviation visual only score | .079
(.528) |
−.119
(.341) |
−.246*
(.048) |
−.096
(.453) |
Note. Data are Pearson product correlation coefficients, with significance values in parentheses. SES = socioeconomic status; REA = right-ear advantage.
p < .05.
p < .01.
Interhemispheric Connectivity
The ANOVA for dual-task interference scores revealed a significant main effect of hand use, F(1, 54) = 14.83, MSE = 2296.5, η2 = .22, p < .0001, and a significant interaction of hand use with diagnostic group, F(1, 54) = 8.09, η2 = .13, p < .006, confirming our a priori hypothesis for dual-task scores (see Figure 1). Post hoc analyses demonstrated significantly greater interference in the control group for right-hand compared with left-hand performance (η2 = .60, p < .0001). In the RTS group, in contrast, interference during right- and left-hand performance did not differ significantly from one another (η2 = .01, p = .50). Although the magnitude of interference scores for right-hand performance did not differ between groups (η2 = .03, p = .17), interference scores for left-hand performance were significantly greater in the TS subjects compared with the controls (η2 = .08, p < .03).
Figure 1.
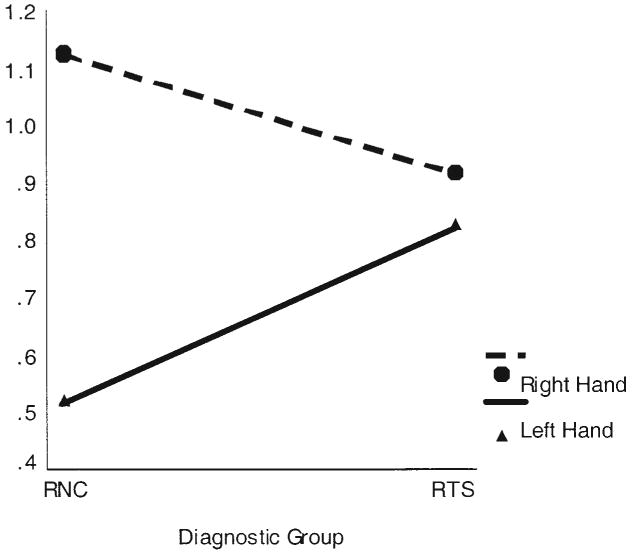
Right- and left-hand interference scores on the dual task by diagnostic group: right-handed subjects with Tourette syndrome (RTSs) and right-handed normal controls (RNCs).
For the Purdue Pegboard, a repeated measures analysis of covariance (ANCOVA) with hand use as a within-subject factor (right, left, bimanual), diagnostic group as a between-subjects factor (RNC, RTS), and one covariate (sex) detected significant main effects of hand use, F(2, 102) = 125.00, MSE = 0.8, η2 = .70, p < .0001; diagnostic group, F(1, 51) = 7.74, MSE = 4.9, η2 = .13, p < .008; and sex, F(1, 51) = 8.49, η2 = .14, p < .005; as well as a significant interaction of hand use with diagnostic group, F(2, 102) = 3.26, η2 = .06, p = .04, thus confirming our a priori hypothesis for Purdue Pegboard scores (see Figure 2). Performance with the dominant, right hand was significantly slower in the RTS group (η2 = .15, p < .004) than in the RNC group, but performance with the left hand did not differ significantly between groups (η2 = .05, p = .11). We also detected significantly slower bimanual performance in the RTS group (η2 = .09, p < .03).
Figure 2.
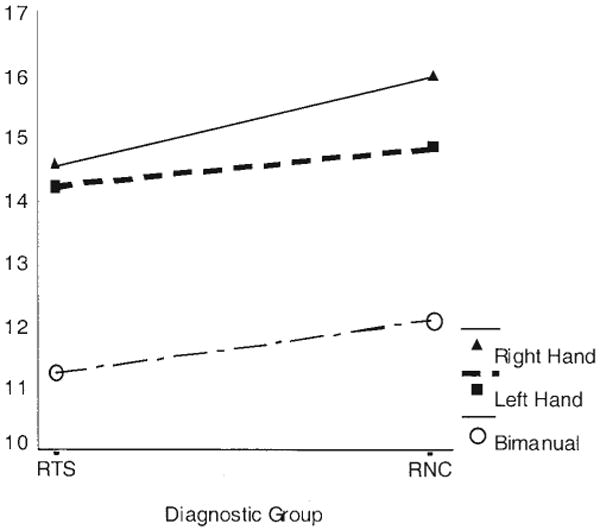
Mean number of pegs placed on the Purdue Pegboard task by each diagnostic group: right-handed subjects with Tourette syndrome (RTSs) and right-handed normal controls (RNCs).
