Abstract
One of the major targets for breast cancer therapy is the epidermal growth factor receptor (EGFR) and related receptors, which signal via different signal transduction pathways including the mitogen-activated protein kinase (MAPK) pathway. This study determined whether there is a correlation between EGFR/HER2 status and MAPK (ERK1/2) phosphorylation in breast cancer cells, and how this affects the response to an inhibitor of the receptors. Expression of EGFR, HER2 and phosphorylated ERK1/2 were measured by immunoblotting in a panel of breast cancer cell lines. Several lines expressed high levels of pERK1/2, with no obvious correlation with the level of EGFR/HER2. The EGFR tyrosine kinase inhibitor PKI166 inhibited growth and induced apoptosis in some cells with high levels of growth factor receptors (MDA-MB-468, SUM149, SKBR3), but was less effective in cells that also had high basal ERK1/2 activity (MDA-MB-231). The combination of an inhibitor of MAPK signalling (U0126) and PKI166 produced significantly more inhibition and apoptosis than either agent alone. This suggests that constitutive activation of the MAPK pathway may bypass inhibition of EGFR/HER2 tyrosine kinases, and lead to insensitivity to agents targeting the receptors. However, inhibiting both EGFR/HER2 and MAPK signalling can result in significant growth inhibition and apoptosis of EGFR-expressing breast cancer cells.
Keywords: mitogen activated protein kinase, epidermal growth factor receptor, HER2, targeted therapy
The high mortality rate from breast cancer metastasis has led to the intensive search for molecular alterations contributing to metastatic progression, with the aim of designing targeted therapies (Dillon et al, 1998; Hortobagyi, 2000). Many of these are now in clinical trials (Kerbel, 2000; Mendelsohn and Baselga, 2000; Posey et al, 2001). Epidermal growth factor receptor (EGFR) (also known as erbB1) and HER2 (or erbB2) are two widely studied molecules that are prototypic members of the erbB family of tyrosine kinase receptors (Olayioye et al, 2000). Other family members are erbB3 and erbB4. Amplification of the EGFR or erbB2 genes, leading to protein overexpression, occurs frequently in several human cancers. In breast cancer, EGFR or erbB2 are overexpressed between 20 and 50% of cases, and increased expression is associated with shortened disease-free and overall survival, pointing to involvement in growth regulation of the tumours (Slamon et al, 1987; 1990; Lacroix et al, 1989; Klijn et al, 1992). The EGFR is an Mr 170 000 plasma membrane glycoprotein that dimerises upon binding ligand (e.g. EGF or TGF-α), resulting in activation of intrinsic tyrosine kinase activity and tyrosine autophosphorylation. This triggers a cascade of biochemical and physiological responses that constitute the mitogenic signal transduction of the cells. Extensive preclinical studies have shown that these signalling cascades regulate multiple cellular processes such as proliferation, differentiation, survival and transformation. Similar details of HER2 function are less well known and no soluble ligand for this receptor has been identified. However, this receptor plays a pivotal role in EGF-mediated signalling, as it is the preferred and most potent heterodimerisation partner for EGFR (Graus-Porta et al, 1997).
Several kinase cascades have been implicated in signal transduction through the erbB receptors (Moghal and Sterenberg, 1999). One of these is the RAS-RAF pathway, leading to the activation of extracellular signal-regulated kinases (ERKs), one of the mitogen activated protein kinase (MAPK) cascades (Campbell et al, 1995). This pathway has been the subject of intense interest because of its role in the regulation of proliferation, differentiation and cell–matrix interactions. ERK1 and ERK2 are dually phosphorylated (pERK) on threonine and tyrosine residues by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK). Activated ERK1/2 phosphorylate and activate a variety of substrates including transcription factors, protein kinases and phosphotyrosine protein phosphatases, leading to positive or negative regulation of signalling cascades (Campbell et al, 1995). The mitogenic effect of EGF on normal and malignant mammary epithelia is dependent, at least in part, on ERK activation (Klapper et al, 2000). Furthermore, mammary tumour epithelium may exhibit an elevation in basal ERK activity and sustained ERK activation when stimulated by EGF (Xing and Imagawa, 1998). The sustained ERK activation may reflect a difference in the regulation of EGFR activity in the tumour cells vs normal breast epithelium. Elevated ERK1/2 activity has been noted in a proportion of clinical breast cancers vs benign disease or cancer-associated normal epithelium (Sivaraman et al, 1997; Santen et al, 2002), and a relationship between elevated MAPK activity and shorter disease-free survival in primary breast cancer has been reported (Mueller et al, 2000; Gee et al, 2001; Adeyinka et al, 2002). Thus, hyperactivity of ERK1/2 may play a role in breast cancer progression. It has been suggested that this increased activity is related to the pathological hyperexpression of EGFR and/or HER2 (Von Lintig et al, 2000). If so, the inhibition of the EGFR/HER2 tyrosine kinase activity should lead to a decrease in the pERK activity (Albanell et al, 2001). However, if the activation of pERK1/2 is independent of EGFR/HER2 signalling then increased pERK activity can theoretically bypass or over-ride, at least partially, the inhibition of these growth factor receptors.
Several agents that target one or more members of the ErbB family of tyrosine kinase receptors are currently undergoing preclinical and clinical investigations (Mendelsohn, 2001; Slichenmyer and Fry, 2001). In the present study we have used PKI166 {4-(R)-phenethylamino-6-(hydroxy)phenyl-7H-pyrrolo[2,3-d]-pyrimidine}, an inhibitor of the pyrrolopyrimidine class that has been shown to inhibit the intracellular domain of the EGF-R kinases with an IC50 of 0.7 nM, with less activity against other tyrosine kinases (Traxler et al, 2001). The goals of the present study were to characterise the pattern of expression of activated ERK1/2 in established breast cancer cell lines and determine whether this correlates with EGFR or HER2 status. Further, to explore whether activation status of ERK1/2 can be used as a marker of EGFR kinase inhibition and antiproliferative effects of agents targeting the growth factor receptors. Our results indicate that high basal activity of ERKs give some breast cancer cells a mechanism to escape the inhibitory effects of EGFR/HER2 tyrosine kinase inhibition. However, a combination of agents to inhibit both EGFR/HER2 and pERK signalling resulted in significant growth inhibition and induction of apoptosis.
