Abstract
TNF-related apoptosis-inducing ligand (TRAIL, also known as Apo-2L) is a promising novel anticancer agent that selectively induces apoptosis in tumour cells and the activity of which can be enhanced by combined treatment with chemo- or radiotherapy. For therapeutic purposes, the use of full-length TRAIL may be favourable to recombinant TRAIL based on its increased tumour cell killing potential, and the delivery of TRAIL at the tumour site by adenovirus vectors may provide an approach to overcome the short half-life of recombinant TRAIL and hepatocyte toxicity in vivo. Here, we constructed an adenoviral vector expressing full-length TRAIL (AdTRAIL) and studied the potential of chemo- and radiotherapy in enhancing AdTRAIL-induced apoptosis in non-small cell lung cancer (NSCLC) H460 cells and normal cells and, in addition, investigated the mechanism of AdTRAIL-induced apoptosis. AdTRAIL effectively killed H460 cells, which we previously showed to have a deficiency in mitochondria-dependent apoptosis by downstream activation of caspase-8 rather than caspase-9. Further analyses revealed that AdTRAIL induces death receptor- and mitochondria-dependent apoptosis that could be partially suppressed by Bcl2 overexpression. Combined treatment with doxorubicin (DOX), cisplatin (CDDP), paclitaxel (PTX) and radiation strongly enhanced AdTRAIL-induced cytotoxicity in a synergistic way. Synergy was accompanied by the cleavage of Bid and an increase in caspase-8 processing that was abolished by Bcl2 overexpression, indicating that the Bid-mitochondrial amplification loop is functional in H460 cells. Moreover, combination treatment did not alter the tumour selectivity of AdTRAIL since normal human fibroblasts (NHFs) remained resistant under these conditions. These findings further indicate that the combined use of chemo/radiotherapy and adenovirus-produced full-length TRAIL may provide a valuable treatment option for NSCLC.
Keywords: NSCLC, full length TRAIL, adenovirus, synergy, Bcl-2
The treatment of advanced cancer, including non-small-cell lung cancer (NSCLC), is often hampered by the intrinsic or developing resistance against the anticancer drugs used (De Vita et al, 2001). In this context, deregulated apoptosis in cancer can contribute to drug resistance since the apoptosis-inducing ability of therapeutic agents is at least partially responsible for drug efficacy. Efforts to circumvent drug resistance include the combined use of different drugs with different mechanisms of action to enhance the overall antitumour effect, which for example can be based on the activation of distinct or overlapping apoptotic pathways that in a cooperative manner can trigger apoptosis more effectively.
TNF-related apoptosis-inducing ligand (TRAIL, also known as Apo-2L) represents a novel promising anticancer agent whose activity is solely dependent on its ability to induce apoptosis in tumour cells (Ferreira et al, 2002; MacFarlane, 2003). Unlike other members of the TNF super family (TNF and FasL), TRAIL acts as a specific antitumour agent without harming normal cells (Huang et al, 2002; Lin et al, 2002a, 2002b). TNF-related apoptosis-inducing ligand is a type-II transmembrane protein and shows the highest homology to FasL. The extracellular domain of TRAIL forms a soluble molecule upon cleavage (Wiley et al, 1995; Pitti et al, 1996). Both soluble and full-length TRAIL bind to their cognate cell surface receptors in the target cell to engage the apoptotic pathway (Ashkinazi et al, 1999) although the two TRAIL variants have been reported to possess different apoptosis-inducing capacities in cancer cells suggesting currently unresolved differences in their mechanism of action (Voelkel-Johnson et al, 2002; Seol et al, 2003). Of the five TRAIL receptors identified so far, TRAIL-R1 (DR4), TRAIL-R2 (DR5/TRICK/KILLER) and TRAIL-R4 (DcR1/TRUNDD) encode classical type-I transmembrane proteins (Pan et al, 1997a, 1997b). The TRAIL-R1 and -R2, which signal for apoptosis, have a complete cytoplasmic death domain (DD) while R3 and R4 act as decoy receptors having either no or a truncated cytoplasmic domain, respectively.
In animal experiments, TRAIL did not cause systemic toxicity (Kagawa et al, 2001). It was however reported that recombinant his-tagged TRAIL could cause toxicity to human, but not murine - or non-human primate hepatocytes in vitro (Jo et al, 2000), which has been assigned to conformational changes in TRAIL structure caused by the histidine tag (Lawrence et al, 2001). Apart from the issue of hepatotoxicity, soluble TRAIL has demonstrated a potent antitumour activity against a wide range of tumours both in vitro and in vivo (Pitti et al, 1996; Nimmanapalli et al, 2001; Rohn et al, 2001; Naka et al, 2002).
The applicability of soluble TRAIL in cancer therapy, however, is limited by its short half-life in vivo (Kelley et al, 2001) that may be overcome by the production of TRAIL at the tumour site by for example a nonreplicating adenoviral vector, which will also reduce the risk of hepatotoxicity (see also Griffith et al, 2000; Lin et al, 2002a).
The mechanism of TRAIL-induced apoptosis has been studied and the majority of cells can be classified as type I, that is, TRAIL-induced apoptosis is solely mediated by the death receptor pathway (Walczak et al, 2000). In this case, the binding of TRAIL to its death receptors triggers the aggregation of the death-inducing signal complex (DISC) with subsequent caspase-8 activation and the activation of executioner caspases and consequently irreversible apoptosis. On the other hand, type-II cells, including several colon carcinoma, neuroblastoma and NSCLC cell lines, are characterized by a strong involvement of the mitochondrial pathway via the caspase-8-dependent activation of the proapoptotic Bcl2 family member Bid, also known as the amplification loop (Sun et al, 2001; Fulda et al, 2002; Ozoren and El-Deiry, 2002). In addition, the combined treatment with different chemotherapeutic agents or ionising radiation is known to enhance the antitumour activity of soluble TRAIL in additive or synergistic manners, both in vitro and in vivo models, and has been related to the increased activation of the mitochondrial pathway but also to the enhanced expression of TRAIL receptors (Gibson et al, 2000; Held and Schulze-Osthoff, 2001; Mitsiades et al, 2001; Xu et al, 2003).
In this study, we examined the apoptosis-inducing effect of a constructed adenoviral vector expressing full-length TRAIL in the NSCLC cell line NCI-H460 and in normal cells when applied alone or in combination with different types of chemotherapeutic agents and radiation. It should be noted that NSCLC cells have a deficiency in the mitochondrial apoptotic pathway in that caspase-8 is activated rather than caspase-9 caused by a yet unknown disturbance in apoptosome functioning (Ferreira et al, 2000). Owing to this and the relative lack of knowledge on the mechanism underlying full-length TRAIL-induced apoptosis, we also addressed the functioning of the mitochondria amplification loop in this context.
