Abstract
A randomised phase III trial was conducted to assess the role of interferon-alpha (INFα) 2a as maintenance therapy following surgery and/or chemotherapy in patients with epithelial ovarian carcinoma. Patients were randomised following initial surgery/chemotherapy to interferon-alpha 2a as 4.5 mega-units subcutaneously 3 days per week or to no further treatment. A total of 300 patients were randomised within the study between February 1990 and July 1997. No benefit for interferon maintenance was seen in terms of either overall or clinical event-free survival. We conclude that INF-α is not effective as a maintenance therapy in the management of women with ovarian cancer. The need for novel therapeutics or strategies to prevent the almost inevitable relapse of patients despite increasingly effective surgery and chemotherapy remains.
Keywords: ovarian cancer, interferon-alpha, maintenance therapy
Although epithelial ovarian cancer is a relatively chemosensitive disease, the disease ultimately relapses and leads to the death of most patients. The median duration of remission following first-line chemotherapy with platinum/taxane combinations for patients with advanced disease is between 12 and 24 months. A variety of strategies have been employed to maintain the benefits achieved with chemotherapy in advanced ovarian cancer. The dose and duration of chemotherapy have both been increased, but increases in response rates have not been associated with improvement in survival (Eltabbakh et al, 1998; Umesaki et al, 1999; McGuire, 2000).
The concept of additional therapy to maintain the response achieved with chemotherapy has been assessed in a number of disease and clinical settings. In the 1980s, interferon-alpha (INFα) was assessed as a maintenance therapy for patients with multiple myeloma. As a single agent, interferon has a low response rate in myeloma patients with responses of approximately 15%. However, in patients who demonstrated no evidence of disease progression following primary chemotherapy, maintenance therapy with low-dose subcutaneous INFα improved progression-free and overall survival (Mandelli et al, 1990).
Interferon-α has limited activity in active advanced ovarian cancer (Freedman et al, 1983; Einhorn et al, 1988). However, we hypothesised that subcutaneous INFα may act as an effective maintenance therapy in patients with advanced ovarian cancer and thus improve overall survival. To test this hypothesis, a prospective randomised trial was performed in patients with FIGO stage Ic–IV epithelial ovarian cancer, in whom no evidence of disease progression was seen following surgery and/or chemotherapy.
PATIENTS AND METHODS
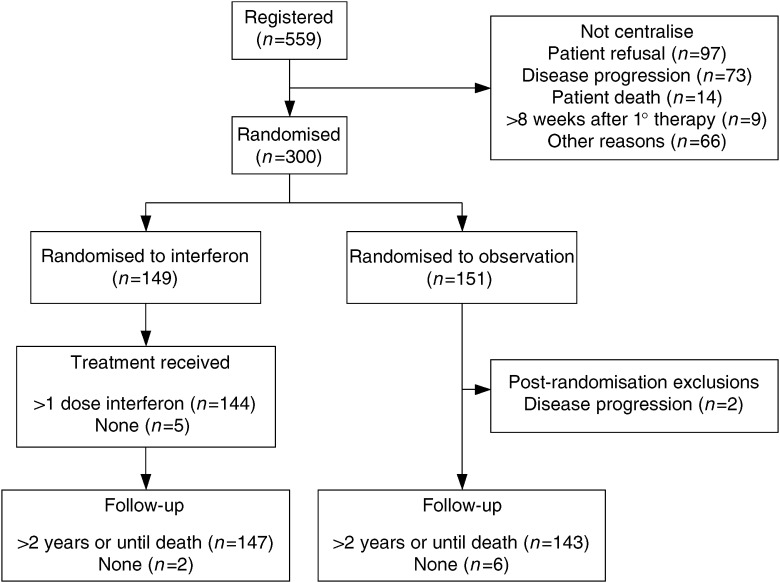
The study was approved by the Local Research Ethics Committee of each participating centre and written informed consent was obtained prior to randomisation. Patients eligible for the study had histologically proven epithelial ovarian cancer that showed no evidence of disease progression (on clinical, radiological or CA-125 assessment) following postoperative chemotherapy. Patients were randomised within 8 weeks of completing chemotherapy to treatment with INF-α 2a (Roferon-A, Roche) (4.5 mega-units subcutaneously 3 days per week) or to a no maintenance treatment control group. Interferon was continued until disease progression, or in response to toxicity or patient request. The study was designed to detect a 50% increase in median survival and therefore required 300 randomised patients. The trial profile is shown in Figure 1.
Figure 1.