MATERIALS AND METHODS
Breast cancer cell lines
A panel of breast cancer cell lines was used in an initial screen of cells to study further, based on expression of EGFR, HER2 and ERK activity. The lines were obtained from the American Type Culture Collection (Rockville, MD, USA) (T47D, SKBR3, Hs578T, MCF-7, ZR-75, BT-20), or provided by the Goodwin Institute (Plantation, FL, USA) (GI101A), Dr Stephen Ethier (University of Michigan) (SUM149), or Dr Relda Cailleau (UT MD Anderson Cancer Center) (MDA-MB-231, MDA-MB-435, MDA-MB-468, MDA-MB-361). Cells were maintained in medium (either MEM, or DMEM-F12) with 5 or 10% foetal bovine serum and L-glutamine, in a humidified incubator at 37°C with 5%-CO2. Cells of five cell lines (MDA-MB-231, MDA-MB-435, MDA-MB-468, SUM149 and GI101A) were injected in the mammary fatpads of nude mice, as described previously (Price, 1996). The resulting tumours were collected for preparation of tissue lysates. The use of animals was approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Antibodies and inhibitors
Anti-total ERK1/2 and phosphorylated-p44/42 MAPK (Thr202/Tyr204) antibodies, anti-EGFR and pEGFR antibodies, anti-HER2 and pHER2, anti-p27kip1, and the MEK1/2 inhibitor UO126 were purchased from Cell Signalling Technology, Inc., Beverly, MA, USA. A polyclonal antibody to β-actin was purchased from Sigma Chemical Co., St Louis, MO, USA). Novartis Pharmaceutical (through Dr IJ Fidler, UT MD Anderson Cancer Center) provided PKI166, and a working solution of the tyrosine kinase inhibitor (5 mM in DMSO) was prepared immediately before use.
In vitro growth
Breast cancer cells were plated in 96-well culture plates at an initial density of 2 × 103 cells per well, and allowed to attach for 24 h. The culture medium was changed and the cells were incubated for a further 72 h in the following: medium alone, or with DMSO (0.1% vol vol−1), or PKI166 (0.5 or 5.0 μM), or UO126 (10 μM), or a combination of UO126 and PKI166. Relative cell numbers were determined using MTT. The conversion of MTT to formazan in metabolically viable cells was monitored with an MR-5000 microtiter plate reader reading at 570 nm (Dynatech, Inc Chantilly, VA, USA). All assays were performed in triplicate, with a minimum of three independent experiments.
Measurement of apoptosis
Apoptosis was assessed by measuring DNA fragmentation by propidium iodide staining and FACS analysis (Nicoletti et al, 1991). Cells were incubated with PKI166 (0.5 or 5.0 μM), with U0126 (10 μM), or a combination for 48 h. The cells were harvested, pelleted by centrifugation and resuspended in PBS with 50 μg ml−1 propidium iodide, 0.1% Triton X-100 and 0.1% sodium citrate. Samples were stored overnight at 4°C and vortexed before analysis.
Immunoblotting
Expression of EGFR, HER2, pERK1/2, total ERK1/2 and p27kip1 were detected by immunoblotting. Protein lysates were prepared from cultures of cells that were at 80% confluency. For detection of the effect of PKI166 and/or UO126 on phosphorylation of EGFR and ERK1/2, lysates were prepared from cultures of cells that were grown in serum-free medium for 24 h prior to addition of the inhibitors. After 1 h incubation with the inhibitors, the cells were stimulated with 50 ng ml−1 EGF for 15 min, for assays of EGFR activation. For protein analysis of tumour lysates, fresh tumour tissue was homogenised in lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA and freshly added inhibitors (1 mM Na3VO4, 1 mM PMSF, 1 μg ml−1 pepstatin A, 2 μg ml−1 aprotinin and 0.5 μg ml−1 leupeptin). After homogenisation, NP-40 was added (1% vol vol−1) and the lysate was mixed, cooled on ice for 30 min, and centrifuged at 10 000 g for 10 min Aliquots of 20 mg (tumour lysate) or 20 μg (cell culture lysate) of protein were separated on 7.5% SDS–polyacrylamide gels and electrophoretically transferred onto nitrocellulose membranes. The membranes were hybridised with a polyclonal antibody to EGFR, HER2, pERK1/2 or total ERK1/2, then incubated with an HRP-conjugated anti-rabbit IgG, and antibodies detected with the Amersham ECL system (Amersham, Arlington Heights, IL, USA), following the manufacturer's recommended procedure. Antibodies were removed by incubating filters in stripping buffer (62.5 mM Tris-HCl, pH 6.7, 2% sodium dodecyl sulphate, 100 mM β-mercaptoethanol). The filters were then hybridised with another primary antibody, or with antibody to actin to demonstrate equal loading and transfer of proteins. Immunoreactive bands were quantified by densitometry using ImageQuant software (Molecular Dynamics, Sunnyvale, CA, USA).
RESULTS
Differences in activity of ERK1/2 in the breast cancer cell lines
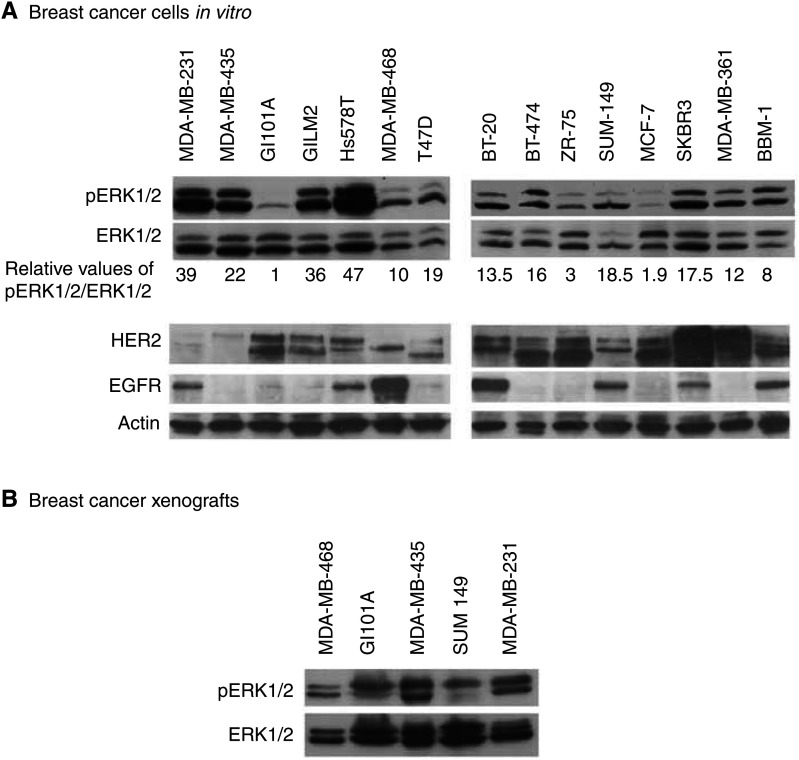
Immunoblotting revealed differences in basal levels of ERK1/2 phosphorylation in different breast cancer cell lines, while the expression of ERK1/2 protein, normalised to actin expression, was relatively consistent (Figure 1A). To test whether the ERK1/2 activity was only a tissue culture phenomenon, selected cell lines were injected into the mammary fatpads of nude mice, and protein lysates were prepared from the tumours. Taking into account the fact that lysates were of a mixture of tumour cells and surrounding stromal and infiltrating host cells, the immunoblotting of the tumour-derived proteins showed similar results to those obtained using lysates of cultured cells. MDA-MB-231 and MDA-MB-435 tumour lysates showed high levels of p-ERK1/2 in comparison to MDA-MB-468 and GI101A tumours (Figure 1B).