Cerebral Lateralization
Performance on lateralizing tasks did not differ significantly between groups. On the dichotic listening test, an ANOVA with diagnostic group as the between-subjects factor did not detect significant differences in performance among groups on MISER or REA scores (η2 = .001, p = .99; and η2 = .001, p = .85, respectively). On the line bisection test, a repeated measures ANCOVA with condition as a within-subject variable (right hand, left hand, or visual only), diagnostic group as the between-subjects factor, and one covariate (SES) did not detect differences between the groups (η2 = .01, p = .44). On finger tapping, a repeated measures ANCOVA with condition as the within-subject variable (right hand or left hand), diagnostic group as the between-subjects factor, and two covariates (gender and full-scale IQ) did not detect significant differences between groups (η2 = .06, p = .09).
To assess any influence of cerebral lateralization on our measures of interhemispheric connectivity, we examined the degree to which behavioral laterality predicted performance on dual-task interference scores. We regressed difference scores for dual-task interference on four independent variables: diagnostic group; dominant, right-hand percentage deviation scores for the line bisection task; dominant, right-hand scores for the finger tapping task; and MISER scores for the dichotic listening task. Estimates for percentage deviation scores, finger tapping scores, and MISER scores were not significant (r = −.04, p = .75; r = .14, p = .66; and r = .20, p = .21, respectively), indicating that cerebral dominance in language, spatial, and motor abilities did not account significantly for variance in scores for dual-task interference. The estimate for diagnostic group remained significant (r = .32, p = .04).
Performance of Left-Handed Subjects
To examine preliminarily the performance of LNCs (n = 4) and LTSs (n = 10), we crossed diagnosis and handedness to yield four groups: RNCs, LNCs, RTSs, and LTSs. A repeated measures ANOVA of dual-task interference scores with hand use (right, left) as the repeated measure and group as a between-subjects factor detected a significant Hand Use × Group interaction, F(3, 64) = 5.15, η2 = .19, p < .003. Inclusion of MISER scores as a covariate did not alter the model, nor was the main effect of the covariate significant (η2 = .01, p = .42); thus, variation in language dominance did not account for differences in dual-task interference. In the control group, left- versus right-hand performance on the dual task differed significantly as a function of hand dominance. Interference scores differed significantly between use of the left and right hands in the RNC subjects (p < .0001) but not in the LNC subjects (p = .47). In contrast to this dependence of interference scores on hand dominance in the control subjects, performance with the two hands did not vary with hand dominance across the RTS and LTS groups (left-hand use, p = .47; right-hand use, p = .76). Furthermore, interference with use of the right hand did not differ significantly across the four groups (p = .27). A statistical trend toward differences across groups in interference scores with use of the left hand approached significance (p < .09), such that the LNC, LTS, and RTS subjects experienced greater interference with their left hand than did the RNC group. Graphing the means (see Figure 3) for interference scores in each group indicated that differences in interference scores across hands, on average, were in opposite directions in left-handed compared with right-handed subjects, regardless of diagnostic group.
Figure 3.