Our findings indicate that adenoviral expression of full-length TRAIL in combination with chemo/radiotherapy may provide an effective and selective strategy for the treatment of NSCLC.
MATERIALS AND METHODS
Cell culture and treatment
Human non-small-cell lung adenocarcinoma (NSCLC) NCI-H460 cells and Bcl2 stable overexpressing derivatives (H460Bcl2, described earlier (Ferreira et al, 2000) were cultured in RPMI 1640 medium (Invitrogen, Breda, The Netherlands) and normal human fibroblasts (NHF) in Nutrient mixture F10 (Invitrogen). Both media were supplemented with 10% heat-inactivated foetal calf serum (Invitrogen, Breda, The Netherlands), 50 IU ml−1 penicillin, 50 μg streptomycin and 1 μg ml−1 puromycin (only for H460Bcl2 cells) and cells were grown at 37°C in a humidified air with 5% CO2. Cell lines were routinely tested for the absence of mycoplasma infection before use. The expression of Bcl2 protein was confirmed by immunohistochemistry before starting the experiments (data not shown). For optimal adenoviral infection, near-confluent cell cultures were used throughout the study. Cells were treated with doxorubicin (DOX, purchased from Pharmacia Upjohn BV (Woerden, The Netherlands), paclitaxel (PTX, purchased from Sigma, Zwijndrecht, The Netherlands), cisplatin (CDDP, purchased from Pharma Chemie BV, Harlem, The Netherlands) or 6 Gy ionising radiation (80-kV orthovolt X-ray source (Pantak Therapax SXT 150)). For caspase inhibition, the synthetic inhibitor zVAD-fmk (Enzyme System Products, Livermore, CA, USA) was used.
Adenovirus construction
The TRAIL open reading frame (ORF), kindly provided by Dr H Yagita, Juntendo University School of Medicine, Japan, was cloned under the control of the immediate early cytomegalovirus (CMV) promoter in the pShuttle plasmid and subsequently recombined with the pAdeasy plasmid for the production of replication incompetent AdTRAIL The adenovirus vectors were propagated in 293 cells and purified by CsCl density gradient. The viral preparations were dialysed and stored at −80°C until use.
The titer of AdTRAIL stock, as determined by the limiting dilution assay, was 3.5 × 109 plaque forming unit (pfu)/ml and the viral particle to pfu ratio measured at OD260 was less than 60. The adenovirus stocks were free of replication competent adenovirus (RCA) as tested by PCR using primers flanking the AdE1A region (Abou El Hassan et al, 2003). The adenovirus expressing green fluorescence protein (GFP) was used as a control and was described earlier (Van Beusechem et al, 2000).
Infection and cytotoxicity measurement
H460, H460Bcl2 and NHF cultured in 96-well plates were incubated with different multiplicity of infection, that is, virus to cell ratio (MOI) of AdTRAIL as indicated in growth medium (50 μl/well) at 37°C. At 2 h postinfection, another volume of virus-free growth medium was added. Cytotoxicity of AdTRAIL alone or combined with other treatments was determined by MTT assays as described previously (Abou El Hassan et al, 2003a).
The percentage survival (taking the blank as 100% survival) was plotted as a function of MOI or drugs concentration. The LC50 values of each drug with (out) preinfection with AdTRAIL – that is, the concentration of drug required to kill 50% of the cultured cells – were determined and the derived sensitisation factors were calculated as the ratio of the LC50 of drug alone/LC50 of drug with AdTRAIL infection.
Western blotting
Treated cells were lysed 24, 48 and 72 h postinfection with RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1%. SDS, 0.5% sodium deoxycholate (DOC, Fluka Biochemika, Buchs, Switzerland) and 1% nonidet P40 (NP40, Fluka Biochemika)). The cellular lysates were immediately stored at −80°C. Protein (50 μg) of each sample (determined by Biorad total protein assay (Bio-Rad Laboratories BV, Veenendaal, The Netherlands) were separated on a 12.5% SDS–PAGE. Thereafter, proteins were blotted on Immobilon membrane (Millipore BV, Etten-Leur, The Netherlands) and subsequently incubated in blocking solution containing 5% nonfat milk in TBST (0.2% Tween 20, 150 mM NaCl and 10 mM Tris-HCl, pH 8).
The following primary antibodies were used: rabbit polyclonal anti-human TRAIL (dilution 1 : 1000; PeproTechLTD, London, UK), rabbit polyclonal anti PARP (dilution 1 : 2000; Roche, Basel, Switzerland), anti-caspase 8 mAb (dilution 1 : 2000; Immunotech, Prague, Czech Rep), rabbit polyclonal anti-Bid (dilution 1 : 2000; Roche) and anti-β-actin mAb (dilution 1 : 7500; Sigma, St Louis, MO, USA). After incubation for 1–2 h with the primary antibody and washing in TBST, the blots were incubated with peroxidase-conjugated goat anti-rabbit/rabbit anti-mouse 2ry antibody (DAKO, Glostrup, Denmark) (1 : 1250 dilution). For chemoluminescence detection, blots were immersed in Lumi-light plus mix (Roche) and exposed to hyperfilm (Amersham Pharmacia UK Ltd., Bukinghamshire, UK).
Immunohistochemistry
H460 and NHF cells cultured in 96-well plate were infected with AdTRAIL (MOI 100). At 3 days postinfection, cells were fixed with chilled methanol : acetone mix (1 : 1, v v−1). TNF-related apoptosis-inducing ligand expression was monitored with rabbit polyclonal anti-human TRAIL antibody (dilution 1 : 200; PeproTechLTD), for 1 h at 37°C. After washing with PBS, cells were incubated with peroxidase-conjugated goat anti-rabbit secondary antibody. The stained cells were visualised using AEC substrate chromogen (DAKO).