Trial profile.
To further analyse the effect of maintenance interferon, the disease status of each patient was determined following surgery and/or chemotherapy. Patients in whom there was no evidence of disease either clinically or radiologically following surgery and/or chemotherapy were designated as disease-free (DF). Those patients in whom disease was apparent clinically or radiologically following surgery and/or chemotherapy were designated as disease-present (DP).
For additional detail regarding patient selection, dose modifications, study design and statistical methods, see supplementary information.
RESULTS
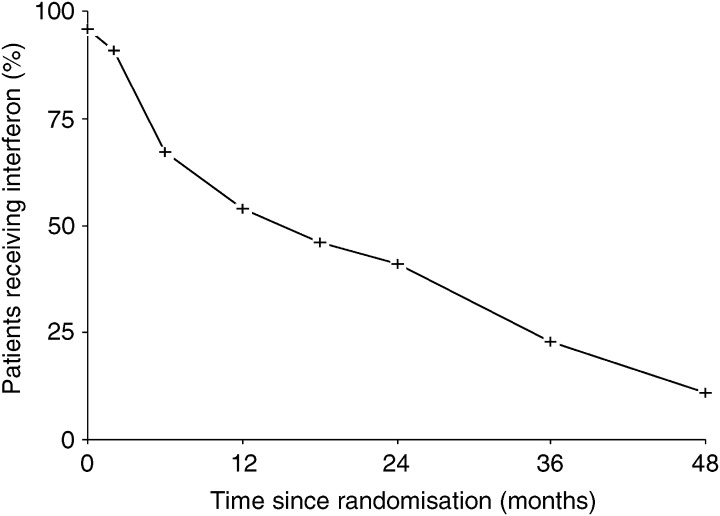
Between February 1990 and July 1997, 300 patients were randomised by 23 consultants from 14 centres across the UK (for participating centres and consultants, see supplementary information), 151 to observation, 149 to interferon. The patients in the two treatment arms were similar with respect to baseline characteristics (Table 1). The median follow-up time for interferon patients was 27.0 months (range 2.3–149.7), and for observation patients 32.2 months (range 1.7–141.7). Eight patients (two interferon and six observation) died without attending any follow-up visits. Of the 149 patients randomised to receive maintenance INFα, 144 received at least one injection (see supplementary information). The duration of treatment and the reason for stopping is shown in Table 2. Figure 4 shows compliance with interferon maintenance over time in those patients who remained progression free. The most commonly reported toxicity/adverse event for both interferon and observation patients was fatigue (72 and 46%, respectively) (Table 3). Dose modification of interferon was made in 18 (12.5%) patients due to toxicity.
Table 1. Patient characteristics by treatment group.
| Interferon | Observation | |
|---|---|---|
| (n=149) | (n=149) | |
| Median (range) age at diagnosis (years) | 58 (31–76) | 57 (33–78) |
| FIGO stage at diagnosis | ||
| I | 11 (7%) | 12 (8%) |
| II | 22 (14%) | 19 (13%) |
| III | 94 (63%) | 95 (64%) |
| IV | 22 (15%) | 23 (15%) |
| Residual disease following surgery (cm) | ||
| 0 | 23 (18%) | 26 (20%) |
| <2 | 44 (35%) | 51 (40%) |
| 2–5 | 24 (19%) | 13 (10%) |
| >5 | 36 (28%) | 40 (31%) |
| Histology | ||
| Serous | 67 (45%) | 72 (48%) |
| Mucinous | 10 (7%) | 12 (8%) |
| Endometrioid | 27 (18%) | 34 (23%) |
| Clear cell | 4 (3%) | 4 (3%) |
| Other | 20 (13%) | 10 (7%) |
| Undifferentiated | 3 (2%) | 2 (1%) |
| Not stated | 18 (12%) | 15 (10%) |
| Tumour grade | ||
| 1 | 11 (9%) | 14 (11%) |
| 2 | 47 (38%) | 43 (33%) |
| 3 | 66 (53%) | 75 (57%) |
| Not stated | 25 | 17 |
| WHO performance status pre-chemotherapy | ||
| 0 | 84 (60%) | 83 (56%) |
| 1 | 46 (32%) | 46 (31%) |
| 2 | 7 (4%) | 17 (12%) |
| 3 | 3 (2%) | 1 (1%) |
| 4 | 3 (2%) | 0 |
| Not stated | 6 | 2 |
| Chemotherapy regimen | ||
| Platinum single agent | 89 (60%) | 88 (59%) |
| Platinum combination | 44 (29%) | 37 (25%) |
| Platinum/taxane | 15 (10%) | 20 (13%) |
| Taxane single agent | 0 | 1 (1%) |
| Other single agent | 1 (1%) | 3 (2%) |
| Response to surgery and/or chemotherapy | ||
| Disease free | 89 (61%) | 95 (65%) |
| Disease present | 58 (39%) | 52 (35%) |
| Not evaluable | 2 | 2 |
| CA125 after surgery and/or chemotherapy | ||
| <35 U ml−1 | 47 (77%) | 62 (78%) |
| 35–65 U ml−1 | 2 (3%) | 9 (11%) |
| 65–200 U ml−1 | 8 (13%) | 5 (6%) |
| >200 | 4 (7%) | 4 (5%) |
| Not stated | 88 | 69 |
| WHO performance status at randomisation | ||
| 0 | 113 (76%) | 114 (79%) |
| 1 | 32 (22%) | 28 (19%) |
| 2 | 3 (2%) | 2 (1%) |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| Not stated | 1 | 5 |
Table 2. Duration of interferon treatment and reason for stopping treatment.