Figure 1.
(A) Expression of EGFR, HER2, pERK1/2 and total ERK1/2 in lysates of breast cancer cell lines, determined by immunoblotting. Filters hybridised with antibody against pERK1/2 were re-probed with antibody against total ERK1/2. Densitometry of the immunoreactive bands generated the values shown under the ERK1/2 panel; these represent phosphorylation of ERK1/2 relative to total ERK1/2 and corrected for equal loading relative to actin (actin for the ERK1/2 hybridisations not shown). The pERK1/2/ERK1/2 values were then normalised to the value for GI101A cells, the line with the lowest value and assigned the value=1. The protein samples used for EGFR and HER2 and actin immunoblots were from different preparations, and are representative of the results from repeated experiments. (B) Immunoblot showing pERK1/2 and total ERK1/2 in lysates prepared from xenografts of human breast cancer cells.
Elevated ERK activity does not necessarily correlate with the status of EGFR and HER2 in breast cancer cells
Since ERK1/2 can be activated via EGFR and HER2 signalling, relative expression levels of these growth factor receptors were measured in the panel of cell lines, to test if there was a correlation between ERK activation and receptor expression levels. As expected from the heterogeneity seen in clinical specimens of breast cancer, there was variability in expression of EGFR, from high expression in MDA-MB-468 and minimal expression in MDA-MB-435 cells (Figure 1A). Comparing these results with the level of pERK1/2 indicated that there was no direct correlation between levels of these growth factor receptors and basal levels of ERK1/2 phosphorylation. Thus, while the MDA-MB-231 cell line with highly activated ERK1/2 expressed a relatively high level of EGFR, other combinations occur. High pERK1/2 levels were detected in MDA-MB-435 cells, which have very little EGFR, in contrast to the SUM149 cells with high EGFR expression and low ERK1/2 activity. Similarly, no correlation was found between the expression of HER2 receptor and the status of pERK (Figure 1A).
PKI166 inhibition of breast cancer cell proliferation
Six cell lines with different levels of EGFR expression were selected for treatment with PKI166. Initial studies used a dose range of 0.1–5.0 μM (data not shown), and the results of treating cells with 0.5 and 5.0 μM are shown in Table 1. Growth inhibition was determined from the results of MTT assays, comparing PKI166 treated cells with cells exposed to medium with 0.1% DMSO. Treatment with 0.5 μM PKI166, a concentration less than plasma and tumour concentrations achieved in preclinical models from oral administration of the drug, and the higher dose of 5.0 μM, produced different levels of growth inhibition in different cell lines. As expected, cells expressing low levels of EGFR and HER2, GI101A, MDA-MB-435 showed least growth inhibition. However, not all of the high EGFR-expressing lines were sensitive to PKI166. The lower dose produced 46 and 21% growth inhibition of SUM149 and MDA-MB-468 cells, respectively, but had little effect (3.3% inhibition) on the growth of MDA-MB-231 cells. The SKBR3 cells, expressing EGFR and also high levels of HER2, were most sensitive, showing 55% growth inhibition with 0.5 μM and 76% inhibition with 5.0 μM PKI166.
Table 1. Percent growth inhibition of cells of six breast cancer cell lines by the EGFR tyrosine kinase inhibitor PKI166 and the MEK inhibitor U0126.
|
% Mean growth inhibition (s.d.) |
|||||
|---|---|---|---|---|---|
| U0 | PKI (0.5 μM) | PKI (0.5 μM)+U0 | PKI (5.0 μM) | PKI (5.0 μM)+U0 | |
| MDA-MB-231 | 8.5 (1.1) | 3.3 (0.5) | 26.2 (3.5)a | 33.7 (8.1) | 61.5 (8.65)b |
| MDA-MB-468 | 24.3 (2.5) | 21.1 (1.2) | 54.2 (4.2)a | 48.2 (6.8) | 63.6 (4.2)b |
| SUM149 | 20.0 (8.9) | 46.7 (13.8) | 63.2 (5.2)a | 70.8 (4.2) | 80.7 (4.2)b |
| SKBR3 | 17.5 (1.3) | 55.0 (15.5) | 66.3 (4.6) | 76.3 (5.3) | 86.7 (6.4)b |
| MDA-MB-435 | 68.4 (8.2) | 5.2 (2.1) | 67.2 (6.3)a | 32.2 (4.2) | 74.0 (7.8)b |
| GI101A | 12.0 (2.3) | 13.7 (1.8) | 10.0 (1.2) | 18.5 (2.3) | 23.5 (2.6) |
Growth inhibition of cells cultured in the presence of PKI166 (0.5 or 5.0 μM) or U0126 (10 μM), or the combination for 72 h, calculated from MTT assays, and expressed as a percent growth inhibition compared with cells grown in the presence of DMSO (0.1%) as control. Values shown are mean values from triplicate wells, and are representative of repeated experiments.
Inhibition by PKI (0.5 μM) vs inhibition by PKI (0.5 μM)+U0 (P<0.05).
Inhibition by PKI (5.0 μM) vs inhibition by PKI (5.0 μM)+U0 (P<0.05), analysed using Student's t-tests.