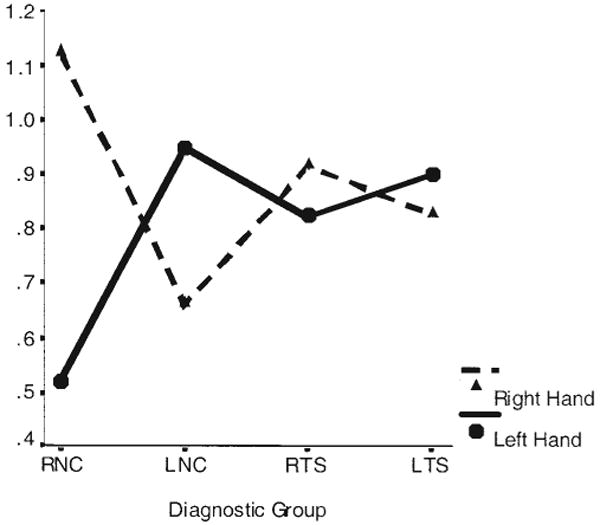
Right- and left-hand interference scores on the dual task by diagnostic group: right-handed subjects with Tourette syndrome (RTSs), left-handed subjects with Tourette syndrome (LTSs), right-handed normal controls (RNCs), and left-handed normal controls (LNCs).
We performed a similar analysis for measures from the Purdue Pegboard. A repeated measures ANCOVA with hand use (preferred, nonpreferred, bimanual) as the repeated measure, group as between-subjects factor, and sex as a covariate detected significant main effects of hand use, F(2, 122) = 103.40, MSE = 0.7, η2 = .63, p < .0001; group, F(3, 61) = 5.90, MSE = 4.7, η2 = .23, p < .001; and sex, F(1, 61) = 12.95, η2 = .18, p < .001. The interaction of hand use with group was not significant, F(6, 122) = 1.70, η2 = .07, p = .10. Across the four levels of group, we observed highly significant differences in hand use (ps < .0001). Performance with the preferred hand was significantly better than performance with the nonpreferred hand; unimanual performance was significantly better than bimanual performance. Across the three levels of hand use, the control groups performed better than the TS groups; the RNC group performed better than the RTS and LTS groups (ps < .006 and .002, respectively), as did the LNC group (ps < .02 and .005, respectively). Performance on lateralizing tasks did not differ significantly among groups. We detected no significant differences among the four levels of group in REA, MISER, or dominant hand percentage deviation scores for the line bisection task (ps = .43, .55, and .81, respectively), which indicates that abnormalities in cerebral lateralization did not characterize differences among the groups.
Neuroanatomical Correlates
We regressed the total cross-sectional area of the CC on the interaction of diagnostic group (RNC or RTS) with the measure of difference between right- and left-hand interference scores, along with the component main effects. We included WBV as a covariate to control for overall scaling effects in the brain. The interaction with diagnosis was not significant, (r = .18, p > .33), indicating no discernible effect of diagnosis on the association of CC size with left-hand advantage on the dual task. When we left the interaction term out of the model, the main effect of the difference scores was not significant (r = .03, p > .25). Nevertheless, the main effect of group was significant (r = −.10, p < .05), indicating a difference between groups in size of the CC (larger in the TS group), consistent with the findings from a larger sample of TS adults of which the present sample was a subset (Plessen et al., 2004).
We investigated the possibility that performance varied with the size of the prefrontal cortex. The prefrontal cortex subserves performance that requires the individual to overcome cognitive interference, such as the cognitive interference associated with published (nonlaterality) dual-task paradigms (Dove et al., 2003; Dreher & Grafman, 2003; Herath et al., 2001; Kensiger et al., 2003; Kondo, Morishita, et al., 2004; Kondo, Osaka, & Osaka, 2004; Marcantoni et al., 2004; Peterson et al., 1999, 2002; Schubert & Szameitat, 2003). We regressed volumes of the prefrontal cortex separately on the interaction between left-hand interference scores and diagnostic group, with full-scale and WBV included as covariates. The main effects of diagnostic group and interference scores were significant for both right-sided (r = .20, p = .02; and r = −.10, p = .01, respectively) and left-sided volumes (r = .26, p = .003; and r = −.10, p = .02, respectively). The significant main effect of group indicated that mean right and left DAPFCs were smaller in the TS group (47.3 cm3 and 48.6 cm3, respectively) than in the control group (53.3 cm3 and 56.0 cm3, respectively), just as they were in the larger sample of TS adults from which this sample was drawn (Peterson et al., 2001), which suggests that our sample is likely representative of this larger sample of symptomatic adults. We observed significant interactions of left-hand interference scores with diagnostic group for both right-and left-sided volumes (r = .04, p = .009; and r = .11, p = .02, respectively), which indicates that DAPFC volumes may account, in part, for group differences in left-hand interference scores. The direction of the effect was such that larger prefrontal volumes accompanied less dual-task interference in the RNC group (see Figure 4) but not in the RTS group (see Figure 5). In addition, both of the covariates, full-scale IQ and WBV, were significantly correlated with right-sided (r = .04, p = .001; and r = .69, p < .0001, respectively) and left-sided volumes (r = −.05, p < .0001; and r = .67, p < .0001, respectively).