RESULTS
Construction and characterisation of AdTRAIL
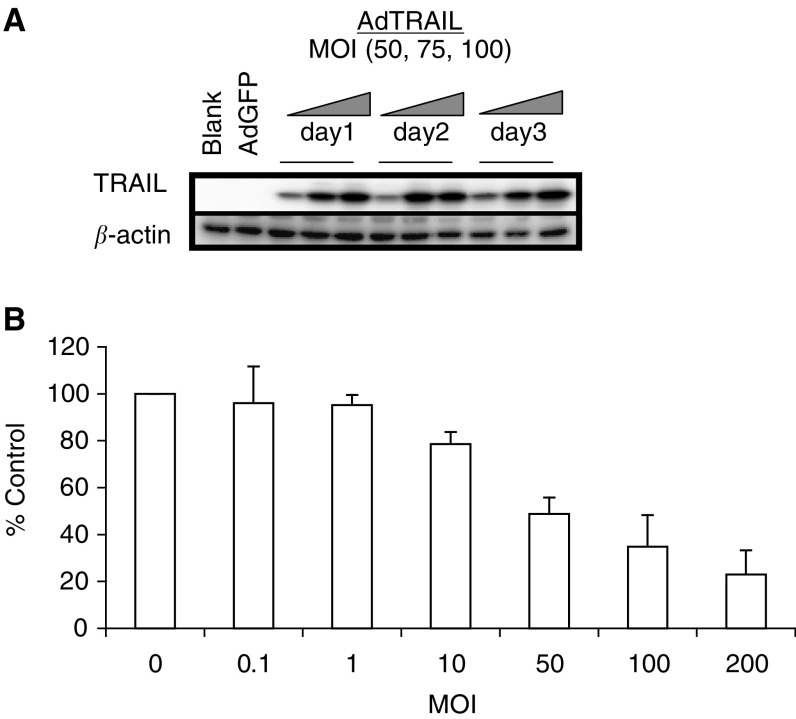
An adenoviral vector expressing full-length TRAIL was constructed as described in the Materials and Methods section. Figure 1A shows the expression of TRAIL in H460 cells followed up to 3 days postinfection with different MOIs of AdTRAIL. The cellular expression of TRAIL was MOI dependent and reached its maximal level at 1 day postinfection. Further increases in TRAIL expression were likely limited by the toxic effect of TRAIL on the infected producer cells. The MOI-dependent killing of H460 cells by AdTRAIL was confirmed 3 days postinfection by MTT assays as shown in Figure 1B.
Figure 1.
TNF-related apoptosis-inducing ligand expression and survival of Ad-TRAIL-infected H460 cells. Cells were infected with different MOIs of AdTRAIL, and TRAIL expression was determined by Western blotting at 1, 2 and 3 days postinfection (A). Survival of infected cells was determined by MTT assays at 3 days postinfection (B).
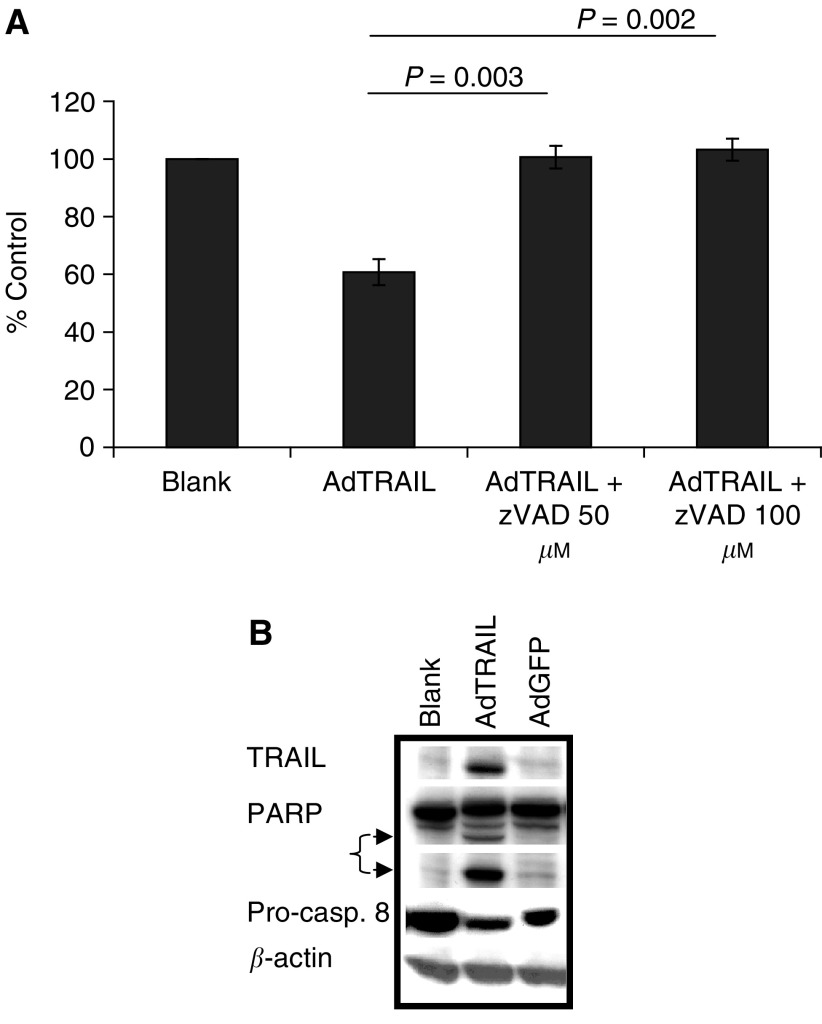
To demonstrate apoptosis activation triggered by adenovirus-produced TRAIL, we tested whether the broad caspase inhibitor zVAD-fmk would protect against AdTRAIL toxicity. A complete abrogation of AdTRAIL-induced apoptosis was observed by cotreating H460 cells with different concentrations of zVAD-fmk at 3 days posttreatment (Figure 2A), indicating the sole involvement of caspases in mediating AdTRAIL-induced apoptosis. To further characterise caspase dependency of AdTRAIL-induced apoptosis, the cleavage of procaspase-8 and PARP was determined in H460 cells 3 days postinfection with AdTRAIL by Western blotting. AdTRAIL specifically induced procaspase-8 and PARP cleavage as indicated in Figure 2B, when compared to uninfected or AdGFP-infected cells.
Figure 2.
AdTRAIL induces caspase-dependent apoptosis in H460 cells. H460 cells were treated with AdTRAIL (MOI 10) in the presence or absence of zVAD-fmk, and after 3 days postinfection cell viability was determined (A). Values are the mean (n=3)±s.d., and P-values were determined by the Student's t-test. Western blot indicating the cleavage of procaspase-8 and PARP (B). Arrows indicate the cleaved products of PARP.
