Abstract
We examined the potential of quantitative epidermal growth factor receptor (EGFR, synonym: c-erbB-1) and c-erbB-2 (synonym: HER2/neu) mRNA expression to predict minor or major histopathologic response to neoadjuvant radiochemotherapy (cis-platinum, 5-FU, 36 Gy), followed by radical surgical resection, in patients with oesophageal cancer. Tissue samples were collected by endoscopic biopsy prior to treatment. RNA was isolated from biopsies and quantitative real-time reverse transcriptase–polymerase chain reaction assays were performed to determine c-erbB-1 and c-erbB-2 mRNA expression. Relative expression (tumour/paired normal tissue ratio standardised for β-actin) was calculated for EGFR and c-erbB-2 mRNA. Expression levels were correlated with the objective histopathologic response in resected specimens. Histomorphologic regression was defined as major response when resected specimens contained less than 10% of residual vital tumour cells, or in case a pathologically complete response was achieved. Expression of c-erbB-1 mRNA was not associated with the degree of histomorphological response. In contrast, the relative expression levels of c-erbB-2 mRNA >1 were not associated with major histopathologic responses (sensitivity 41.6%, specificity 100%), and 10 out of 36 (28%) patients could be unequivocally identified, whose tumours did not respond well to the delivered neoadjuvant radiochemotherapy (P<0.01). Quantitative expression levels of c-erbB-2, but not c-erbB-1 mRNA, in pretreatment biopsies appear to predict minor histopathologic response to our neoadjuvant radiochemotherapy protocol. This test could be used to prevent expensive, noneffective and potentially harmful therapies in approximately one-fourth of our patients, and leads to a more individualised type of combined modality treatment.
Keywords: growth factor receptor, gene expression, response prediction, radiosensitivity, chemosensitivity, multimodality treatment
Patients with locally advanced oesophageal cancers have a poor prognosis when treated exclusively by surgical resection. Therefore, many investigators apply neoadjuvant treatment strategies in an effort to improve survival (Sherman et al, 2002). Results from phase III randomised trials are encouraging; however, they revealed that only patients with major histopathologic response will eventually benefit from treatment (Walsh et al, 1996; Urba et al, 2001; MRC Esophageal Study Group, 2002). In addition, these therapies are expensive and associated with increased therapy-induced complication rates (Kelsen, 2000). Predictive molecular markers indicating response or nonresponse to neoadjuvant treatment would be extremely helpful in selecting patients for future treatment protocols.
Epidermal growth factor receptor (EGFR, synonym: c-erbB-1) and c-erbB-2 (synonym: HER2/neu) are members of the type I growth factor receptor gene family (Gullick, 2001). The c-erbB-1 gene encodes a 170 kDa membrane protein. Under physiologic conditions, binding of epidermal growth factor (EGF) or transforming growth factor-α (TGF-α) leads to receptor kinase activity, and subsequently a complex cascade of events that can induce cellular proliferation (Harris et al, 2003; Jorissen et al, 2003). The c-erbB-2 gene encodes a 185 kDa transmembrane glycoprotein (p185) with tyrosine kinase activity (Wang and Hung, 2001).
Overexpression of c-erbB-1 or c-erbB-2 mRNA and/or protein has been implicated in the pathogenesis of various solid tumours including breast, ovarian, lung and oesophageal cancers; however, association with prognosis varied substantially between studies and tumour entities (Meden et al, 1994; Singleton et al, 1994; Schneider et al, 2000; Brabender et al, 2001; Esteva et al, 2002; Lau et al, 2002).
In tumour cell lines, the sensitivity to several chemotherapeutic agents (Kroning et al, 1995; Dixit et al, 1997; Nouri et al, 2000) and radiotherapy (Kwok and Sutherland, 1989; Bonner et al, 2002) was significantly modified by EGF or EGFR expression levels. In addition, there is evidence that blocking EGFR could further increase chemo- and radiosensitivity of tumour cells expressing high receptor levels in vitro and in vivo (Anderson and Jankowski, 2003; Gee and Nicholson, 2003).
Several studies demonstrated an association between the expression of c-erbB-2 and the response to adjuvant chemotherapy in breast cancer (Allred et al, 1992; Muss et al, 1994; Miles et al, 1999; Menard et al, 2001). These results, however, are challenged by negative data in other adjuvant or neoadjuvant trials (Rozan et al, 1998; Wang et al, 2002; Zhang et al, 2003). Trial results might also depend on the chemotherapeutic agents used (Muss et al, 1994; Wang et al, 2002). In addition, anti-p185-specific antibodies enhanced cis-platin (CDDP) sensitivity (Hancock et al, 1991; Pietras et al, 1994) and radiosensitivity (Pietras et al, 1999) in vitro, and promising results have already been reported in a clinical phase II trial using a combination of recombinant anti-p185-HER2/neu monoclonal antibody plus CDDP in refractory metastatic breast cancer (Pegram et al, 1998).
The purpose of this prospective study was to investigate the potential of quantitative c-erbB-1 and c-erbB-2 mRNA expression in pretreatment biopsies to predict a minor or major histopathologic response to neoadjuvant therapy with CDDP, 5-fluorouracil (5-FU) and simultaneous radiation (36 Gy), followed by surgical resection in oesophageal cancers.
PATIENTS AND METHODS
Study population, demographic data and neoadjuvant therapy
All patients were recruited from an ongoing clinical trial on neoadjuvant radiochemotherapy for oesophageal cancer. None of the patients had prior radio- and/or chemotherapy.