| Number of patients | Median (range) time (in months) on interferon | |
|---|---|---|
| All patients | 144 | 5 (<1–88) |
| Interferon stopped | ||
| Disease progression | 61 | 8 (1–66) |
| Toxicity | 50 | 3 (<1–37) |
| Patient desire | 14 | 7 (<1–48) |
| Other | 8 | 38 (1–71) |
| Death of patient | 4 | 8 (3–14) |
| Not stated | 4 | 22 (7–51) |
| Treatment status unknown at last review | 3 | 54 (51–88) |
Figure 4.
Compliance with interferon maintenance. The percentage of patients still receiving interferon in the absence of disease progression is shown.
Table 3. Adverse events/toxicity (all grades).
| Interferon | Observation | ||
|---|---|---|---|
| (n=144) | (n=149) | Significance | |
| Toxicity/adverse event | |||
| Flu-like symptoms | 102 (69%) | 47 (31%) | P<0.001 |
| Fatigue | 106 (71%) | 71 (47%) | P<0.001 |
| Nausea/vomiting | 61 (41%) | 52 (34%) | Not significant |
| Neurological | 6 (4%) | NR | — |
| Dyspnoea | 9 (6%) | NR | — |
| Depression/anxiety | 9 (6%) | NR | — |
| Skin change/rash | 7 (5%) | NR | — |
| Alopecia | 7 (5%) | NR | — |
| Arthalgia/arthritis | 4 (3%) | NR | — |
| Insomnia | 3 (2%) | NR | — |
| Haematological | 3 (2%) | NR | — |
| Hepatic | 1 (1%) | NR | — |
| Injection site reaction | 2 (1%) | NR | — |
| Other | 19 (13%) | NR | — |
| Not stated | 4 (3%) | NR | — |
NR=not recorded.
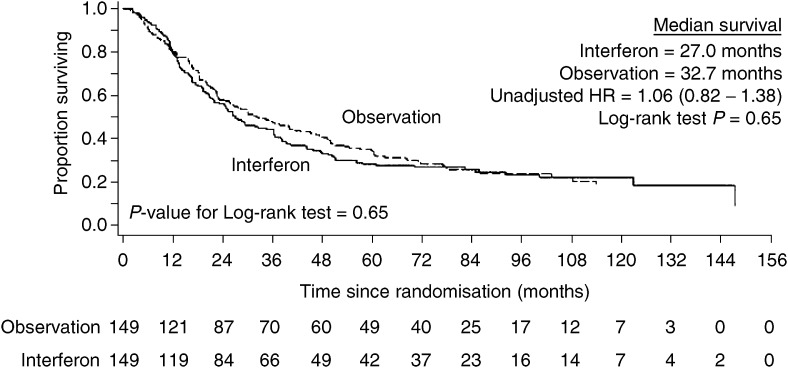
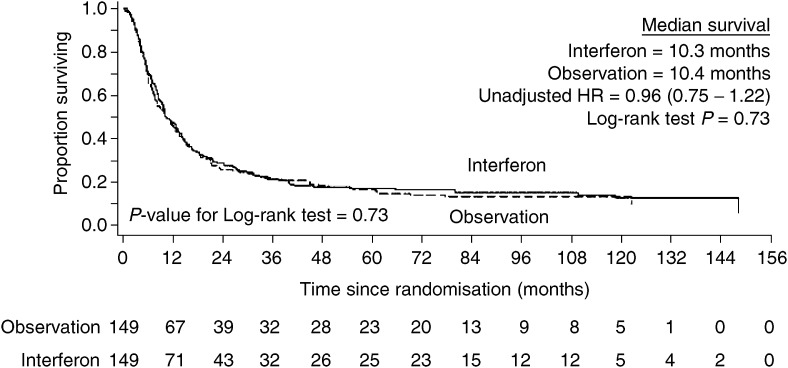
No benefit for interferon maintenance was seen in terms of either overall or clinical event-free survival. The median overall survival in the interferon arm was 27.0 months and in the observation arm 32.7 months. The median clinical event-free survival in the interferon arm was 10.3 months and 10.4 months in the observation arm. The Kaplan–Meier survival curves for overall and clinical event-free survival (Figure 2 and Figure 3) showed no significant difference (overall survival – unadjusted HR=1.06, 95% C.I.=0.82–1.38, Log-rank test P=0.65; clinical event-free survival – unadjusted HR=0.96, 95% C.I.= 0.75–1.22, Log-rank test P=0.73). When hazard ratios were adjusted for prognostic factors previously defined, the results were similar.