Combination treatment of AdTRAIL with chemo(radio)therapy
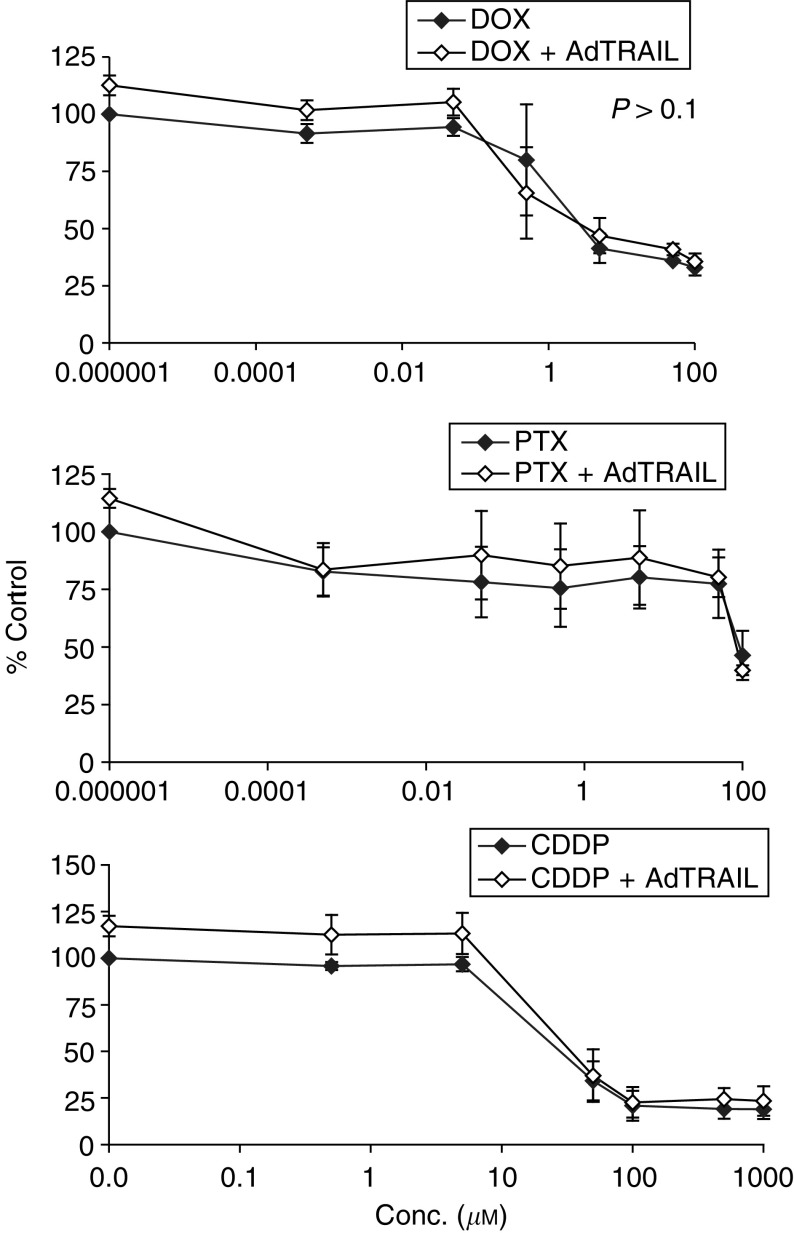
Figure 3 shows the combined effect of different types of chemotherapeutic drugs, DOX, PTX, CDDP or 6 Gy irradiation with AdTRAIL (MOI 10) on the viability of H460 cells at 3 days after treatment. Under these conditions, AdTRAIL caused moderate cell killing in H460 cells with a viability of 78.6±6.9% of control noninfected cells. Doxorubicin, PTX or CDDP alone induced a concentration-dependent cell kill of H460 cells with LC50 values of 1.5, 0.3 and 15 μM, respectively (see also Table 1 ). Interestingly, the combined treatment with subtoxic concentrations of DOX, PTX or CDDP already sensitised H460 cells to AdTRAIL-induced apoptosis (P⩽0.05, Figure 3). Accordingly, the LC50 values of DOX, PTX and CDDP were reduced 100-, 150- and 10-fold, respectively (Table 1). Likewise, the use of 6 Gy ionising radiation, which did not result in cytotoxicity in H460 cells, augmented the apoptotic effect of AdTRAIL in H460 cells at 3 days postinfection.
Figure 3.
Cytotoxic effect of AdTRAIL (MOI 10) alone or in combination with different concentrations of DOX, PTX, CDDP or 6 Gy irradiation in H460 cells at 3 days postinfection. Values are the mean (n=3)±s.d. The P-values were calculated by comparing the slopes of the multiple regression of ln (viability2) of H460 cells treated with AdTRAIL alone or combined with DOX, PTX or CDDP vs ln (drug conc.2) by Student's t-test. For the irradiated groups, the P-value was calculated by comparing the viability of cells treated with AdTRAIL alone or in combination with 6 Gy irradiation using Student's test.
Table 1. AdTRAIL-induced sensitisation of H460, H460Bcl2 and NHF cells to DOX, PTX or CDDP 2 days after treatment.
|
LC50 (mM) |
||||||
|---|---|---|---|---|---|---|
|
DOX |
PTX |
CDDP |
||||
| − | +AdTRAIL | − | +AdTRAIL | − | +AdTRAIL | |
| H460 | 1.5 | 0.015 | 0.3 | 0.002 | 15 | 1.5 |
| SFa | 100 | 150 | 10 | |||
| H460Bcl2 | 0.5 | 0.25 | 0.1 | 0.005 | 15 | 15 |
| SFa | 2 | 20 | 1 | |||
| NHF | 3 | 3 | 90 | 85 | 28 | 35 |
| SFa | 1 | 1.1 | 0.8 | |||
Sensitisation factor.
DOX=doxorubicin; CDDP=cisplatin; PTX=paclitaxel; LC50=the concentration of drug required to kill 50% of the cultured cells; NHFs=normal human fibroblasts.