Figure 4.
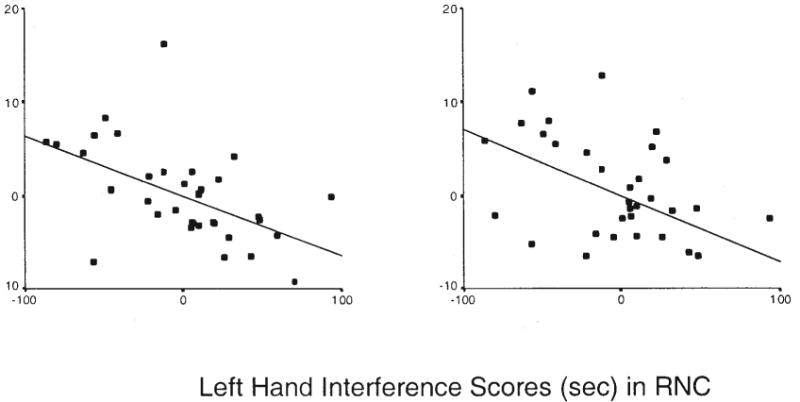
Partial correlation of left-hand interference scores with left and right dorsal anterior prefrontal cortex (DAPFC) volumes in right-handed normal controls (RNCs). Residual DAPFC volumes are adjusted for whole brain volume and full-scale IQ scores and thus can be positive or negative.
Figure 5.
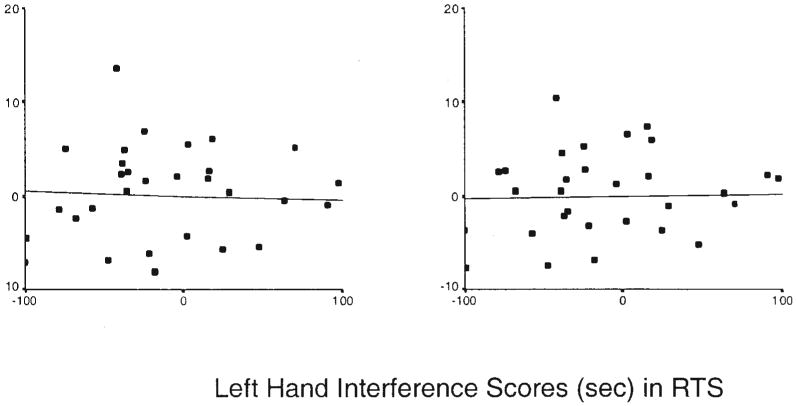
Partial correlation of left-hand interference scores with volumes of the left and right dorsolateral prefrontal cortex (DAPFC) volumes in right-handed Tourette syndrome subjects (RTSs). Residual DAPFC volumes are adjusted for whole brain volume and full-scale IQ scores and thus can be positive or negative.
Comorbidity and Medication Effects
A lifetime diagnosis of comorbid OCD or ADHD was not associated with performance on the dual task either as a main effect (η2 = .00, p > .96; and η2 = .04, p > .16, respectively) or as an interaction with performance (η2 = .00, p > .77; and η2 = .04, p > .14, respectively). Similarly, a lifetime diagnosis of comorbid OCD or ADHD was not associated with performance on the Purdue Pegboard either as a main effect (η2 = .01, p > .41; and η2 = .001, p > .99, respectively) or as an interaction with performance (η2 = .02, p > .38; and η2 = .01, p > .61, respectively).