Figure 2.
Overall survival by randomised treatment group.
Figure 3.
Clinical event-free survival by randomised treatment group.
The effect of treatment on overall survival and clinical event-free survival was compared separately among patients who were DF after surgery and/or chemotherapy and those who had DP after surgery and/or chemotherapy. This comparison was unplanned and performed only as a hypothesis generating analysis. No significant differences for interferon maintenance were identified (DF patients – clinical event-free survival – unadjusted HR=0.88, 95% C.I.=0.63–1.22).
The multivariate analysis of prognostic factors and treatment received, performed on the 298 patients in the intention to treat population (Table 4), demonstrated that receiving interferon maintenance was not related to either overall or clinical event-free survival (see supplementary information).
Table 4. Multivariate analysis of treatment received and prognostic factors for overall and clinical event-free survival (n=298).
|
Overall survival |
Clinical event-free survival |
|||
|---|---|---|---|---|
| Adjusted HR (95% C.I.) | P | Adjusted HR (95% C.I.) | P | |
| Randomised treatment | ||||
| Observation | 1.00 | 1.00 | ||
| Interferon | 1.10 (0.83–1.45) | 0.51 | 0.92 (0.71–1.20) | 0.55 |
| Response–1° treatment | ||||
| Disease-free | 1.00 | 1.00 | ||
| Disease-present | 1.53 (1.12–1.65) | 0.01 | 1.84 (1.37–2.49) | <0.001 |
| Age at diagnosis (years) | ||||
| <55 | 1.00 | 1.00 | ||
| 55–65 | 1.51 (1.10–2.07) | 1.13 (0.83–1.52) | ||
| >65 | 1.36 (0.92–2.01) | 0.034 | 1.14 (0.79–1.65) | 0.67 |
| FIGO stage | ||||
| Ic | 1.00 | 1.00 | ||
| II | 0.80 (0.33–2.74) | 1.02 (0.51–2.06) | ||
| III | 2.11 (1.04–4.29) | 2.48 (1.28–4.80) | ||
| IV | 2.82 (1.32–6.03) | <0.001 | 2.92 (1.42–5.59) | <0.001 |
| WHO performance status | ||||
| 0 | 1.00 | 1.00 | ||
| 1 | 1.16 (0.82–1.65) | 1.16 (0.82–1.65) | ||
| 2/3/4 | 1.74 (1.22–2.49) | 0.035 | 1.74 (1.22–2.49) | 0.62 |
| Size of residual disease | ||||
| 0 | 1 | 1 | ||
| <2 cm | 1.02 (0.63–1.64) | 1.02 (0.63–1.64) | ||
| >2 cm | 1.10 (0.67–2.44) | 1.09 (0.67–1.77) | ||
| Not stated | 1.69 (0.97–2.93) | 0.17 | 1.69 (0.97–2.93) | 0.74 |
| Tumour type | ||||
| Serous | 1.00 | 1.00 | ||
| Mucinous | 1.32 (0.78–2.22) | 1.15 (0.68–1.93) | ||
| Endometrioid | 1.16 (0.79–1.70) | 1.06 (0.72–1.56) | ||
| Clear cell | 1.69 (0.65–4.41) | 1.39 (0.49–3.93) | ||
| Other | 0.86 (0.53–1.40) | 0.89 (0.56–1.43) | ||
| Undifferentiated | 1.09 (0.37–3.81) | 1.36 (0.52–3.57) | ||
| Not stated | 1.59 (1.00–2.43) | 0.33 | 1.39 (0.87–2.21) | 0.74 |
| Tumour grade | ||||
| Well differentiated | 1.00 | 1.00 | ||
| Moderately differentiated | 0.83 (0.45–1.54) | 0.87 (0.54–1.52) | ||
| Poorly differentiated | 1.33 (0.73–2.41) | 1.32 (0.77–2.24) | ||
| Not stated | 0.92 (0.46–1.84) | 0.033 | 0.85 (0.45–1.60) | 0.038 |
DISCUSSION
This trial was designed to determine whether the use of low-dose subcutaneous INFα could improve the overall survival of patients with epithelial ovarian cancer following primary therapy with surgery and/or chemotherapy. Patients with all stages of disease at presentation and with no evidence of disease progression after primary therapy were randomised within the trial. The overall and clinical event-free survival of ovarian cancer patients studied within this trial was typical of results obtained with platinum combination chemotherapy in the 1990s. However, survival times were not improved by the addition of maintenance low-dose subcutaneous IFNα following primary therapy.