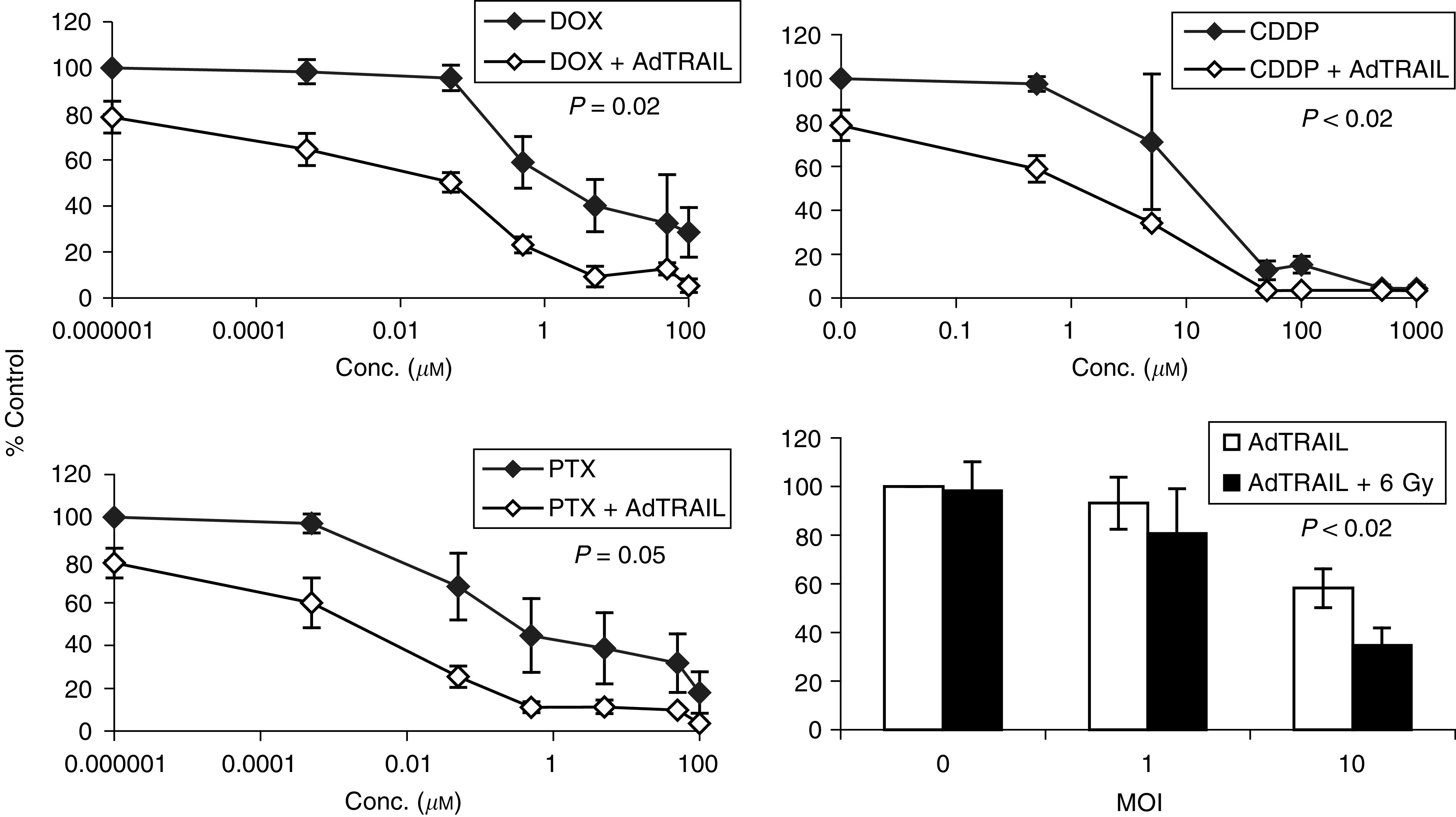
In order to study whether the mitochondria amplification loop mediates the observed synergistic effects in the mitochondria/caspase-9 pathway impaired H460 cells, Bcl2-overexpressing cells (H460Bcl2) were treated with AdTRAIL alone or combined with chemotherapy or irradiation. The overexpression of Bcl2 significantly protected H460 cells against AdTRAIL-induced apoptosis (compare Figures 3 and 4).
Figure 4.
Cytotoxic effect of AdTRAIL (MOI 10) alone or in combination with different concentrations of DOX, PTX, CDDP OR 6 Gy irradiation in H460Bcl2 cells at 3 days postinfection. Values are the mean (n=3)±s.d. The P-value was calculated by comparing the slopes of the multiple regression of ln (viability2) of H460Bcl2 cells treated with AdTRAIL alone or combined with DOX, PTX or CDDP vs ln (drug conc.2) by Student's t test. For the irradiated groups, the P-value was calculated by comparing the viability of cells treated with AdTRAIL alone or in combination with 6 Gy irradiation using Student's test.
In addition, H460Bcl2 cells could not be sensitised to AdTRAIL by DOX, CDDP and 6 Gy-irradiation, whereas the sensitising effect of PTX was strongly reduced but not completely abolished (P>0.1, Figure 4 and Table 1). As control, the combined treatment with chemo (radio)therapy did not sensitise H460 cells infected with the AdGFP (MOI 100) control virus (data not shown).
Bcl2 overexpression prevents caspase-8 activation and Bid cleavage
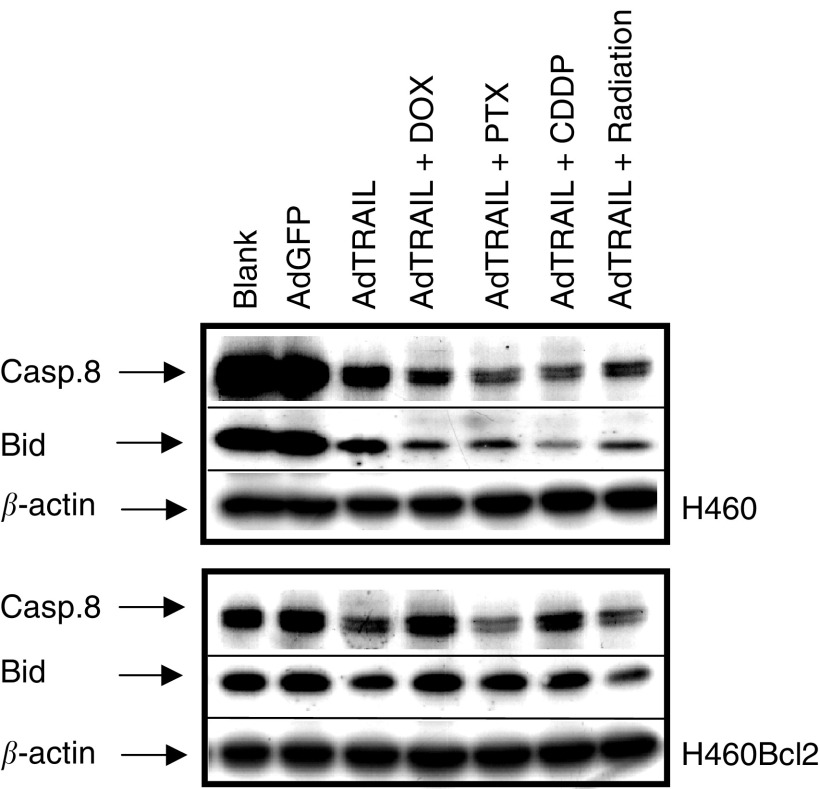
To further examine the role of Bcl2 in preventing the potentiating effect of chemotherapy and ionising radiation to AdTRAIL-induced apoptosis, we studied the cleavage of procaspase-8 and Bid in H460 and H460Bcl2 cells after infection with AdTRAIL (MOI 10) alone or in combination with 15 nM DOX or PTX, 15 μM CDDP or 6 Gy irradiation 3 days postinfection. Figure 5 shows that control adenovirus infection (AdGFP) did not induce caspase-8 activation or Bid cleavage in H460 and H460Bcl2 cells as indicated by the constant levels of the nonprocessed forms. AdTRAIL infection induced caspase-8 and Bid cleavage, which was enhanced upon combined treatment with chemo (radio) therapy in H460 cells. The overexpression of Bcl2 almost completely prevented the cleavage of Bid in cells treated with AdTRAIL alone and when combined with chemo (radio) therapy, and also reduced procaspase-8 cleavage. This indicates that the lack of chemo/radiation-dependent sensitisation of H460Bcl2 cells to AdTRAIL is related to the Bcl2-dependent prevention of the enhanced processing of procaspase-8 and Bid.