PKI166 inhibits phosphorylation of EGFR and HER2 in breast cancer cells
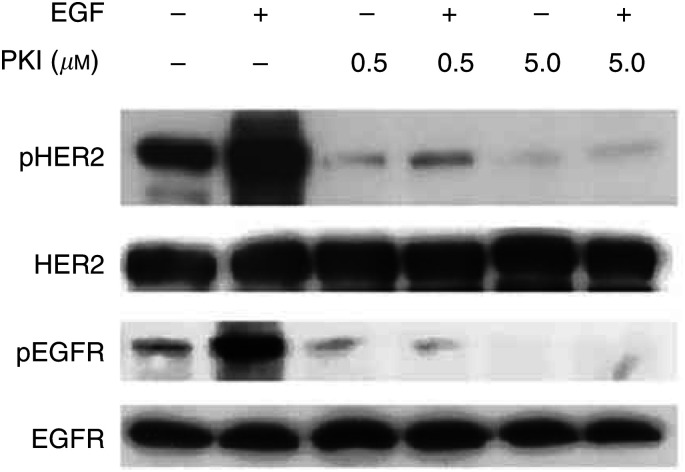
To demonstrate inhibition of EGFR and HER2 phosphorylation by the concentrations of PKI166 used for the growth inhibition assays, cell lysates were prepared from MDA-MB-231, MDA-MB-468, SUM149 and SKBR3 cells after treatment with PKI166 and stimulation with EGF, and phosphorylation of the receptors assessed. PKI166 inhibited ligand-induced EGFR phosphorylation in a dose dependent manner in these four cell lines, and also phosphorylation of HER2 in SKBR3 cells, in the absence or presence of 50 ng ml−1 EGF (Figure 2) (data for other cell lines not shown).
Figure 2.
PKI166 inhibits phosphorylation of HER2 and EGFR in SKBR3 cells. The cells were serum-starved for 24 h before incubation with PKI166 (0.5 or 5.0 μM) for 1 h, then stimulation with EGF (50 ng ml−1) for 15 min before preparation of protein lysates. Phosphorylation of the growth factor receptors was detected with antibodies recognising the activated forms of HER2 and EGFR. The same filters were then reprobed with antibodies recognising the ‘total’ receptors.
Constitutive ERK1/2 phosphorylation as a potential escape mechanism from inhibition by PKI166
Inhibition of growth by PKI166 was most effective in cells with high levels of EGFR and nonactivated ERK1/2 (SUM149, MDA-MB-468) when compared with cells with high EGFR and high basal level of phosphorylated ERK1/2 (MDA-MB-231). To test whether the basal ERK1/2 activity was providing an escape mechanism from inhibition by PKI166, cells were treated with a combination of PKI166 and UO126, an inhibitor of MEK (Table 1). GI101A cells, with low EGFR and nonactivated ERK1/2, showed modest growth inhibition when treated with an individual inhibitor and no significant difference with the combination of the two. MDA-MB-435 cells were significantly inhibited by U0126 alone, and the addition of PKI166 made no difference. The combination of agents significantly increased the antiproliferative action of PKI166 at the 0.5 and 5.0 μM doses in cells expressing higher levels of EGFR or HER2 (SUM149, MDA-MB-468, SKBR3), including MDA-MB-231 cells. Treating the MDA-MB-231 cells with U0126 alone produced 8.5% inhibition, which was not significantly different from control values. The addition of U0126 to 0.5 or 5.0 μM PKI166 significantly increased the growth inhibition produced by the receptor tyrosine kinase inhibitor alone (Table 1).
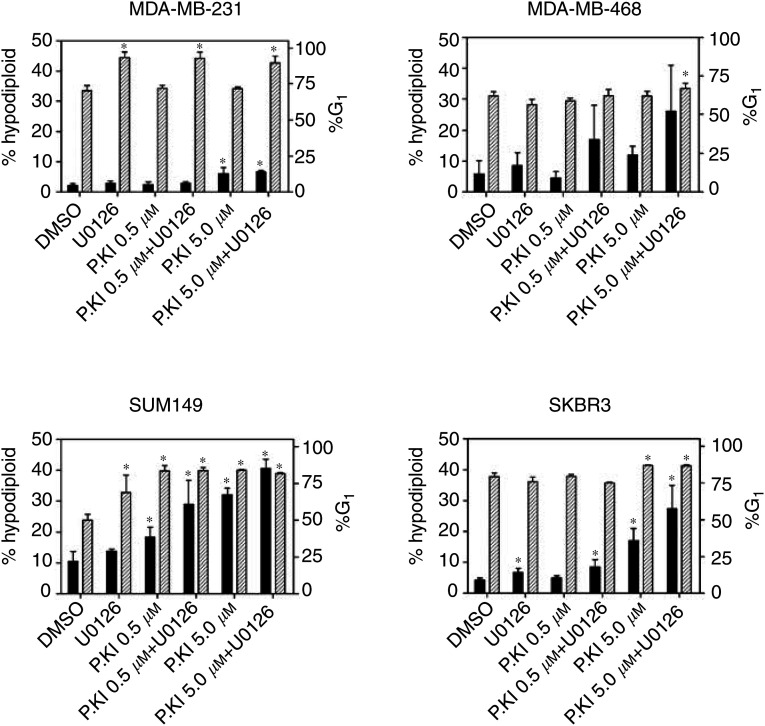
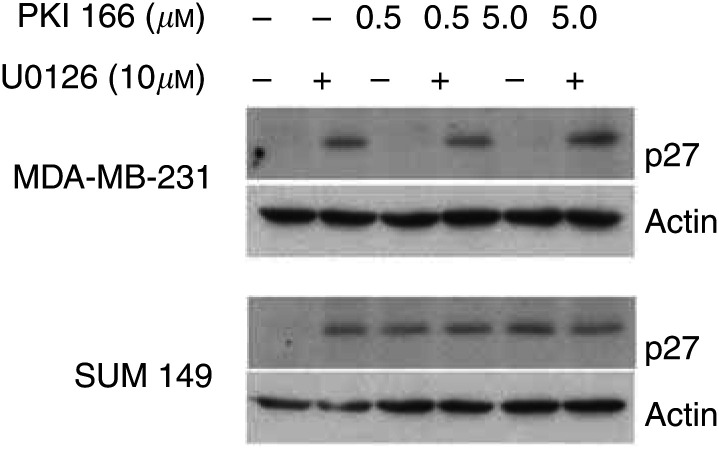
Apoptosis induced by PKI166 and U0126 was assessed by measuring DNA fragmentation by propidium iodide staining and FACS analysis, and determining the proportions of hypodiploid cells. This showed that PKI166 alone or in combination with U0126 induced apoptosis in the EGFR or HER2 expressing cell lines MDA-MB-231, MDA-MB-468, SKBR3 and SUM149 cells (Figure 3), although the proportions of hypodiploid cells varied between the different lines. Similar to the MTT results in Table 1, SKBR3 and SUM 149 cells were most sensitive to treatment with the inhibitors, while the proportions of hypodiploid MDA-MB-231 cells were lower. Treatment with U0126 alone significantly increased the numbers of MDA-MB-231 in the G1 phase of the cell cycle (89–93% compared with 70–72% of control or PKI 166 treated cells, P<0.001). The proportion of SUM149 cells in G1 was significantly increased by treatment with either inhibitor alone and the combination, while apoptosis was significantly increased in cells exposed to PKI166, with or without U0126. Induction of the cyclin-dependent kinase inhibitor p27KIP1 generally corresponded with increases in the proportion of cells in G1, as shown for MDA-MB-231 and SUM149 (Figure 4).