Interferon-α has been shown to have an in vitro activity against ovarian cancer cell lines (Epstein et al, 1980) and a limited clinical benefit in advanced ovarian cancer (Freedman et al, 1983; Einhorn et al, 1988). Clinical studies have assessed the effect of intraperitoneal INFα in a number of phase II studies both after and in combination with platinum chemotherapy (Berek et al, 1985; Nardi et al, 1990; Willemse et al, 1990; Bruzzone et al, 1997; Berek et al, 1999). These studies have confirmed that such a treatment can be delivered although its benefit over standard treatment has not been assessed. No clinical trial of subcutaneous INFα in ovarian cancer has previously been reported. However, an alternative immunoregulatory cytokine, interferon-γ has been assessed in a randomised phase III trial as an addition to primary chemotherapy with cisplatin and cylophosphamide (Windbichler et al, 2000). This showed a significant improvement in progression-free survival. However, improvements in clinical response rate and overall survival did not achieve statistical significance.
Experience with biological therapy has suggested that the benefits of such therapy are often seen in patients with small volume or clinically undetectable disease. Although this effect has also been seen in previous trials of cytokine therapy in ovarian cancer (Berek et al, 1985; Pujade-Lauraine et al, 1996), the recent study of interferon-γ showed equal efficacy in patients with both small and large volume disease (Windbichler et al, 2000). To mirror the initial study of interferon maintenance in myeloma, patients with no evidence of disease progression following primary therapy were randomised and therefore, many patients with residual disease were randomised. However, a subgroup analysis of the patients in complete clinical remission within this study failed to identify a significant benefit for those patients.
Compliance with interferon maintenance was poor (Figure 4). At 6 months, only 67% of patients with no evidence of disease progression remained on interferon maintenance. This is likely to be due to the fact that this cohort of patients had undergone both surgical intervention and chemotherapy prior to commencing interferon treatment. This is in contrast to studies in myeloma, renal cancer and melanoma in which treatment rarely follows both extensive surgery and chemotherapy. Pegylated interferon has recently been introduced into clinical trials. The half-life of these modified forms of interferon are significantly increased allowing weekly administration. It may be that the use of such treatment would improve compliance by reducing both the frequency of administration and the toxicity associated with the treatment.
Despite modern chemotherapy combinations, the natural history of ovarian carcinoma remains one of relapse following initial response to chemotherapy. A potential benefit for maintenance paclitaxel chemotherapy for some patients with ovarian cancer has recently been reported although at the cost of clinically significant toxicity (Markman et al, 2003). The need for an effective nontoxic maintenance therapy is clear. This study has demonstrated that subcutaneous INF-α given following surgery and chemotherapy is not the answer to this ongoing dilemma. However, the recent development of new, targeted molecular therapies may allow the concept of maintenance therapy to be examined further.
Acknowledgments
Support for this project was provided by Roche Pharmaceuticals UK and the Yorkshire Cancer Research/Imperial Cancer Research Fund/Cancer Research UK.
Footnotes
Supplementary information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc).