In all, 36 consecutive patients (median age: 59.6 years, range 29.5–72.7; gender: 29 men, seven women) with locally advanced, resectable oesophageal cancers (cT2-4, Nx, M0, UICC/AJCC TNM Classification) in good general health condition (ECOG performance status 0–1) and normal to moderate risk factors for oesophageal surgery (Bollschweiler et al, 2000) were offered standardised neoadjuvant radiochemotherapy. Clinical staging was based on barium swallow, endoscopic ultrasound and CT of the chest and abdomen. Diagnostic laparoscopy was performed in all patients with adenocarcinomas to exclude peritoneal carcinomatosis. CDDP (20 mg m2 day−1) was administered as short-term infusion on days 1–5 and 5-fluorouracil (1000 mg m2 day−1) as continuous infusion over 24 h on days 1–5. Radiation therapy was administered by linear accelerators with 10–15 MV photons. Radiation therapy was simulated to encompass the tumour volume with 5 cm cephalo-caudad-margins and 2 cm radial margins, and treatment ports were designed to include enlarged regional nodes based on CT evaluation and endoscopic ultrasound. Radiation was delivered in daily fractions of 1.8 Gy (days 1–5, 8–12, 15–19 and 22–26) to a total dose of 36 Gy using a multiple-field technique. Surgical resection was performed 4–5 weeks following completion of chemoradiation after clinical restaging using the same procedures as for staging, except for laparoscopy. Standardised transthoracic en bloc oesophagectomy with two-field lymphadenectomy and reconstruction by gastric tube interposition with either left cervical or high intrathoracic anastomosis was performed in all patients (Schroeder et al, 2002). Clinical data of the patients are summarised in Table 1 .
Table 1. Clinical and histopathologic parameters (patients with tumour resection: 36).
| Parameter | Subtype | Number | % |
|---|---|---|---|
| Histology | Squamous | 23 | 63.9 |
| Adeno | 13 | 36.1 | |
| ypT-categorya | T0 | 2 | 5.6 |
| T1 | 1 | 2.8 | |
| T2 | 7 | 19.4 | |
| T3 | 25 | 69.4 | |
| T4 | 1 | 2.8 | |
| ypN-categoryb | N0 | 15 | 41.7 |
| N1 | 21 | 58.3 | |
| R-categoryc | R0 | 31 | 86.1 |
| R1 | 5 | 13.9 | |
| R2 | 0 | 0 | |
| Grading | G1 | 0 | 0 |
| G2 | 12 | 33.3 | |
| G3 | 24 | 66.7 | |
| Regression graded | I | 15 | 41.7 |
| II | 9 | 25 | |
| III | 10 | 27.8 | |
| IV | 2 | 5.6 |
Histopathologic tumour category after neoadjuvant therapy according to UICC (Union Internationale Contre Le Cancer, 5th edition, 1997).
Histopathologic lymph node category after neoadjuvant therapy according to UICC (Union Internationale Contre Le Cancer, 5th edition, 1997).
Residual tumour category according to UICC (Union Internationale Contre Le Cancer, 5th edition, 1997).
Grade I: >50% vital residual tumour cells (VRTC), grade II: 10–50% VRTC, grade III: nearly complete response (NCR) with <10% VRTC and grade IV: complete response (pCR, ypT0).
Informed consent was obtained from each patient and the scientific protocol was approved by the local ethic committee.
Histomorphologic grading of tumour regression
Since clinical response evaluation after neoadjuvant therapy for oesophageal cancer was known to be highly inaccurate (Adelstein et al, 1997), objective histopathologic response analysis was applied using morphologic criteria described by Junker et al (1997).
The resected specimens were fixed in 10% formaldehyde, en bloc embedded in paraffin and sectioned into 5-μm slices which were stained with haematoxylin and eosin. These sections were used for both histopathologic staging according to the TNM classification system (UICC 5th edition, 1997) and histomorphologic evaluation of the effect of radiochemotherapy. The degree of histomorphologic regression was classified into four categories: grade I: >50% vital residual tumour cells (VRTC), grade II: 10–50% VRTC, grade III: nearly complete response (NCR) with <10% VRTC and grade IV: complete response (pCR, ypT0). This analysis was performed by two independent staff pathologists who were blinded for all other clinical data (SEB and HPD), and there was no interobserver variation in response classification.
Regression grades III and IV were considered as major histomorphologic response (MaHR) compared to grades I and II constituting minor histopathologic response (MiHR).
Tissue acquisition and RNA isolation
Tissue samples from oesophageal cancers and corresponding normal tissues were collected by endoscopic biopsy prior to starting neoadjuvant treatment. Samples were snap-frozen in liquid nitrogen and stored at −80°C until further processing. Samples were carefully chosen after control staining with haematoxylin and eosin of individual biopsies, and contained >50% tumour cells.
Total cellular RNA was isolated using Trizol reagent (Life Technologies/GIBCO, Grand Island, NY, USA) and quantitated at A260/280 nm (Smart Spec; Biorad, Hercules, CA, USA).