The stability of parameter estimates and significance values for dual-task and Purdue Pegboard analyses including and excluding subjects with comorbid ADHD and OCD did not differ appreciably, which further suggests that comorbid illnesses had minimal influence on our findings. Lifetime diagnoses of comorbid ADHD or OCD also were not associated with performance on cerebral lateralization measures.
We evaluated medication effects in all final models, with no observable effects on our findings. Current use of medication was not associated with performance on the dual task as a main effect (η2 = .02, p > .32) or as an interaction (η2 = .02, p > .55,) or with performance on the Purdue Pegboard, either as a main effect (η2 = .02, p > .07) or as an interaction (η2 = .05, p > .12).
Discussion
Right-handed individuals with TS performed abnormally on both behavioral measures of interhemispheric connectivity. They showed as much between-hemispheres as within-hemisphere interference on the verbal–manual interference task—they were insufficiently able to inhibit interhemispheric interference between right-hemisphere motor regions and left-hemisphere language systems. They were slower on the bimanual portion of the Purdue Pegboard, and thus they were insufficiently able to integrate motor performance across hemispheres. Neuroanatomically, the TS group exhibited a larger CC in cross-section than did controls but smaller DAPFC volumes. To help discern how these anatomical deviations might relate to the observed deficits in performance, we assessed correlations of performance with anatomical measures.
Neuroanatomical Correlates
Performance on the behavioral tasks did not correlate significantly with size of the CC, and the association of CC size with performance measures did not differ significantly across diagnostic groups. We therefore did not confirm findings from our earlier study of 11 healthy adults, in which interference on the dual task correlated inversely with CC size (Yazgan et al., 1996). We regard the results of the present study as more valid than those of our prior study because our current sample size more than triples the prior sample size and because our current measures of the CC are superior to those of the older study, being obtained from true midline sagittal images on higher resolution MRI scans. Either the CC does not have the role we attributed to it, or its enlargement in TS has no functional implications (at least on the present variables).
Left-hand interference on the dual task did vary inversely with DAPFC volumes in the RNC group, though not in the RTS group. Right-hand performance did not correlate with DAPFC volumes in either group, which suggests that right-handed performance might not have benefited from contributions of the executive system, regardless of diagnosis. Perhaps the within-hemisphere functional interference associated with use of the right hand overwhelmed the regulatory capacities of the frontal executive system. Performance of the RTS group was similar when subjects used either the left or the right hand, which suggests the presence of a functional impairment in resolving the cognitive interference created by time-sharing tasks. The absence of a significant correlation of left-hand performance with DAPFC volume in RTS subjects may represent a floor effect, because in this group, the interference was as great between as within hemispheres.
The dual task requires both time sharing across tasks and the successful resolution of the cognitive interference that accompanies the simultaneous use of two cognitive systems, behaviors that the anterior prefrontal cortex is thought to subserve (Ramnani & Owen, 2004). The bimanual portion of the Purdue Pegboard test requires the coordination of two separate actions into a single, fluid movement and could also depend on executive functioning of the DAPFC. DAPFC volumes are smaller in adults with TS (Peterson et al., 2001; Spessot et al., 2004), and that was true in the present sample. Furthermore, smaller volumes of the DAPFC may reduce self-regulatory control and executive functioning in adults with TS (Peterson et al., 2001; Spessot et al., 2004). Our prior work indicates that reduced self-regulatory control associated with smaller prefrontal volumes in adults with TS contributes to more severe illness. A larger CC in those same subjects may compensate by bolstering activity in the otherwise insufficiently active prefrontal cortex. We detected both significantly larger CCs and smaller DAPFCs in the present sample. The greater interference that TS subjects experienced when they used the left hand in the dual task might have been caused by the presence of diminished prefrontal control of interhemispheric communication, which is itself mediated by a hypertrophied, though otherwise functionally normal, CC.