Figure 3.
PKI166 and U0126 induce apoptosis and increase proportions of cells in G1. The percentages of cells in sub-G1/hypodiploid (solid bars) and in G1 (cross-hatched bars) following 48 h treatment with PKI166 (0.5 or 5.0 μM) or U0126 (10 μM), and the combination were determined by FACS analysis of propidium iodide stained cells. Values shown are the mean and SD from three independent experiments, and * indicates a significant difference from the control values, P<0.05, Student's t-test.
Figure 4.
Induction of p27KIP1 by PKI166 and U0126, detected by immunoblotting lysates of cells from the same experiment described in Figure 3. Either inhibitor induced expression of the cyclin-dependent kinase inhibitor in SUM149 cells, while only U0126 produced detectable protein in MDA-MB-231 cells.
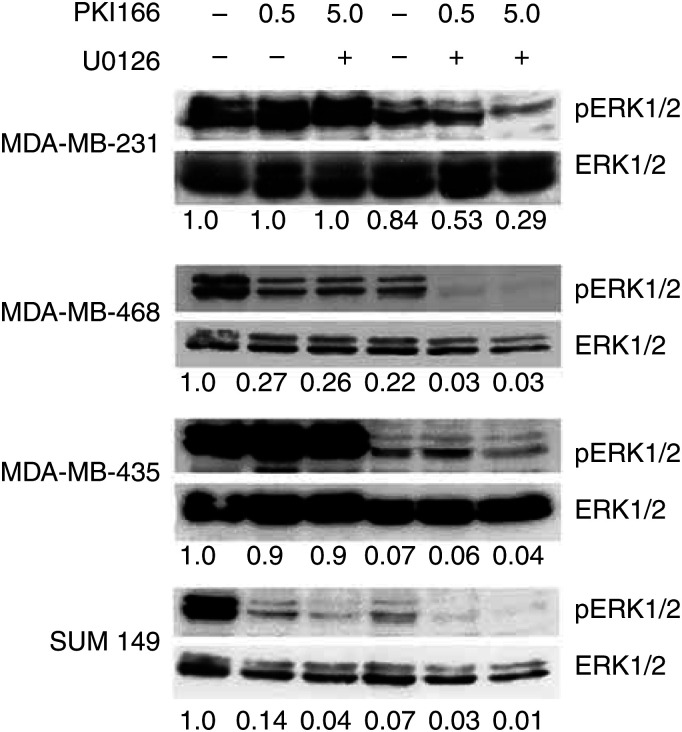
Differential effect of PKI166 on ERK1/2 phosphorylation
To evaluate whether the antiproliferative effects of EGFR inhibition involve ERK1/2 activation, the status of pERK1/2 was determined in cells exposed to the same concentrations of PKI166 used for the growth inhibition assays, in the presence and absence of U0126 (10 μM) (Figure 5). U1026 alone inhibited ERK1/2 phosphorylation in MDA-MB-435 cells, with PKI 166 having no effect, as expected from minimal expression of EGFR in these cells. PKI166 inhibited ERK1/2 phosphorylation in SUM149 cells, as did U0126 alone, and further inhibition by the combination of drugs was barely discernible. Treatment of MDA-MB-468 with either drug resulted in similar inhibition of ERK1/2 phosphorylation, with almost complete elimination of phosphorylated proteins by the combination. PKI166 alone minimally altered the ERK1/2 status in the MDA-MB-231 cells, and U0126 produced some inhibition, while the combination resulted in a substantial reduction, reflecting the effect on cell proliferation and apoptosis. For SUM149 and MDA-MB-468 cells the combination of the inhibitors almost completely eliminated ERK1/2 phosphorylation after 1 h incubation, although growth inhibition over 72 h was 54–63% with 0.5 μM PKI166 plus 10 μM U0126, and 63–81% with 5.0 μM PKI166 plus 10 μM U0126 (Table 1). Recovery of ERK1/2 phosphorylation in the U0126-treated cells over the period of the growth inhibition assays was not investigated, but the data may also suggest that other signal pathways were contributing to the growth and survival of the cells. The effects of the inhibitors were not related to downregulation of total ERK1/2 proteins, as the levels did not decrease with treatment (Figure 5).
Figure 5.
Immunoblots showing pERK1/2 and total ERK1/2 in lysates of cells serum-starved for 24 h, then treated with PKI166 (0.5 or 5.0 μM) or UO126 (10 μM) as indicated, for 1 h before preparation of the lysates. Values of the relative expression of pERK1/2 expressed as a ratio of total ERK1/2 expression, and corrected for equal loading relative to actin (not shown) indicated below the immunoblots were determined by densitometry, and expressed as proportion of the control values (cells treated with DMSO).
DISCUSSION
Due to growing disappointment with current therapies for breast cancer, which have not lead to significant alleviation of metastatic disease, more attention has been focused on developing novel targeted therapies (Bange et al, 2001). Major targets are members of the erbB family of growth factor receptors, principally EGFR and HER2, which are known to play important roles in breast cancer pathogenesis (Lacroix et al, 1989; Slamon et al, 1990; Klijn et al, 1992). As the targeted agents move into clinical trials it will be important to have a clear understanding of the EGFR-dependent pathways and their patterns of expression and activation. The present study focuses on the relationship between EGFR and the MAPK pathway. This choice was not arbitrary, as this pathway is one of the major downstream signalling pathways from EGFR. Activated ERK1/2 control many processes that are central to malignant progression, including cell growth, apoptosis and migration (Pages et al, 1993; Campbell et al, 1995; Hoshino et al, 1999; Schramek, 2002).