Real-time RT–PCR assay
Total cellular RNA (0.5 μg) was reverse-transcribed using an oligo (dT)18 primer and Moloney murine leukaemia virus (MMLV) reverse transcriptase (Clontech Lab, Palo Alto, USA), according to the manufacturer's recommendation. Placenta RNA from this kit was used to prepare standard curves. An amount of 25 ng of cDNA was taken for real-time PCR using the Light Cycler System (Roche, Mannheim, Germany). Amplification was monitored by SybrGreen intercalation. For hot start LC-DNA, Master SYBR Green was preincubated with TaqStart™ antibody (Clontech Lab, Palo Alto, USA), as suggested by the manufacturer. In addition, the 10 μl reaction volume contained 2 mM MgCl2 and 1 μM of each primer. Primers used for PCR amplification were chosen to encompass intron between exon sequences to identify false-positive DNA amplification.
Three sets of published primers EGFR-F/EGFR-R (Pawlowski et al, 2000), ERB-2/ERB-3 (Ninomiya et al, 1992) and P5/P6 (Tokuchi et al, 1999) were purchased (Eurogentec, Seraing, Belgium) and used to amplify EGFR mRNA, c-erbB-2 mRNA and β-actin mRNA, respectively. A 250 bp amplification product was obtained for c-erbB-1 mRNA, a 183 bp product for c-erbB-2 and a 276 bp product for β-actin mRNA. We used the expression of β-actin mRNA to normalise the level of expression of EGFR and c-erbB-2 mRNA. Thermal cycling conditions for EGFR were 30 s at 95°C for initial denaturation, followed by 35 cycles of 95°C for 0 s, 68°C for 20 s and 72°C for 20 s; for c-erbB-2, 30 s at 95°C for initial denaturation, followed by 35 cycles of 95°C for 0 s, 64°C for 20 s and 72°C for 20 s; and for β-actin, 30 s at 95°C, followed by 40 cycles of 95°C for 0 s, 63°C for 20 s and 72°C for 20 s. We used serial dilutions of standard cDNA synthesised from human placenta total cellular RNA (Clontech Lab Inc, Palo Alto, CA, USA) for the quantification analysis. Triplicates of the clinical samples and duplicates of standard cDNA dilutions were assayed in each run. Product purity was controlled by melting point analysis and PCR products were further analysed electrophoretically in 2% agarose gels using a Horizon 58 electrophoresis chamber (GIBCO/BRL, Eggenstein, Germany) and visualised by ethidium bromide staining.
Absolute mRNA expression levels were calculated as c-erbB-1 or c-erbB-2/β-actin in tumour and normal tissue, respectively, and relative mRNA expression levels (ratio tumour/normal) were calculated as (c-erbB-1 or c-erbB-2/β-actin in tumour)/(c-erbB-1 or c-erbB-2/β-actin in paired normal tissue).
Statistical analysis
Gene expression levels were described using the median as point estimator and the range of values. Cutoff values for discrimination of mRNA expression levels and histopathologic response were derived from receiver operating curve (ROC) data (area under the curve and the 95% confidence interval) according to Metz et al (1973).
Associations between dichotomised gene expression levels and clinico-pathological parameters were evaluated using χ2-analysis and Fisher's exact test for significance (Software Package SPSS for Windows, Version 11.0, Chicago, IL, USA).
P-values are given for two-sided testing and the level of significance was set to P<0.05.
RESULTS
Distribution of c-erbB-1 and c-erbB-2 mRNA expression levels
Quantitative expression levels of c-erbB-1 and c-erbB-2 mRNA were evaluated in 36 patients in tumour and corresponding normal tissues from pretreatment biopsies. The Median absolute c-erbB-1 mRNA expression levels standardised for β-actin were 0.115 (min. 0.001, max. 2) in tumour and 0.103 (min. 0.003, max. 1.467) in the corresponding normal tissue. The calculated median relative expression level (T/N ratio) was 1.082 (min. 0.008, max. 27.148). For c-erbB-2 mRNA expression, median absolute levels standardised for β-actin were 0.401 (min. 0.002, max. 7) in tumour and 0.634 (min. 0.11, max. 62.091) in paired normal tissues. The calculated median relative c-erbB-2 mRNA expression level (T/N ratio) was 0.595 (min. 0.007, max. 7.873).
Histopathologic response to radiochemotherapy and c-erbB-1 mRNA expression
Relative overexpression of c-erbB-1 mRNA with a T/N ratio >1.0 was detected in 20 out of 36 (55.6%) tumours and normal to low relative expression with a T/N ratio ⩽1.0 in 16 out of 36 (44.4%), respectively. The association of dichotomised relative expression levels (T/N ratio⩽1.0 vs >1) and histopathologic response grades is shown in Table 2 . This association is statistically not significant (P=1), with a low sensitivity (54.1%) and specificity (65%) to predict minor histopathologic response.
Table 2. c-erbB-1 mRNA expression and regression (sensitivity and specificity of response prediction).
|
Regression grading |
|||
|---|---|---|---|
| c-erbB-1a | Grades I/II (MiHRb) | Grades III/IV (MaHRc) | Total: n (%) |
| ⩽1: n (%) | 11 (30.6%) | 5 (13.9%) | 16 (44.4%) |
| >1: n (%) | 13 (36.1%) | 7 (19.4%) | 20 (55.6%) |
| Total: n (%) | 24 (66.7%) | 12 (33.3%) | 36 (100%) |
Dichotomised c-erbB-1 mRNA levels.
Minor histopathologic response.
Major histopathologic response.
Prediction of MiHR (c-erbB1>1): sensitivity 54.1%, specificity: 65% χ2-analysis (Fisher's exact test): P=1.