This interpretation of our findings is consistent with a large number of prior studies suggesting that the prefrontal cortex mediates the resolution of cognitive interference (Dove et al., 2000; Dreher & Grafman, 2003; Herath et al., 2001; Kensiger et al., 2003; Kondo, Morishita, et al., 2004; Kondo, Osaka, & Osaka, 2004; Marcantoni et al., 2004; Peterson et al., 1999, 2002; Schubert & Szameitat, 2003). It is also consistent with functional imaging studies that have suggested the participation of prefrontal cortex in the programming of sequential movements similar to those of the Purdue Pegboard (Lepage et al., 1999). Indeed, individuals with frontal lobe epilepsy have slower bimanual performance on the Purdue Pegboard than do healthy controls or subjects with temporal lobe epilepsy (Hernandez et al., 2002). Perhaps the RTS group also found bimanual coordination on the Purdue Pegboard difficult because of deficient assistance by prefrontal control systems.
Although we have assumed in our interpretation that our findings on this particular dual-task paradigm and the Purdue Pegboard arose from a common underlying cause (structural differences in the prefrontal cortex), it is possible that these findings instead derived from diverse and independent causes. Nevertheless, the findings and our interpretation of them are consistent with increasing evidence that disturbances in self-regulatory processes based within the prefrontal cortex and associated basal ganglia circuitry contribute to the behavioral disturbances and pathophysiology of TS. Longitudinal studies will reveal whether poor executive functioning emerges as a function of the persistence of the tic disorder or, conversely, contributes to its persistence. Unrelated to our a priori hypotheses but notable is the high correlation between DAPFC size and IQ, consistent with the recent emphasis on working memory and self-regulatory capacity as a major contributor to general intelligence (Duncan, Emslie, Williams, Johnson, & Freer, 1996; Pennington, 1994).
Handedness
Our small sample of left-handed subjects afforded preliminary information about the role of hand dominance. LNCs evidenced larger interference scores when using their left hand, a pattern of asymmetry on the dual task that was equal in magnitude but opposite in direction to that of the RNCs (see Figure 3). Whether this indicates representation of expressive language in the right cerebral hemisphere that would interfere with left-hand use awaits further study. LNCs and RNCs did not differ in receptive laterality in dichotic listening scores.
LTSs performed similarly to the RTSs on the dual task, regardless of hand use (see Figure 3). Neither of the TS handedness groups demonstrated a hand advantage on this task, and their interference scores were similar in magnitude to those for right-hand use in normal controls, for whom, again, the absence of a hand advantage may derive from impaired prefrontal control.
Limitations
Our results could have been confounded by differences in functional asymmetries in cerebral lateralization across diagnostic groups. We were unable to detect differences between the groups, however, in commonly used measures of cerebral lateralization, line bisection, and dichotic listening. Other possible confounds include influences on neuropsychological test performance in the TS sample from the effects of motor tics, motor side effects of tic-suppressant medications, or the effects of comorbid illnesses. Statistical modeling, however, was unaffected by inclusion of medication use, and findings persisted in analyses of subjects without comorbid illnesses or medication use, which suggests that medications and comorbid illnesses did not influence our findings appreciably.
Our sample represents only those people who have a lifetime diagnosis of TS; the symptoms of most children with TS substantially improve or remit by adulthood (Leckman et al., 1998). This constitutes an ascertainment bias associated with symptom persistence. Longitudinal studies of more representative nonclinical samples will determine whether the findings of this study are generalizeable to the larger population of individuals with TS.
Finally, functional imaging studies might reveal more directly than our morphological measures the contributions of the CC and prefrontal cortices to performance on interhemispheric tasks. Similarly, diffusion tensor imaging may more directly assess the disturbances in interaction of the DAPFC with the CC that our findings suggest. Nevertheless, interpreting correlations between brain measures and behavior is always difficult, including the correlations of functional MRI activations and performance, particularly when these correlations are used to discriminate patient and control groups who differ in their performance on baseline tasks or in their underlying brain anatomy.
Acknowledgments
This work was supported in part by National Institute of Mental Health Grants K02-74677, MH01232, MH59139, and MH068318; by the Suzanne Crosby Murphy Endowment at the Columbia University College of Physicians and Surgeons; and by the Thomas D. Klingenstein and Nancy D. Perlman Family Fund.
Contributor Information
Amy Margolis, Brooklyn Learning Center, Brooklyn, NY.
Mireille Donkervoort, Yale Child Study Center, New Haven, CT.
Marcel Kinsbourne, New School University, New York, NY.