Disrupting regulation of the MAPK pathway can predispose cells to undergo tumorigenic transformation, as illustrated by the position of the ras oncogene upstream of ERK (Zhang et al, 1993). Transfection with constitutively active forms of MEK resulted in transformation, increased sensitivity to, or independence from growth factors in vitro, and tumorigenicity in vivo (Brunet et al, 1994; Mansour et al, 1994). A few studies have assessed the expression of activated MAPK in different clinical tumour specimens, and asked if this has any clinical or biological correlation. In renal cell carcinoma, MAPK activation correlated with MAPK kinase activation and Raf-1 activation (Oka et al, 1995), while for hepatocarcinomas a relationship was reported between ERK1/2 activation and expression of the transcription factor c-Fos and cyclin D1 (Ito et al, 1998). High levels of activated MAPK were found in high grade and advanced stage prostate cancers, suggesting a link between elevated ras signalling and advanced disease (Gioeli et al, 1999). Activated MAPK was also reported in glial tumours and in a group of oligodendrogliomas, an increase in the proportions of cells expressing activated MAPK corresponded with malignant progression (Mandell et al, 1998). Sivaraman et al (Itoh et al, 2002) provided the first demonstration of MAPK activation in human breast cancer tissues, comparing primary breast cancer with benign lesions using substrate-based MAPK enzyme assays and immunoblotting. They found significantly less MAPK activity to be in benign breast tissues compared with invasive breast cancers. In another study, a 2.5-fold increase in activated MAPK was seen in breast cancer specimens compared to normal breast tissue, and this was associated with poor prognosis and decreased sensitivity to endocrine therapy, and with expression of phosphorylated c-jun, a transcription factor activated by MAPK (Gee et al, 2000; Donovan et al, 2001). Phosphorylated MAPK is also thought to be involved in acquisition of resistance to antioestrogen treatment in ER-positive cells, as it has been shown to be one of the characteristics of advanced breast cancer and involved in the progression to oestrogen-independence (Jeng et al, 2000; Gee et al, 2001). In a recent study of human breast cancer specimens, activated MAPK detected by immunohistochemistry was increased in lymph node metastases compared with primary tumours, suggesting a role in the metastatic process (Adeyinka et al, 2002).
The causes of MAPK activation in human cancers vary among the different types of cancer. In many, ERK activation reflects the activity of mutated forms of ras. However, in breast cancer activating ras mutations are relatively rare, reported in only 5% of cases (Bos, 1989; Dickson et al, 1991), leading to the view that ras mutations do not have an important role in this disease. However, it is not easy to distinguish whether an apparent constitutive elevation in basal activity of ERK1/2 is due to an inherent alteration in the pathway regulation, or if the pathway is more sensitive to stimulation by an exogenous ligand. One possible explanation can still be connected to ras, as any one of the three major genes (H-ras, K-ras, N-ras) may be overexpressed in breast cancer, and this correlates with cancer progression (Von Lintig et al, 2000). Wild-type ras is subject to regulation by GTPase activating proteins and guanine nucleotide exchange factors by different upstream receptors, one of which is EGFR. A correlation between overexpression of EGFR and enhanced ERK activity has been previously suggested (Xing and Imagawa, 1998), and the present study explores the relationship further. The results, using several different cell lines in an attempt to mimic the heterogeneity of clinical breast cancer cases, do not fully support the previous report. We found that the levels of activated ERK1/2 did not uniformly correlate with EGFR or HER2 status in the different cell lines, and that different combinations of EGFR and low pERK1/2, or low EGFR and high pERK1/2 were found in the panel of cell lines studied. The results suggest that although EGFR and HER2 can signal through ERK1/2, an increase in receptors does not necessarily result in sustained ERK1/2 activity. Also, the hyperactivity of ERK1/2 in some of the cells studied may be secondary to other genetic, or epigenetic, alterations, for example the K-ras mutation in the MDA-MB-231 cell line (Davidson et al, 1987). Another potential explanation for elevation in ERK1/2 activation is a change in the expression or activation of specific threonine/tyrosine phosphatases that inactivate pERK1/2 (Tonks and Neel, 1996; Cook et al, 1997). Recently, it was shown that the expression of the MKP-1 phosphatase in Rat-1 cells was controlled by growth factors acting via ERK- and calcium-dependent pathways. Treatment with the phosphatase inhibitor sodium orthovanadate elevated basal ERK1/2 activity in the absence of growth factors (Cook et al, 1997). Thus, an attenuation of phosphatase expression or activity might be manifest as a rise in basal ERK1/2 activation.
Administration of PKI166 has been shown to be effective in controlling tumour growth and metastasis when used in combination with chemotherapy agents in preclinical models, including pancreatic cancer (Bruns et al, 2000), bladder and renal cell cancer (Baker et al, 2002). While used to target EGFR phosphorylation, PKI166 also inhibits phosphorylation of HER2 (Traxler et al, 2001). In this study the high HER2-expressing SKBR3 cell line was one of the more sensitive to growth inhibition and apoptosis induced by PKI166. As previously reported (Moasser et al, 2001; Bishop et al, 2002), and supported by the results of this study, a relatively high level of the target is needed to see the efficacy of an anti-EGFR agent. Similar to these previous reports, we also found that not every cell line expressing high levels of EGFR was growth inhibited by PKI166. Insensitive lines either had relatively little receptor expression or had a high basal activity of ERK1/2, the latter of which appeared to protect the cells from EGFR-signalling blockade. The prediction from this in the clinical situation is that while some tumours expressing high levels of EGFR will respond well to EGFR inhibitors, others may not. Determination of the levels of pERK1/2, in addition to expression of EGFR, in pretreatment biopsies may allow prediction of the response of a patient to anti-EGFR therapy.
Blocking EGFR signalling has been shown to stabilise the cyclin-dependent kinase inhibitor p27KIP1 and leads to G1 cell cycle arrest (Busse et al, 2000; Di Gennaro et al, 2003). PKI166 alone, and in combination with U0126 reduced ERK1/2 activity, and induced p27KIP1 expression in SUM149 cells. In contrast, only treatment with U0126 was able to induce G1 arrest and p27KIP1 in MDA-MB-231 cells, which were relatively insensitive to PKI166 alone. U0126 blocks MAPK signalling pathways by preventing activation of MEK1/2 (Davies et al, 2000), and it is also reported to block ERK5/BMK1 phosphorylation at the concentration used in this study (Kamakura et al, 1999). Signalling through ERK5 contributes to cyclin D1 regulation in breast cancer cells (Mulloy et al, 2003). Thus, the antiproliferative effects of U0126 may be due to the inhibition of ERK1/2 and ERK5 mediated signalling.
This study focused on the consequences of blocking EGFR and ERK signalling. However, even in the most sensitive cell lines, such as SUM149 and MDA-MB-468, in which ERK1/2 phosphorylation was almost completely inhibited, the growth inhibition or apoptosis induction were not more than 50–80% of control values. One explanation is that additional signalling pathways, insensitive to either PKI166 or U0126, were maintaining the viability of the cells. The phosphoinositide 3-kinase (PI3K) pathway is sensitive to EGFR-signalling blockade, promoting cell death through inhibition of AKT (Busse et al, 2000; Anderson et al, 2001; Moasser et al, 2001; Clark et al, 2002). PKI166 reduced basal levels of AKT activity in SUM 149, SKBR3 and MDA-MB-468, and inhibited EGF-induced AKT activity in MDA-MB-231 cells (data not shown). Similar to what this study found for ERK1/2 activity, the activation status of the PI3K/AKT pathway may influence responses to inhibitors. For example, Moasser et al (2001) found that MDA-MB-468 cells were relatively resistant to the tyrosine kinase inhibitor ZD1839, and this was attributed to the high basal AKT activity, resulting from deletion of the PTEN tumour suppressor (Lu et al, 1999).