Figure 5.
Bid and procaspase-8 cleavage in H460 and H460Bcl2 cells 3 days postinfection with AdTRAIL (MOI 10) with (out) 15 nM of DOX or PTX, 15 μM CDDP or 6 Gy irradiation.
Effect of AdTRAIL combination treatment on normal cells
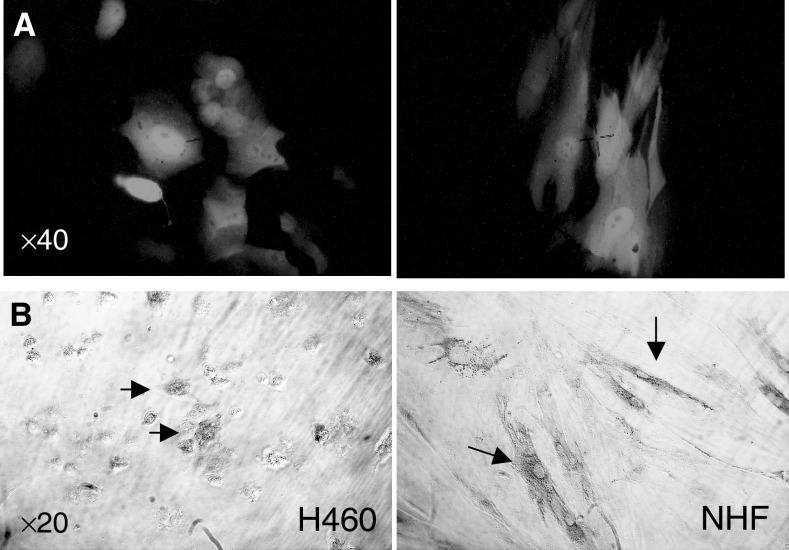
To study the possibility that under conditions of chemotherapy enhanced Ad TRAIL-induced H460 cell killing the sensitivity to normal cells could also be altered, we treated NHF cells in the same way and determined cytotoxicity. For this purpose, first the infection efficiency of NHF was examined. Figure 6A shows a considerable level of AdGFP infection at MOI 100 in NHF cells of approximately 20%, whereas H460 cells demonstrated an infection efficiency of around 70%.
Figure 6.
AdGFP and AdTRAIL infection of H460 and NHF cells. Cells were infected at MOI 100, and after 3 days, GFP expression (A) or TRAIL expression (B) was determined.
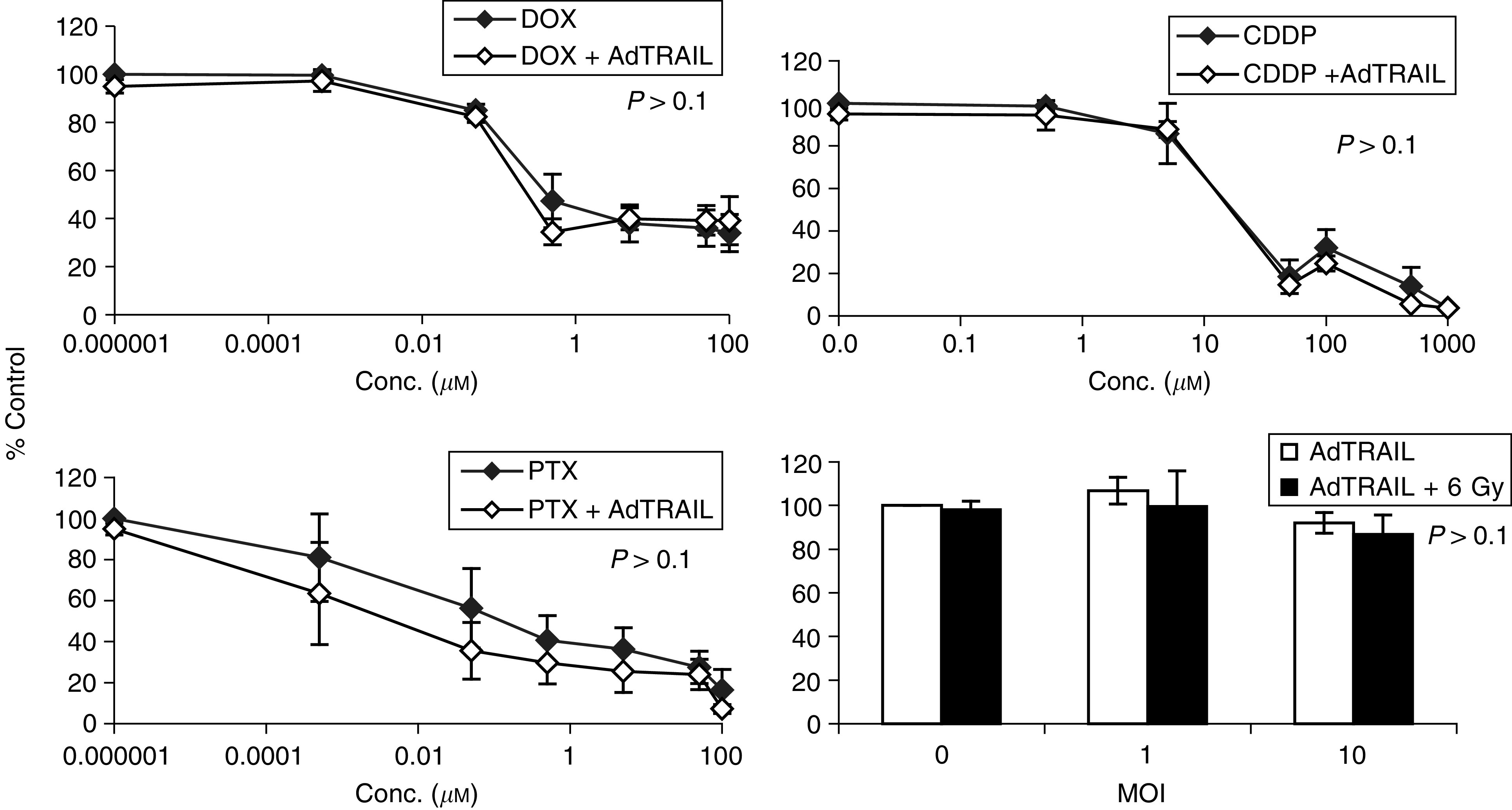
Infection with AdTRAIL eradicated most of the H460 cells but left the NHF intact as indicated by the few remaining cells that were either positive or negative for TRAIL expression in H460 cells as determined by immunohistochemistry (Figure 6B). Subsequently, the effect of combined chemotherapy at different concentrations with AdTRAIL on NHF cells was examined (Figure 7). Each of the drugs alone exerted a concentration-dependent cytotoxicity on NHF cells 2 days post-treatment. Normal human fibroblasts were relatively refractory to the damage induced by the chemotherapeutic agents (especially to PTX) when compared to H460 cells (see Table 1). The cotreatment of NHF with different concentrations of DOX, PTX or CDDP 24 h postinfection with AdTRAIL did not result in extra cytotoxicity either compared to AdTRAIL alone or chemotherapy alone. Instead, perhaps even an increase in viability after AdTRAIL infection could be appreciated (Figure 7). These data show that adenovirus-expressed TRAIL combined with chemotherapy does not alter the sensitivity of normal cells.