Bradley S. Peterson, Division of Child and Adolescent Psychiatry, Department of Psychiatry, New York State Psychiatric Institute, and the College of Physicians and Surgeons, Columbia University
References
- Adcock RA, Constable RT, Gore JC, Goldman-Rakic PS. Functional neuroanatomy of executive processes involved in dual-task performance. Proceedings of the National Academy of Sciences, USA. 2000;97:3567–3572. doi: 10.1073/pnas.060588897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Caviness VSJ, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Clarke LP, Velthuizen RP, Camacho MA, Heine JJ, Vaidyanathan M, Hall LO, et al. MRI segmentation: Methods and applications. Magnetic Resonance Imaging. 1995;13:343–368. doi: 10.1016/0730-725x(94)00124-l. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995 November 16;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon YD. Prefrontal cortex activation in task switching: An event-related fMRI study. Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cerebral Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. Parent and teacher ratings of ADHD symptoms: Psychometric properties in a community-based sample. Journal of Clinical Child Psychology. 1991;20:245–253. [Google Scholar]
- Fagard J, Hardy-Leger I, Kervella C, Marks A. Changes in interhemispheric transfer rate and the development of bimanual coordination during childhood. Journal of Experimental Child Psychology. 2001;80:1–22. doi: 10.1006/jecp.2000.2623. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS. The young adult human brain: An MRI-based morphometric analysis. Cerebral Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Singer HS, Cooper KL, et al. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58:85–89. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. Journal of Psychosomatic Research. 2003;55(1):13–22. doi: 10.1016/s0022-3999(02)00581-0. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale–Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Halwes T. User's guide for the MISER program, MISER PC Version 5.2. New Haven, CT: Precision Neurometrics; 1990. [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: An fMRI study. Cerebral Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- Hernandez MT, Sauerwein HC, Jambaque I, DeGuise E, Lussier F, Lortie A, et al. Deficits in executive functions and motor coordination in children with frontal lobe epilepsy. Neuropsychologia. 2002;40:384–400. doi: 10.1016/s0028-3932(01)00130-0. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette's syndrome: A quantitative MRI study of monozygotic twins. Neurology. 1995;45:1176–1182. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Press GA, Hesselink JR. Methods for measuring brain morphologic features on magnetic resonance images. Validation and normal aging. Archives of Neurology. 1990;47:27–32. doi: 10.1001/archneur.1990.00530010035015. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Developmental Medical Child Neurology. 1990;32:379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Resolving dual-task interference: An fMRI study. Neuroimage. 2004;22:748–754. doi: 10.1016/j.neuroimage.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VSJ. Gyri of the human neocortex: An MRI-based analysis of volume and variance. Cerebral Cortex. 1998;8:372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- Kensiger E, Clark RJ, Corkin S. What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm. Journal of Neuroscience. 2003;23:2407–2415. doi: 10.1523/JNEUROSCI.23-06-02407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M, Cook J. Generalized and lateralized effects of concurrent verbalization on a unimanual skill. Quarterly Journal of Experimental Psychology. 1971;23:341–345. [Google Scholar]
- Kinsbourne M, Hicks RE. Functional cerebral space: A model for overflow, transfer and interference effects in human performance. In: Requin J, editor. Attention and performance VII. Hillsdale, NJ: Erlbaum; 1978. pp. 342–362. [Google Scholar]
- Kinsbourne M, Hiscock M. Asymmetries of dual-task performance. In: Hellige JB, editor. Cerebral hemisphere asymmetry: Method, theory, and application. New York: Praeger; 1983. [Google Scholar]
- Klingberg T, Roland PE. Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Cognitive Brain Research. 1997;6(1):1–8. doi: 10.1016/s0926-6410(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H. Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage. 2004;21:2–14. doi: 10.1016/j.neuroimage.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23:670–679. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Kosaka B, Hiscock M, Strauss E, Wada JA, Purves S. Dual task performance by patients with right or left speech dominance as determined by carotid amytal tests. Neuropsychologia. 1993;31:127–136. doi: 10.1016/0028-3932(93)90041-w. [DOI] [PubMed] [Google Scholar]