A cutoff value for relative c-erbB-1 mRNA expression and discrimination of minor histopathologic response was derived from ROC curves for a relative c-erbB-1 mRNA expression level of 3.318 (area under the curve: 0.476; 95% confidence interval: 0.29–0.66). The results are summarised in Table 3 and show no statistically significant association between dichotomised expression levels (T/N ratio⩽3.318 vs >3.318). The sensitivity to predict minor histopathologic response was 20.8%, with a specificity of 100% (P=0.15).
Table 3. c-erbB-1 mRNA expression and regression (sensitivity and specificity of response prediction for maximum cutoff).
|
Regression grading |
|||
|---|---|---|---|
| c-erbB-1a | Grades I/II (MiHRb) | Grades III/IV (MaHRc) | Total: n (%) |
| ⩽3.318: n (%) | 19 (52.8%) | 12 (33.3%) | 31 (86.1%) |
| >3.318: n (%) | 5 (13.9%) | ∅ | 5 (13.9%) |
| Total: n (%) | 24 (66.7%) | 12 (33.3%) | 36 (100%) |
Dichotomised c-erbB-1 mRNA levels.
Minor histopathologic response.
Major histopathologic response.
Prediction of MiHR (c-erbB1>3.32): sensitivity 20.8%, specificity: 100% χ2-analysis (Fisher's exact test): P=0.15.
There was no significant association between dichotomised mRNA levels for T/N ratios ⩽1.0 vs >1 and histologic type (P=0.17), pT categories (P=1), pN categories (P=0.25) and grading (P=1) and T/N ratios ⩽3.32 vs >3.32 and histologic type (P=0.63), pT categories (P=0.0.63), pN categories (P=1) and grading (P=0.65) by Fisher's exact test.
Histopathologic response to radiochemotherapy and c-erbB-2 mRNA expression
The response frequencies for the 36 tumours analysed for c-erbB-2 expression were as follows: 12 out of 36 (33.3%) of the tumours demonstrated major (grades III and IV) and 24 out of 33 (66.7%) minor histopathologic response (grades I and II) to our neoadjuvant treatment regimen.
Relative overexpression of c-erbB-2 mRNA with a T/N ratio >1.0 was detected in 10 out of 36 (27.8%) tumours, and normal to low relative expression with a T/N ratio ⩽1.0 in 26 out of 36 (72.2%), respectively. In addition, a cutoff value for relative c-erbB-2 mRNA expression and maximum discrimination of minor histopathologic response was calculated from ROC curves for a relative c-erbB-2 mRNA expression level of 1.06 (area under the curve: 0.62; 95% confidence interval: 0.42–0.81). This calculated cutoff value, however, showed identical results when compared to dichotomised expression levels at T/N ratios ⩽1.0 vs >1 (Table 4 ).
Table 4. c-erbB-2 mRNA expression and regression (sensitivity and specificity of response prediction).
|
Regression grading |
|||
|---|---|---|---|
| c-erbB-2a | Grades I/II (MiHRb) | Grades III/IV (MaHRc) | Total: n (%) |
| ⩽1 or ⩽1.06: n (%)d | 14 (38.9%) | 12 (33.3%) | 26 (72.2%) |
| >1 or >1.06: n (%)d | 10 (27.8%) | ∅ | 10 (27.8%) |
| Total: n (%) | 24 (66.7%%) | 12 (33.3%) | 36 (100%) |
Dichotomised c-erbB-2 mRNA levels.
Minor histopathologic response.
Major histopathologic response.
All values remain identical if maximum cutoff at 1.06 was chosen from ROC curves. χ2-analysis (Fisher's exact test): P=0.015.
Prediction of MiHR (c-erbB2 >1): sensitivity 41.6%, specificity: 100%.
There was no significant association between dichotomised mRNA levels for T/N ratios ⩽1.0 vs >1 or ⩽1.06 vs >1.06 and histologic types (P=1.0), pT categories (P=0.93), pN categories (P=0.21) and grading (P=1).
Table 4 shows the association between major and minor histopathologic response groups and dichotomised relative c-erbB-2 expression levels for the whole study group (n=36), Table 5 for patients with squamous cell histology (n=23) and Table 6 for adenocarcinomas (n=13). The sensitivity for detection of a minor histopathologic response was 41.6% for the whole group, 42.8% for squamous cell cancers and 40% for adenocarcinomas with a specificity of 100% in all the three groups. This association of dichotomised c-erbB-2 mRNA levels and histopathologic response was significant for the whole group of tumours (P<0.015) and the subgroup of squamous cell cancers (P<0.048). Statistical analysis was not possible for adenocarcinomas due to small (n=13) patient numbers.
Table 5. c-erbB-2 mRNA expression and regression in SCCa (sensitivity and specificity of response prediction).
|
Regression grading |
|||
|---|---|---|---|
| c-erbB-2b | Grades I/II (MiHRc) | Grades III/IV (MaHRd) | Total: n (%) |
| ⩽1 or ⩽1.06e: n (%) | 8 (34.8%) | 9 (39.1%) | 17 (73.9%) |
| >1 or >1.06e: n (%) | 6 (26.1%) | ∅ | 6 (26.1%) |
| Total: n (%) | 14 (60.9%) | 9 (39.1%) | 23 (100%) |
Squamous cell cancer.
Dichotomised c-erbB-2 mRNA levels.
Minor histopathologic response.
Major histopathologic response.