The present study underscores the fact that the overexpression of EGFR or HER2 does not predict sensitivity to a therapy targeted to these receptors. Moreover, strategies designed to block more than one protein or pathway are likely to potentiate antiproliferative responses. Considering that cells in advanced breast cancers can have multiple mutations and genetic alterations, it is likely that therapeutic combinations targeting multiple pathways or key proteins will be more effective than single or nontarget-specific agents. Although ERK1/2 are not abnormal proteins, expression at abnormally high and sustained levels may be a potential target for pharmacological intervention for proliferative diseases, including cancer. The blockade of the MAPK pathway with an MEK inhibitor given orally suppressed the growth of colon tumours transplanted in mice, with no apparent side effects (Sebolt-Leopold et al, 1999). In our study, the combination of U0126 with PKI166 resulted in significant growth inhibition and apoptosis in cells expressing EGFR and pERK1/2. These results suggest that there is a strong molecular rationale supporting the continued development of inhibitors of the MAPK pathway, and for using them in combination with inhibitors of growth factor receptors such as the tyrosine kinase inhibitor PKI 166.
Acknowledgments
We thank Dr IJ Fidler for providing PKI 166, and gratefully acknowledge the technical assistance of Galina Kiriakova, and Karen Ramirez for expert assistance with FACS analyses. The work was supported in part by DAMD17-00-1-0315 from the US Army Medical Research and Materiel Command (JEP), an award from the Texas Higher Education Coordinating Board (JEP), RO1-CA46523 (HNA) and Cancer Center Support Core Grant CA 16672 from the National Cancer Institute. D Chelouche Lev was supported by an American Physicians Fellowship.
References
- Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC (2002) Activated mitogen-activated protein kinase expression during breast tumorigenesis and breast cancer progression. Clin Cancer Res 8: 1747–1753 [PubMed] [Google Scholar]
- Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J (2001) Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor α expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res 61: 6500–6510 [PubMed] [Google Scholar]
- Anderson NG, Ahmad T, Chan K, Dobson R, Bundred NJ (2001) ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int J Cancer 94: 774–782 [DOI] [PubMed] [Google Scholar]
- Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, Fidler IJ (2002) Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol 161: 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange J, Zwick E, Ullrich A (2001) Molecular targets for breast cancer therapy and prevention. Nat Med 7: 548–552 [DOI] [PubMed] [Google Scholar]
- Bishop PC, Myers T, Robey R, Fry DW, Liu ET, Blagosklonny MV, Bates SE (2002) Differential sensitivity of cancer cells to inhibitors of the epidermal growth factor receptor family. Oncogene 21: 119–127 [DOI] [PubMed] [Google Scholar]
- Bos JL (1989) Ras oncogenes in human cancer: a review. Cancer Res 49: 4682–4689 [PubMed] [Google Scholar]
- Brunet A, Pages G, Pouyssegur J (1994) Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene 9: 3379–3387 [PubMed] [Google Scholar]
- Bruns CJ, Solorzano CC, Harbison M, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ (2000) Blockade of the epidermal growth factor receptor by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res 60: 2926–2935 [PubMed] [Google Scholar]
- Busse D, Doughty RS, Ramsey TT, Russell WE, Price JO, Flanagan WM, Shawver LK, Arteaga CL (2000) Reversible G1 arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27KIP1 independent of MAPK activity. J Biol Chem 275: 6987–6995 [DOI] [PubMed] [Google Scholar]
- Campbell J, Segar R, Graves J, Graves L, Jensen A, Krebs E (1995) The MAP kinase cascade. Recent Prog Horm Res 50: 131–159 [DOI] [PubMed] [Google Scholar]
- Clark AS, West K, Streicher S, Dennis PA (2002) Constitutive and inducible Akt activity promotes resistance to chemotherapy, Trastuzumab, or Tamoxifen in breast cancer cells. Mol Cancer Ther 1: 707–712 [PubMed] [Google Scholar]
- Cook S, Beltman J, Cadwallader K, McKahon M, McCormick F (1997) Regulation of mitogen-activated protein kinase phosphatase-expression by extracellular signal-related dependent and Ca2+-dependent signal pathways. J Biol Chem 272: 13309–13319 [DOI] [PubMed] [Google Scholar]
- Davidson NE, Gelmann EP, Lippman ME, Dickson RB (1987) Epidermal growth factor receptor gene expression in estrogen receptor-positive and -negative human breast cancer cell lines. Mol Endocrinol 1: 216–223 [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanisms of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro E, Barbarino M, Bruzzese F, de Lorenzo S, Caraglia M, Abbruzzese A, Avallone A, Comella P, Caponigro F, Pepe S, Budillon A (2003) Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 (‘Iressa’), an epidermal growth factor receptor tyrosine kinase inibitor, in head and neck squamous carcinoma cells. J Cell Physiol 195: 139–150 [DOI] [PubMed] [Google Scholar]
- Dickson RB, Gottardis MM, Merlino GT (1991) Molecular insights into breast cancer from transgenic mouse models. BioEssays 13: 591–596 [DOI] [PubMed] [Google Scholar]
- Dillon DA, Howe CL, Bosari S, Costa J (1998) The molecular biology of breast cancer: accelerating clinical applications. Crit Rev Oncogenesis 9: 125–140 [DOI] [PubMed] [Google Scholar]
- Donovan J, Milic A, Slingerland JM (2001) Constitutive MEK/MAPK activation leads to p27kip1 deregulation and antiestrogen resistance in human breast cancer. J Biol Chem 276: 40888–40895 [DOI] [PubMed] [Google Scholar]
- Gee JM, Barroso AF, Ellis IO, Robertson JF, Nicholson RI (2000) Biological and clinical associations of c-jun activation in human breast cancer. Int J Cancer 89: 177–186 [DOI] [PubMed] [Google Scholar]
- Gee JM, Robertson JF, Ellis IO, Nicholson RI (2001) Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer 95: 247–254 [DOI] [PubMed] [Google Scholar]
- Gioeli D, Mandell JW, Petroni GR, Frierson HF, Weber MJ (1999) Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 59: 279–284 [PubMed] [Google Scholar]
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE (1997) Erb-B2, the preferred heterodimerization partner of all erbB receptors, is a mediator of lateral signalling. EMBO J 16: 1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi GN (2000) Developments in chemotherapy of breast cancer. Cancer 88: 3073–3079 [DOI] [PubMed] [Google Scholar]