- Lassonde M, Jeeves MA, editors. Advances in Behavioral Biology. Vol. 42. 1994. Callosal agenesis: A natural split brain? pp. 207–219. [Google Scholar]
- Leckman J, Riddle M, Hardin M, Ort S, Swartz K, Sevenson J, Cohen D. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: The first two decades. Pediatrics. 1998;102(1):14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Lepage M, Beaudoin G, Boulet C, O'Brien I, Marcantoni W, Bourgouin P, et al. Frontal cortex and the programming of repetitive tapping movements in man: Lesion effects and functional neuroimaging. Cognitive Brain Research. 1999;8(1):17–25. doi: 10.1016/s0926-6410(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Marcantoni WS, Lepage M, Beaudoin G, Bourgouin P, Richer F. Neural correlates of dual task interference in rapid visual streams: An fMRI study. Brain and Cognition. 2004;53:318–321. doi: 10.1016/s0278-2626(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: Data and theory. Psychological Bulletin. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Hurst CR. Schedule for Tourette and other behavioral syndromes. New Haven, CT: Yale University Child Study Center; 1996. [Google Scholar]
- Pennington BF. The working memory functions of the prefrontal cortices. In: Haith MM, Benson JB, Roberts RJ, Pennington BF, editors. The development of future-oriented processes. Chicago: University of Chicago Press; 1994. pp. 243–289. [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event related functional MRI study comparing interference effects in Stroop and Simon tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, et al. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in chronic tic, obsessive-compulsive, and attention deficit/hyperactivity disorder. Archives of General Psychiatry. 2000;57:364–372. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, et al. Reduced basal ganglia volumes in Tourette's syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43:941–949. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of General Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, et al. Regional brain and ventricular volumes in Tourette syndrome. Archives of General Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang Z, Bronen R, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Archives of General Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, et al. Altered interhemispheric connectivity in individuals with Tourette's disorder. American Journal of Psychiatry. 2004;161:2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen MA. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead–Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1987. [Google Scholar]
- Roland PE, Zilles K. Structural divisions and functional fields in the human cerebral cortex. Brain Research Review. 1998;26(2–3):87–105. doi: 10.1016/s0165-0173(97)00058-1. [DOI] [PubMed] [Google Scholar]
- Sauerwein HC, Lassonde M. Cognitive and sensori-motor functioning in the absence of the corpus callosum: Neuropsychological studies in callosal agenesis and callosotomized patients. Behavioural Brain Research. 1994;64:229–240. doi: 10.1016/0166-4328(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Schubert T, Szameitat AJ. Functional neuroanatomy of interference in overlapping dual tasks: An fMRI study. Cognitive Brain Research. 2003;17:733–746. doi: 10.1016/s0926-6410(03)00198-8. [DOI] [PubMed] [Google Scholar]
- Singer HS, Reiss AL, Brown JE, Aylward EH, Shih B, Chee E, et al. Volumetric MRI changes in basal ganglia of children with Tourette's syndrome. Neurology. 1993;43:950–956. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- Spessot AL, Plessen KJ, Peterson BS. Neuroimaging of developmental psychopathologies: The importance of self-regulatory and neuroplastic processes in adolescence. In: Dahl RE, Patia Spear L, editors. Annals of the New York Academy of Sciences: Vol 1021. Adolescent brain development: Vulnerabilities and opportunities. New York: New York Academy of Sciences; 2004. pp. 86–104. [DOI] [PubMed] [Google Scholar]
- Szameitat A, Schubert T, Muller K, von Cramon D. Localization of executive functions in dual-task performance with fMRI. Journal of Cognitive Neuroscience. 2002;14:1184–199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue Pegboard: Examiner manual. Chicago: Science Research Associates; 1968. [Google Scholar]
- Wechsler D. The Wechsler Adult Intelligence Scale—Revised manual. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- Yazgan MY, Wexler BE, Kinsbourne M, Peterson B, Leckman FJ. Functional significance of individual variations in callosal area. Neuropsychologia. 1996;33:769–779. doi: 10.1016/0028-3932(95)00018-x. [DOI] [PubMed] [Google Scholar]