All values remain identical if maximum cutoff at 1.06 was chosen from ROC curves. χ2-analysis (Fisher's exact test): P=0.048.
Prediction of MiHR (c-erbB-2 >1): sensitivity 42.8%, specificity: 100%.
Table 6. c-erbB-2 mRNA expression and regression in ACa (sensitivity and specificity of response prediction).
|
Regression grading |
|||
|---|---|---|---|
| c-erbB-2b | Grades I/II (MiHRc) | Grades III/IV (MaHRd) | Total: n (%) |
| ⩽1 or ⩽1.06e: n (%) | 6 (46.2%) | 3 (23.1%) | 9 (69.2%) |
| >1 or >1.06e: n (%) | 4 (30.8%) | ∅ | 4 (30.8%) |
| Total: n (%) | 10 (76.9%) | 3 (23.1%) | 13 (100%) |
Adenocarcinoma.
Dichotomised c-erbB-2 mRNA levels.
Minor histopathologic response.
Major histopathologic response.
All values remain identical if maximum cutoff at 1.06 was chosen from ROC curves. χ2-analysis (Fisher's exact test): not possible (three cells with expected frequencies <5).
Prediction of MiHR (c-erbB-2 >1): sensitivity 40%, specificity: 100%.
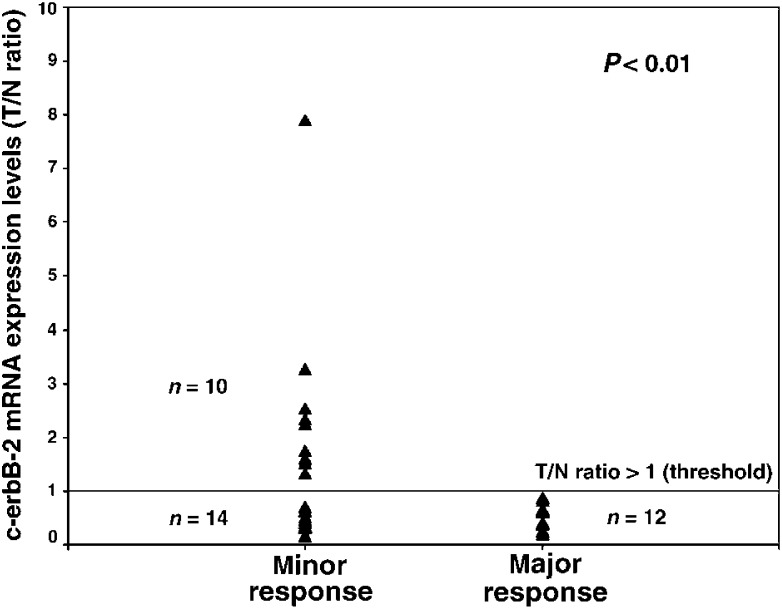
In summary, quantitative c-erbB2 mRNA expression testing unequivocally identified 10 out of 36 (28%) patients, whose tumours did not respond well to the delivered neoadjuvant radiochemotherapy (Figure 1).
Figure 1.
Scattergram showing relative c-erbB-2 mRNA expression levels (T/N: ratio of tumour to normal tissue) in relation to minor and major histopathologic response in resected specimens. C-erbB-2 expression levels >1 are exclusively present in the group of minor histopathologic response (sensitivity: 41.6%, specificity: 100%).
DISCUSSION
A significant association between quantitative c-erbB-2 but not c-erbB-1 mRNA expression levels in oesophageal cancers and minor histopathologic response to CDDP/5-FU-based neoadjuvant radiochemotherapy could be demonstrated. In fact, quantitative c-erbB-2 mRNA expression testing by real-time RT–PCR unequivocally identified 10 out of 36 (28%) patients, whose tumours did not exhibit (specificity 100%) a major histopathologic response to the applied preoperative treatment.
Several in vitro studies demonstrated that EGF and EGFR expression enhanced the sensitivity to various anticancer agents (Dixit et al, 1997; Nouri et al, 2000) and radiotherapy (Kwok and Sutherland, 1989; Bonner et al, 2002). Kroning et al (1995) showed the improvement of drug sensitivity including CDDP and 5-fluorouracil by EGF in various cell lines. Furthermore, they described that enhancement of drug sensitivity depended on the number of receptors. Dixit et al (1997) reported that a critical level of EGFR signalling had an inhibitory effect on the repair of CDDP-damaged DNA. Despite positive in vitro data, clinical trials failed to demonstrate the relation between EGFR expression and response to chemotherapy with CDDP-containing regimens (Goff et al, 1998; Baekelandt et al, 1999). There is a possibility for a more complex in vivo reaction process between EGFR, cytotoxic agents and radiation-induced DNA damage.
Concerning our negative clinical results, it is necessary to point out that EGFR mRNA expression levels were examined in pretreatment biopsies only, so that we cannot rule out that induction of EGFR expression and signalling during radiochemotherapy as shown in vitro could contribute to progressive loss of chemo- and radiation sensitivity during the course of radiochemotherapy. In addition, our data also do not advocate against the potential benefit of an EGFR-targeted therapy (Anderson and Jankowski, 2003; Gee and Nicholson, 2003).
Nevertheless, EGFR mRNA expression testing in pretreatment biopsies did not significantly contribute to the identification of patients with major or minor response to our radiochemotherapy protocol.