- Hoshino K, Chatani Y, Yamori T, Tsuruo T, Hiroya O, Yoshinada O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M (1999) Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 18: 813–822 [DOI] [PubMed] [Google Scholar]
- Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano Y, Yamamoto H, Okamoto E, Hatashi N, Hori M (1998) Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology 27: 951–958 [DOI] [PubMed] [Google Scholar]
- Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M (2002) Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 94: 3127–3134 [DOI] [PubMed] [Google Scholar]
- Jeng MH, Yue W, Eischeid A, Wang JP, Santen RJ (2000) Role of the MAP kinase in the enhanced cell proliferation of long term estrogen deprived human breast cancer cells. Breast Cancer Res Treat 62: 167–175 [DOI] [PubMed] [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E (1999) Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem 274: 26563–26571 [DOI] [PubMed] [Google Scholar]
- Kerbel RS (2000) Tumor angiogenesis: past, present and the near future. Carcinogenesis 21: 505–515 [DOI] [PubMed] [Google Scholar]
- Klapper LN, Kirschenbaum MH, Sela MYY (2000) Biochemical and clinical implications of ErbB/HER signaling network of growth factor receptors. Adv Cancer Res 77: 25–79 [PubMed] [Google Scholar]
- Klijn JG, Berns PM, Schmitz PI, Foekens JA (1992) The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocrine Rev 13: 3–17 [DOI] [PubMed] [Google Scholar]
- Lacroix H, Igelhert JD, Skinner MA, Kraus MH (1989) Over-expression of erbB-2 or EGF receptor proteins in early stage mammary carcinoma is detected simultaneously in matched primary tumors and regional metastases. Oncogene 4: 145–151 [PubMed] [Google Scholar]
- Lu Y, Lin Y-Z, LaPushin R, Cuevas B, Fang X, Yu SX, Davies MA, Khan H, Furui T, Mao M, Zinner R, Hung MC, Steck P, Siminovitch K, Mills GB (1999) The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene 18: 7034–7045 [DOI] [PubMed] [Google Scholar]
- Mandell JW, Hussaini I, Zecevic M, Weber MJ, VandenBerg SR (1998) In situ visualization of intratumor growth factor signaling: immunohistochemical localization of activated ERK/MAP kinase in glial neoplasms. Am J Pathol 153: 1411–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S, Matten W, Hermann A, Candia J, Rong S, Fukasawa K, Vande Woude G, Ahn N (1994) Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265: 966–970 [DOI] [PubMed] [Google Scholar]
- Mendelsohn J (2001) The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer 8: 3–9 [DOI] [PubMed] [Google Scholar]
- Mendelsohn J, Baselga J (2000) The EGF receptor family as targets for cancer therapy. Oncogene 19: 6550–6565 [DOI] [PubMed] [Google Scholar]
- Moasser MM, Basso A, Averbuch SD, Rosen N (2001) The tyrosine kinase inhibitor ZD1839 (‘Iressa’) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res 61: 7184–7188 [PubMed] [Google Scholar]
- Moghal N, Sterenberg PW (1999) Multiple positive and negative regulators of signaling by the EGF-receptor. Curr Opin Cell Biol 11: 190–196 [DOI] [PubMed] [Google Scholar]
- Mueller H, Flury N, Eppenberger-Castori S, Kueng W, David F, Eppenberger U (2000) Potential prognostic value of mitogen-activated protein kinase activity for disease-free survival of primary breast cancer patients. Int J Cancer 89: 384–388 [DOI] [PubMed] [Google Scholar]
- Mulloy R, Salinas S, Philips A, Hipskind RA (2003) Activation of cyclin D1 expression by the ERK5 cascade. Oncogene 22: 5387–5398 [DOI] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139: 271–279 [DOI] [PubMed] [Google Scholar]
- Oka H, Chatani Y, Hoshno R, Ogawa O, Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M, Yoshida O (1995) Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res 55: 4182–4187 [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane AH, Hynes NE (2000) The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur M (1993) Mitogen-activated protein kinases P42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA 90: 8319–8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF (2001) A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm 16: 125–132 [DOI] [PubMed] [Google Scholar]
- Price JE (1996) Metastasis from human breast cancer cell lines. Breast Cancer Res Treat 39: 93–102 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Xinde Song R, McPherson R, Kumar R, Adam L, Jeng MH, Yue W (2002) The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol 80: 239–256 [DOI] [PubMed] [Google Scholar]
- Schramek H (2002) MAP kinases: from intracellular signals to physiology and disease. News Physiol Sci 17: 62–67 [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR (1999) Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med 5: 810–816 [DOI] [PubMed] [Google Scholar]
- Sivaraman V, Wang HY, Nuovo G, Malbon C (1997) Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest 99: 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W (1987) Human breast cancer: correlation of relapse and survival with the amplification of HER2/neu oncogene. Oncogene 235: 177–182 [DOI] [PubMed] [Google Scholar]
- Slamon D, Godolphin W, Jones L, Holt J, Wong S, Keith D, Levin W, Stuart S, Udove J, Ullrich A, Press M (1990) Studies of the HER2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712 [DOI] [PubMed] [Google Scholar]
- Slichenmyer WJ, Fry DW (2001) Anticancer therapy targeting the erbB family of receptor tyrosine kinases. Semin Oncol 28: 67–79 [DOI] [PubMed] [Google Scholar]
- Tonks N, Neel B (1996) From form to function: signaling by protein tyrosine phosphatases. Cell 87: 365–368 [DOI] [PubMed] [Google Scholar]
- Traxler P, Bold G, Buchdunger E, Caravatti G, Furet P, Manley P, O'Reilly T, Wood J, Zimmermann J (2001) Tyrosine kinase inhibitors: from rational design to clinical trials. Med Res Rev 21: 499–512 [DOI] [PubMed] [Google Scholar]
- Von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Darren EC, Boss GR (2000) Ras activation in human breast cancer. Breast Cancer Res Treat 62: 51–62 [DOI] [PubMed] [Google Scholar]
- Xing C, Imagawa W (1998) Altered MAP kinase (ERK1,2) regulation in primary cultures of mammary tumor cells: elevated basal activity and sustained response to EGF. Carcinogenesis 20: 1201–1208 [DOI] [PubMed] [Google Scholar]
- Zhang XF, Settleman J, Kyriakis JM, Takeuchi-Suzuki E, Elledge SJ, Marshall MS, Bruder JT, Rapp UR, Avruch J (1993) Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature 364: 308–313 [DOI] [PubMed] [Google Scholar]