Figure 7.
Chemotherapy in combination with AdTRAIL does not alter the sensitivity of NHF. The cytotoxic effect of AdTRAIL (MOI 100) with (out) different concentrations of DOX, PTX and CDDP was determined at 3 days postinfection. Values are the mean (n=3)±s.d. The P-value was calculated by comparing the slopes of the multiple regression of NHF cells ln (viability2) treated with AdTRAIL alone or combined with DOX, PTX or CDDDP vs. ln (drug conc.2) by Student's t-test.
DISCUSSION
Adenovirus-mediated production of the promising biological anticancer agent TRAIL is a form of proapoptotic gene therapy that has gained considerable attention recently (Griffith et al, 2000; Routes et al, 2000; Lin et al, 2002b). The main reasons for this are the intrinsic tumour-selective activity of TRAIL in a broad range of cancer cells that complements the rather nonselective delivery of viral vectors to tumours and normal tissues, the observed bystander effect of TRAIL resulting in the killing of cells surrounding the infected cells (Kagawa et al, 2001), and the notion that virally produced TRAIL may overcome problems observed with the use of recombinant soluble TRAIL regarding protein instability and resistance (Kelley et al, 2001; Voelkel-Johnson et al, 2002; Seol et al, 2003).
Full-length TRAIL has recently been reported to kill tumour cells that are resistant to soluble TRAIL, suggesting a greater therapeutic potential of this form and indicating the existence of yet not understood mechanistic differences between the two TRAIL variants (Voelkel-Johnson et al, 2002; Seol et al, 2003).
The tumour-selective properties of full-length TRAIL alone or in combination with chemo- and radiation treatment thus far have been mainly investigated and confirmed for recombinant TRAIL, and its mechanism of action has been left unexplored.
In the present study, we generated an adenoviral vector expressing full-length TRAIL to explore its possible use for the treatment of NSCLC. We used the NSCLC cell line H460 as a representative cell line that has been well characterised and in which we found a defect in mitochondria-dependent caspase-9 activation upon treatment with DNA-damaging chemotherapeutic agents that may provide an explanation for chemoresistance of NSCLC in the clinic (Ferreira et al, 2000).
AdTRAIL efficiently killed H460 cells in a MOI-dependent manner, which could be inhibited by the broad-caspase-inhibitor zVAD-fmk as an indication of the activation of caspase-dependent apoptosis. AdTRAIL-induced apoptosis in H460 was associated with caspase-8 activation and PARP cleavage. Subtoxic doses of DOX, PTX, CDDP or ionising radiation sensitised H460 cells to AdTRAIL-induced apoptosis that we found to be a result of augmented processing of procaspase-8 and Bid, indicating that both TRAIL and chemo (radio) therapy-induced apoptotic signals converge at the mitochondrial apoptotic pathway. The most likely explanation for this is that both chemotherapeutic agents and irradiation trigger damage-induced signals that lead to the activation of proapoptotic Bcl2 family members, such as Bax and Bak (Cartron et al, 2003; Kim et al, 2003), and subsequent mitochondria destabilisation that as we reported previously results in the unusual activation of caspase-8 in NSCLC H460 cells by an as yet unresolved mitochondria-dependent mechanism (Ferreira et al, 2000). Consequently, Bid cleavage is enhanced and as a result the tBid-dependent amplification loop.
Confirmation for this notion is provided by our finding that the stable overexpression of Bcl2 in H460 cells completely counteracted the enhancing effect of chemo (radio) therapy, except for PTX. Paclitaxel-induced toxicity was significantly reduced by Bcl2 overexpression, but not completely abrogated as found for the other treatments. This may be related to our previous finding that PTX in contrast to, for example CDDP, induces cell death in H460 cells that is only partially triggered through the mitochondrial pathway and mainly depends on the activation of a caspase-independent pathway (Huisman et al, 2002). The found importance of the tBid-mitochondria amplification loop in mediating AdTRAIL-induced apoptosis in H460 cells classifies them as type-II cells. This observation is in line with a previous report by Sun et al (2001), who showed that Bcl2 overexpression inhibits soluble TRAIL-induced apoptosis in NSCLC cells; however, instead of their suggestion that Bcl2 acts by preventing caspase-7 activation, we find that Bcl2 suppresses procaspase-8 cleavage that as mentioned above we have found to occur in a mitochondria-controlled manner in NSCLC cells (Ferreira et al, 2000).
The notion was also tested whether the use of the full-length TRAIL-encoding virus together with chemotherapy may affect the tumour selectivity of the treatment and result in toxic side effects on normal tissue. We did not observe such a change in cytotoxicity in NHF and rather observed a not understood small increase in viability after AdTRAIL infection.
In conclusion, we showed that the production of full-length TRAIL by an adenoviral vector effectively kills NSCLC H460 cells that can be enhanced by combined treatment with the chemotherapeutic agents CDDP, DOX and PTX and by radiation. The synergistic effects are dependent on the enhanced activation of the mitochondria apoptotic pathway that remains functional in the mitochondria/caspase-9 route-deficient H460 cells. Combination treatment of AdTRAIL and chemo/radiotherapy was not toxic for normal cells indicating that adenovirus-directed expression of full-length TRAIL might provide an attractive strategy for treating NSCLC.
Acknowledgments
We thank Martine Lamfers and Jaap van den Berg for their kind help with the irradiation experiments. We are also grateful to Dr HM Pinedo for his continuous interest and support.