Supplementary Information
References
- Berek JS, Hacker NF, Lichtenstein A, Jung T, Spina C, Knox RM, Brady J, Greene T, Ettinger LM, Lagasse LD (1985) Intraperitoneal recombinant alpha-interferon for ‘salvage’ immunotherapy in stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. Cancer Res 45: 4447–4453 [PubMed] [Google Scholar]
- Berek JS, Markman M, Stonebraker B, Lentz SS, Adelson MD, DeGeest K, Moore D (1999) Intraperitoneal interferon-alpha in residual ovarian carcinoma: a phase II gynecologic oncology group study. Gynecol Oncol 75: 10–14 [DOI] [PubMed] [Google Scholar]
- Bruzzone M, Rubagotti A, Gadducci A, Catsafados E, Foglia G, Brunetti I, Giannessi PG, Carnino F, Iskra L, Rosso R, Martoni A, Pannuti F, De LV, Maltoni R, Ridolfi R, Mammoliti S, Gallo L, Boccardo F, Ragni N, Conte PF (1997) Intraperitoneal carboplatin with or without interferon-alpha in advanced ovarian cancer patients with minimal residual disease at second look: a prospective randomized trial of 111 patients. G.O.N.O. Gruppo Oncologic Nord Ovest. Gynecol Oncol 65: 499–505 [DOI] [PubMed] [Google Scholar]
- Einhorn N, Ling P, Einhorn S, Strander H (1988) A phase II study on escalating interferon doses in advanced ovarian carcinoma. Am J Clin Oncol 11: 3–6 [DOI] [PubMed] [Google Scholar]
- Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Blumenson LE (1998) Prolonged disease-free survival by maintenance chemotherapy among patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol 71: 190–195 [DOI] [PubMed] [Google Scholar]
- Epstein LB, Shen JT, Abele JS, Reese CC (1980) Sensitivity of human ovarian carcinoma cells to interferon and other antitumor agents as assessed by an in vitro semi-solid agar technique. Ann NY Acad Sci 350: 228–244 [DOI] [PubMed] [Google Scholar]
- Freedman RS, Gutterman JU, Wharton JT, Rutledge FN (1983) Leukocyte interferon (IFN alpha) in patients with epithelial ovarian carcinoma. J Biol Resp Mod 2: 133–138 [PubMed] [Google Scholar]
- Mandelli F, Avvisati G, Amadori S, Boccadoro M, Gernone A, Lauta VM, Marmont F, Petrucci MT, Tribalto M, Vegna ML (1990) Maintenance treatment with recombinant interferon alfa-2b in patients with multiple myeloma responding to conventional induction chemotherapy. N Engl J Med 322: 1430–1434 [DOI] [PubMed] [Google Scholar]
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, Jiang C, Alberts D (2003) Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol 21: 2460–2465 [DOI] [PubMed] [Google Scholar]
- McGuire WP (2000) High-dose chemotherapeutic approaches to ovarian cancer management. Semin Oncol 27: 41–46 [PubMed] [Google Scholar]
- Nardi M, Cognetti F, Pollera CF, Giulia MD, Lombardi A, Atlante G, Calabresi F (1990) Intraperitoneal recombinant alpha-2-interferon alternating with cisplatin as salvage therapy for minimal residual-disease ovarian cancer: a phase II study. J Clin Oncol 8: 1036–1041 [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Guastalla JP, Colombo N, Devillier P, Francois E, Fumoleau P, Monnier A, Nooy M, Mignot L, Bugat R, Marques C, Mousseau M, Netter G, Maloisel F, Larbaoui S, Brandely M (1996) Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J Clin Oncol 14: 343–350 [DOI] [PubMed] [Google Scholar]
- Thigpen JT, Vance RB, Lambuth BW (1988) Ovarian carcinoma: the role of chemotherapy. Semin Oncol 15: 16–23 [PubMed] [Google Scholar]
- Umesaki N, Tanaka T, Muso H, Kawamura N, Kanaoka Y, Honda K, Deguchi M, Ishiko O, Ogita S (1999) Intermittent cisplatin therapy for stage-III ovarian cancer patients following clinical remission. Gynecol Obstet Invest 47: 139–143 [DOI] [PubMed] [Google Scholar]
- Willemse PH, de Vries EG, Mulder NH, Aalders JG, Bouma J, Sleijfer DT (1990) Intraperitoneal human recombinant interferon alpha-2b in minimal residual ovarian cancer. Eur J Cancer 26: 353–358 [DOI] [PubMed] [Google Scholar]
- Windbichler GH, Hausmaninger H, Stummvoll W, Graf AH, Kainz C, Lahodny J, Denison U, Muller-Holzner E, Marth C (2000) Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer 82: 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.