Contrary to the c-erbB-1 expression data, increased relative c-erbB-2 mRNA levels prior to the beginning of preoperative radiochemotherapy proved to be a statistically significant factor in predicting minor histopathologic response (P<0.01) in our clinical setting.
It is known from previous studies that c-erbB-2 overexpression can induce chemoresistance against various cytotoxic agents (Allred et al, 1992; Benz et al, 1993; Miles et al, 1999; Menard et al, 2001) and several groups demonstrated that anti-c-erbB2 (p185)-specific antibodies enhanced the cytotoxic sensitivity in high p185 protein-expressing cell lines (Hancock et al, 1991; Pietras et al, 1994; Tsai et al, 1995). Tsai et al (1996) concluded from in vitro experiments using non-small cell lung cancer cell lines that high levels of p185 can promote DNA repair after exposure to cytotoxic agents.
In clinical studies, it has been shown that overexpression of c-erbB-2 protein induced chemoresistance in breast and ovarian cancers (Muss et al, 1994; Meden et al, 1998; Jukkola et al, 2001). Other studies, however, failed to demonstrate a relation between c-erbB-2 expression and chemotherapy response in breast, ovarian or squamous cell head and neck cancers (Makar et al, 1994; Rozan et al, 1998; Giatromanolaki et al, 2000).
Clinical response evaluation after neoadjuvant therapy in solid tumours is highly inaccurate, as shown for oesophageal cancer (Adelstein et al, 1997). In contrast, histopathologic evaluation results in an objective analysis of remission with prognostic importance, as convincingly demonstrated for non-small cell lung cancer (Junker et al, 1997; Thomas et al, 1999). PET imaging might further improve clinical response evaluation, which, however, is currently still under investigation (Bruecher et al, 2001; Downey et al, 2003).
We therefore applied histopathologic criteria instead of inaccurate clinical restaging modalities for objective response evaluation in neoadjuvant-treated oesophageal cancers and identified a significant association between minor histopathologic response and dichotomised relative c-erbB-2 mRNA expression levels (P<0.01).
The sensitivity for the whole group of oesophageal cancers is 41.6%. More important, however, is the specificity of 100%, which would allow the unequivocal identification of a subset of 28% of patients with minor responses prior to treatment. Although median follow-up is too short to allow a definitive evaluation between the association of c-erbB-2 expression levels and survival, it has been convincingly demonstrated in the past that only patients with major histopathologic responses benefit from this type of treatment independent of the applied protocol (Walsh et al, 1996; Urba et al, 2001; MRC Esophageal Study Group, 2002).
The present, still early, results of this ongoing study are promising, and it appears that we could expect to identify approximately one-fourth of patients who in principal fulfil the criteria for neoadjuvant treatment for locally advanced oesophageal cancer, who will, however, not benefit from our treatment protocol. This might prevent a substantial number of our patients from expensive, noneffective and potentially harmful therapies, and could lead to a more individualised type of combined multimodality treatment in the near future. Quantitative real-time RT–PCR is a very efficient and fast technology that could easily be incorporated in a clinical setting, since results can be obtained within 1 or 2 days. A very promising approach could also be the application of specific anti-p185 antibodies or tyrosine kinase inhibitors in addition to radiochemotherapy in patients overexpressing c-erbB-2.
Acknowledgments
This study was supported by grants from the Marga and Walter Boll-Stiftung and the Deutsche Krebshilfe/Dr Mildred Scheel Stiftung (70-2300-Me I). We are grateful to Ms Susanne Neiss and Ms Sabine Berners, Laboratory of Experimental and Molecular Oncology, for their technical assistance.
References
- Adelstein DJ, Rice TW, Becker M, Larto MA, Kirby TJ, Koka A, Tefft M, Zuccaro G (1997) Use of concurrent chemotherapy, accelerated fractionation radiation, and surgery for patients with esophageal carcinoma. Cancer 80: 1011–1020 [PubMed] [Google Scholar]
- Allred DC, Clark GM, Tandon AK, Moliand R, Tormey DC, Osborne CK, Gilchrist KW, Mansour EG, Abeloff M, Eudey L (1992) HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol 10: 599–605 [DOI] [PubMed] [Google Scholar]
- Anderson MR, Jankowski JA (2003) The role of receptor tyrosine kinase inhibition in treating gastrointestinal malignancy. Expert Opin Investig Drugs 12: 577–592 [DOI] [PubMed] [Google Scholar]
- Baekelandt M, Kristensen GB, Trope CG, Nesland JM, Holm R (1999) Epidermal growth factor receptor expression has no independent prognostic significance in advanced ovarian cancer. Anticancer Res 19: 4469–4474 [PubMed] [Google Scholar]
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK (1993) Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 24: 85–95 [DOI] [PubMed] [Google Scholar]
- Bollschweiler E, Schroeder W, Hoelscher AH, Siewert JR (2000) Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br J Surg 87: 1106–1110 [DOI] [PubMed] [Google Scholar]