References
- Abou El Hassan MA, Heijn M, Rabelink MJ, van der Vijgh WJ, Bast A, Hoeben RC (2003) The protective effect of cardiac gene transfer of CuZn-sod in comparison with the cardioprotector monohydroxyethylrutoside against doxorubicin-induced cardiotoxicity in cultured cells. Cancer Gene Ther 10: 270–277 [DOI] [PubMed] [Google Scholar]
- Abou El Hassan MA, Verheul HM, Jorna AS, Schalkwijk C, van Bezu J, van der Vijgh WJ, Bast A (2003a) The new cardioprotector Monohydroxyethylrutoside protects against doxorubicin-induced inflammatory effects in vitro. Br J Cancer 89: 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkinazi A, Pai RC, Fong S, Leung S, Lawrence DA, Maresters S, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron PF, Juin P, Oliver L, Meflah K, Vallette FM (2003) Impact of proapoptotic proteins Bax and Bak in tumor progression and response to treatment. Expert Rev Anticancer Ther 3: 563. [DOI] [PubMed] [Google Scholar]
- De Vita DT, Helman S, Rosenberg SA (2001) Principles and Practice on Oncology, 6th, Chapter 31; Lippincott, Williams & Wilkins: Philadelphia [Google Scholar]
- Ferreira CG, Epping M, Kruyt FA, Giaccone G (2002) Apoptosis: target of cancer therapy. Clin Cancer Res 8: 2024–2034 [PubMed] [Google Scholar]
- Ferreira CG, Span SW, Peters GJ, Kruyt FAE, Giaccone G (2000) Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondrial controlled manner in the non-small cells lung cancer cell line NCI-H460. Cancer Res 60: 7133–7141 [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin K-M (2002) Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 21: 2283–2294 [DOI] [PubMed] [Google Scholar]
- Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL (2000) Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide to TRAIL. Mol Cell Biol 20: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL (2000) Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol 165: 2886–2894 [DOI] [PubMed] [Google Scholar]
- Held J, Schulze-Osthoff K (2001) Potentials and caveats of TRAIL. Drug Resist Updates 4: 243–252 [DOI] [PubMed] [Google Scholar]
- Huang X, Lin T, Gu J, Zhang L, Roth JA, Stephens LC, Yu Y, Liu J, Fang B (2002) Combined TRAIL and Bax gene therapy prolonged survival in mice with ovarian cancer xenograft. Gene Ther 9: 1379–1386 [DOI] [PubMed] [Google Scholar]
- Huisman C, Ferreira CG, Broker LE, Rodriguez JA, Smit EF, Postmus PE, Kruyt FAE, Giaccone G (2002) Paclitaxel triggers cell death primarily via caspase-independent routes in the non-small cell lung cancer cell line NCI-H460. Clin Cancer Res 8: 596–606 [PubMed] [Google Scholar]
- Jo M, Kim T, Seol D, Esplen JE, Dorko K, Billiar TR, Strom S (2000) Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 6: 564–567 [DOI] [PubMed] [Google Scholar]
- Kagawa S, He C, Gu J, Koch P, Rha S, Roth JA, Curley SA, Stephens LC, Fan B (2001) Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res 61: 3330–3338 [PubMed] [Google Scholar]
- Kelley SN, Marris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Therapeut 299: 31–38 [PubMed] [Google Scholar]
- Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T (2003) Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol 50: 343–352 [DOI] [PubMed] [Google Scholar]
- Lawrence D, Shahrokh Z, Marsters S, Achilies K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A (2001) Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med 7: 383–385 [DOI] [PubMed] [Google Scholar]
- Lin T, Gu J, Zhang L, Huang X, Stephens LG, Curely SA, Fang B (2002a) Targeted expression of green fluorescence protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effect on primary human hepatocytes. Cancer Res 62: 3620–3625 [PubMed] [Google Scholar]
- Lin T, Huang X, Gu J, Zhang L, Roth JA, Xiong M, Curley SA, Yu Y, Hunt KK, Fang B (2002b) Long-term tumor-free survival from treatment with the GFP-TRAIL fusion gene expressed from the hTERT promoter in breast cancer cells. Oncogene 21: 8020–8028 [DOI] [PubMed] [Google Scholar]
- MacFarlane M (2003) TRAIL-induced signaling and apoptosis. Toxicol Lett 139: 89–97 [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman RHT, Anderson KC (2001) TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 98: 795–804 [DOI] [PubMed] [Google Scholar]
- Naka T, Suramura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA (2002) Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients colon tumors grown in SCID mice. Cancer Res 62: 5800–5806 [PubMed] [Google Scholar]
- Nimmanapalli R, Porosnicu M, Nguyen D, Worthington E, O’bryan E, Perkins C, Bhalla K (2001) Cotreatment with STI-571 enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL or Apo2L)-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Clin Cancer Res 7: 350–357 [PubMed] [Google Scholar]
- Ozoren N, El-Deiry WS (2002) Defining characteristics of type I and II apoptotic cells in response to TRAIL. Neoplasia 4: 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997a) An antagonistic decoy receptor and a death domain-containing receptor for TRAIL. Science 277: 815–818 [DOI] [PubMed] [Google Scholar]
- Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997b) The receptor for the cytotoxic ligand TRAIL. Science 276: 111–113 [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Dunahue CJ, Moore A, Ashkenazi A (1996) Induction of apoptosis by Apo-2ligand, a new member of tumor necrosis factor cytokine family. J Biol Chem 271: 12687–12690 [DOI] [PubMed] [Google Scholar]
- Rohn TA, Wagenkenecht B, Roth W, Naumann U, Gulbins E, Krammer PH, Walczak H, Weller M (2001) CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p52-independent but may involve enhanced cytochrome c release. Oncogene 20: 4128–4137 [DOI] [PubMed] [Google Scholar]
- Routes JM, Ryan S, Clase A, Miura T, Kuhl A, Potter TA, Cook JL (2000) Adenovirus E1A oncogene expression in tumor cells enhances killing by TNF-related apoptosis-inducing ligand (TRAIL). J Immunol 165: 4522–4527 [DOI] [PubMed] [Google Scholar]
- Seol JY, Park K, Hwang C, Park W, Yoo C, Kim YW, Han SK, Shim YS, Lee C (2003) Adenovirus-TRAIL can overcome TRAIL resistance and induce a bystander effect. Cancer Gen Ther 10: 540–548 [DOI] [PubMed] [Google Scholar]
- Sun S-Y, Yue P, Zhou J-Y, Choi Kim H-R, Lotan R, Wu GS (2001) Overexpression of Bcl2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Comm 280: 788–797 [DOI] [PubMed] [Google Scholar]
- Van Beusechem VW, van Rijswijk AL, van Es HH, Haisma HJ, Pinedo HM, Gerritsen WR (2000) Recombinant adenovirus vectors with knobless fibers for targeted gene transfer. Gene Ther 7: 1940–1946 [DOI] [PubMed] [Google Scholar]
- Voelkel-Johnson C, King DL, Norris JS (2002) Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gen Ther 9: 164–172 [DOI] [PubMed] [Google Scholar]
- Walczak H, Bouchon A, Stahl H, Krammer PH (2000) Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl2 or BclxL-overexpressing chemotherapy resistant tumor cells. Cancer Res 60: 3051–3057 [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3: 673–682 [DOI] [PubMed] [Google Scholar]
- Xu L-HDC-S, Zhu Y-Q, Liu D-Z (2003) Synergistic antitumor effect of TRAIL and doxorubicin on colon cancer cell line SW 480. World J Gastroenterol 9: 1241–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]