- Bonner JA, De Los Santos J, Waksal HW, Needle MN, Trummel HQ, Raisch KP (2002) Epidermal growth factor receptor as a therapeutic target in head and neck cancer. Semin Radiat Oncol 12: 11–20 [DOI] [PubMed] [Google Scholar]
- Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Hoelscher AH, Danenberg PV (2001) Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin Cancer Res 7: 1850–1855 [PubMed] [Google Scholar]
- Bruecher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, Werner M, Zimmerman F, Siewert JR, Schwaiger M (2001) Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg 233: 300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit M, Yang JL, Poirier MC, Price JO, Andrews PA, Arteaga CL (1997) Abrogation of cisplatin-induced programmed cell death in human breast cancer cells by epidermal growth factor antisense RNA. J Natl Cancer Inst 89: 365–373 [DOI] [PubMed] [Google Scholar]
- Downey RJ, Akhurst T, Ilson D, Ginsberg R, Bains MS, Gonen M, Koong H, Gollub M, Minsky BD, Zakowski M, Turnbull A, Larson SM, Rusch V (2003) Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 21: 428–432 [DOI] [PubMed] [Google Scholar]
- Esteva FJ, Sahin AA, Cristofanilli M, Arun B, Hortobagyi GN (2002) Molecular prognostic factors for breast cancer metastasis and survival. Semin Radiat Oncol 12: 319–328 [DOI] [PubMed] [Google Scholar]
- Gee JM, Nicholson RI (2003) Expanding the therapeutic repertoire of epidermal growth factor receptor blockade: radiosensitization. Breast Cancer Res 5: 126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Sivridis E, Fountzilas G (2000) c-erbB-2 oncoprotein is overexpressed in poorly vascularised squamous cell carcinomas of the head and neck, but is not associated with response to cytotoxic therapy or survival. Anticancer Res 20: 997–1004 [PubMed] [Google Scholar]
- Goff BA, Ries JA, Els LP, Coltrera MD, Gown AM (1998) Immunophenotype of ovarian cancer as predictor of clinical outcome: evaluation at primary surgery and second-look procedure. Gynecol Oncol 70: 378–385 [DOI] [PubMed] [Google Scholar]
- Gullick WJ (2001) The type 1 growth factor receptors and their ligands considered as a complex system. Endocr Relat Cancer 8: 75–82 [DOI] [PubMed] [Google Scholar]
- Hancock MC, Langton BC, Chan T, Toy P, Monahan JJ, Mischak RP, Shawver LK (1991) A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res 51: 4575–4580 [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ (2003) EGF receptor ligands. Exp Cell Res 284: 2–13 [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW (2003) Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284: 31–53 [DOI] [PubMed] [Google Scholar]
- Jukkola A, Bloigu R, Soini Y, Savolainen ER, Holli K, Blanco G (2001) c-erbB-2 positivity is a factor for poor prognosis in breast cancer and poor response to hormonal or chemotherapy treatment in advanced disease. Eur J Cancer 37: 347–354 [DOI] [PubMed] [Google Scholar]
- Junker K, Thomas M, Schulmann K, Klinke F, Bosse U, Muller KM (1997) Tumour regression in non-small cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 123: 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsen DP (2000) Multimodality therapy of esophageal cancer: an update. Cancer J 6(Suppl 2): S177–S181 [PubMed] [Google Scholar]
- Kroning R, Jones JA, Hom DK, Chuang CC, Sanga R, Los G, Howell SB, Christen RD (1995) Enhancement of drug sensitivity of human malignancies by epidermal growth factor. Br J Cancer 72: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok TT, Sutherland RM (1989) Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor. J Natl Cancer Inst 81: 1020–1024 [DOI] [PubMed] [Google Scholar]
- Lau CL, Moore MB, Brooks KR, D’Amico TA, Harpole Jr DH (2002) Molecular staging of lung and esophageal cancer. Surg Clin North Am 82: 497–523 [DOI] [PubMed] [Google Scholar]
- Makar AP, Holm R, Kristensen GB, Nesland JM, Trope CG (1994) The expression of c-erbB-2 (HER-2/neu) oncogene in invasive ovarian malignancies. Int J Gynecol Cancer 4: 194–199 [DOI] [PubMed] [Google Scholar]
- Meden H, Marx D, Rath W, Kron M, Fattahi-Meibodi A, Hinney B, Kuhn W, Schauer A (1994) Overexpression of the oncogene c-erb B2 in primary ovarian cancer: evaluation of the prognostic value in a Cox proportional hazards multiple regression. Int J Gynecol Pathol 13: 45–53 [DOI] [PubMed] [Google Scholar]
- Meden H, Marx D, Roegglen T, Schauer A, Kuhn W (1998) Overexpression of the oncogene c-erbB-2 (HER2/neu) and response to chemotherapy in patients with ovarian cancer. Int J Gynecol Pathol 17: 61–65 [DOI] [PubMed] [Google Scholar]
- Menard S, Valagussa P, Pilotti S, Gianni L, Biganzoli E, Boracchi P, Tomasic G, Casalini P, Marubini E, Colnaghi MI, Cascinelli N, Bonadonna G (2001) Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol 19: 329–335 [DOI] [PubMed] [Google Scholar]
- Metz CE, Goodenough DJ, Rossmann K (1973) Evaluation of receiver operating characteristic curve data in terms of information theory, with applications in radiography. Radiology 109: 297–303 [DOI] [PubMed] [Google Scholar]
- Miles DW, Harris WH, Gillett CE, Smith P, Barnes DM (1999) Effect of c-erbB(2) and estrogen receptor status on survival of women with primary breast cancer treated with adjuvant cyclophosphamide/methotrexate/fluorouracil. Int J Cancer 84: 354–359 [DOI] [PubMed] [Google Scholar]
- MRC Esophageal Study Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359: 1727–1733 [DOI] [PubMed] [Google Scholar]
- Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M, Henderson IC (1994) c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 330: 1260–1266 [DOI] [PubMed] [Google Scholar]
- Ninomiya I, Endo Y, Yonemura Y, Noguchi M, Fushida S, Nakai M, Takamura H, Harada F, Suzuki T, Miyazaki I, Sasaki T (1992) Specific detection of c-erbB-2 mRNA expression in gastric cancers by the polymerase chain reaction following reverse transcription. Br J Cancer 66: 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri AM, Zubairi ST, Russell MV, Moss T, Cannell H, Paris AM, Symes M, Oliver RT (2000) Concordance between tumour cell activation by epidermal growth factor and alteration of cell sensitivity to cisplatin and lymphokine-activated killer cell activity. Oncol Rep 7: 197–201 [PubMed] [Google Scholar]
- Pawlowski V, Revillion F, Hebbar M, Hornez L, Peyrat JP (2000) Prognostic value of the type I growth factor receptors in a large series of human primary breast cancers quantified with a real-time reverse transcription–polymerase chain reaction assay. Clin Cancer Res 6: 4217–4225 [PubMed] [Google Scholar]
- Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ (1998) Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 16: 2659–2671 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ (1994) Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 9: 1829–1838 [PubMed] [Google Scholar]
- Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ (1999) Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 59: 1347–1355 [PubMed] [Google Scholar]
- Rozan S, Vincent-Salomon A, Zafrani B, Validire P, De Cremoux P, Bernoux A, Nieruchalski M, Fourquet A, Clough K, Dieras V, Pouillart P, Sastre-Garau X (1998) No significant predictive value of c-erbB-2 or p53 expression regarding sensitivity to primary chemotherapy or radiotherapy in breast cancer. Int J Cancer 79: 27–33 [DOI] [PubMed] [Google Scholar]
- Schneider PM, Praeuer HW, Stoeltzing O, Boehm J, Manning J, Metzger R, Fink U, Wegerer S, Hoelscher AH, Roth JA (2000) Multiple molecular marker testing (p53, C-Ki-ras, c-erbB-2) improves estimation of prognosis in potentially curative resected non-small cell lung cancer. Br J Cancer 83: 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W, Moenig SP, Baldus SE, Gutschow C, Schneider PM, Hoelscher AH (2002) Frequency of nodal metastases to the upper mediastinum in Barrett's cancer. Ann Surg Oncol 9: 807–811 [DOI] [PubMed] [Google Scholar]
- Sherman CA, Turrisi AT, Wallace MB, Reed CE (2002) Locally advanced esophageal cancer. Curr Treat Options Oncol 3: 475–485 [DOI] [PubMed] [Google Scholar]
- Singleton TP, Perrone T, Oakley G, Niehans GA, Carson L, Cha SS, Strickler JG (1994) Activation of c-erbB-2 and prognosis in ovarian carcinoma. Comparison with histologic type, grade, and stage. Cancer 73: 1460–1466 [DOI] [PubMed] [Google Scholar]
- Thomas M, Rube C, Semik M, von Eiff M, Freitag L, Macha HN, Wagner W, Klinke F, Scheld HH, Willich N, Berdel WE, Junker K, Schulmann K, Bosse U, Muller KM (1999) Impact of preoperative bimodality induction including twice-daily radiation on tumor regression and survival in stage III non-small cell lung cancer. Tumour regression in non-small cell lung cancer following neoadjuvant therapy. Histological assessment. J Clin Oncol 17: 1185. [DOI] [PubMed] [Google Scholar]
- Tokuchi Y, Hashimoto T, Kobayashi Y, Hayashi M, Nishida K, Hayashi S, Imai K, Nakachi K, Ishikawa Y, Nakagawa K, Kawakami Y, Tsuchiya E (1999) The expression of p73 is increased in lung cancer, independent of p53 gene alteration. Br J Cancer 80: 1623–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CM, Levitzki A, Wu LH, Chang KT, Cheng CC, Gazit A, Perng RP (1996) Enhancement of chemosensitivity by tyrphostin AG825 in high-p185(neu) expressing non-small cell lung cancer cells. Cancer Res 56: 1068–1074 [PubMed] [Google Scholar]
- Tsai CM, Yu D, Chang KT, Wu LH, Perng RP, Ibrahim NK, Hung MC (1995) Enhanced chemoresistance by elevation of p185neu levels in HER-2/neu-transfected human lung cancer cells. J Natl Cancer Inst 87: 682–684 [DOI] [PubMed] [Google Scholar]
- Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M (2001) Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19: 305–313 [DOI] [PubMed] [Google Scholar]
- Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP (1996) A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335: 462–467 [DOI] [PubMed] [Google Scholar]
- Wang J, Buchholz TA, Middleton LP, Allred DC, Tucker SL, Kuerer HM, Esteva FJ, Hortobagyi GN, Sahin AA (2002) Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer 94: 3107–3114 [DOI] [PubMed] [Google Scholar]
- Wang SC, Hung MC (2001) HER2 overexpression and cancer targeting. Semin Oncol 28: 115–124 [DOI] [PubMed] [Google Scholar]
- Zhang F, Yang Y, Smith T, Kau SW, McConathy JM, Esteva FJ, Kuerer HM, Symmans WF, Buzdar AU, Hortobagyi GN, Pusztai L (2003) Correlation between HER-2 expression and response to neoadjuvant chemotherapy with 5-fluorouracil, doxorubicin, and cyclophosphamide in patients with breast carcinoma. Cancer 97: 1758–1765 [DOI] [PubMed] [Google Scholar]