Abstract
Intravesical instillation of Bacillus Calmette-Guérin (BCG) is used for the treatment of superficial bladder cancer, both to reduce the recurrence rate of bladder tumour and to diminish the risk of progression. Since its first therapeutic application in 1976, major research efforts have been directed to decipher the exact mechanism of action of the BCG-associated antitumour effect. Bacillus Calmette-Guérin causes an extensive local inflammatory reaction in the bladder wall. Of this, the massive appearance of cytokines in the urine of BCG-treated patients stands out. Activated lymphocytes and macrophages are the most likely sources of these cytokines, but at present other cellular sources such as urothelial tumour cells cannot be ruled out. Bacillus Calmette-Guérin is internalised and processed both by professional antigen-presenting cells and urothelial tumour cells, resulting in an altered gene expression of these cells that accumulates in the presentation of BCG antigens and secretion of particular cytokines.
Keywords: Bacillus Calmette-Guérin, bladder cancer, immunotherapy, cytokine
Bladder cancer is detected in 75% of patients at an early, superficial stage. The majority of the superficial urothelial cell carcinomas (TCCs) consists of papillary bladder carcinomas (Figure 1A), while the remaining (10%) is called carcinoma in situ (CIS), a high-grade diffuse surface-spreading lesions. The standard treatment is trans-urethral resection (TUR) followed by intravesical instillation with adjuvant, chemo- or immunotherapeutic drugs (Kurth et al, 2000). For treatment of the surgically nonaccessible CIS, these latter modalities are the only bladder sparing options available. Although the therapeutic efficacy is well recognised, a high recurrence rate of 60–70% and a progression rate of 15% dictate the need for lifelong follow-up and treatment.
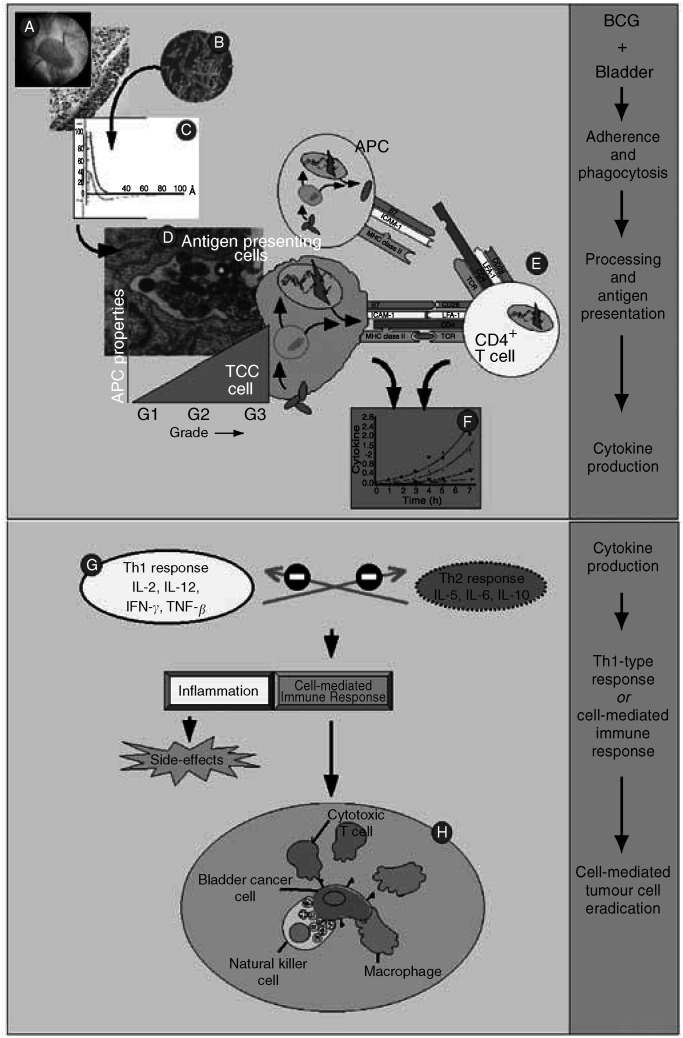
Figure 1.
Simplified scheme of the supposed mechanism of action of BCG in tumour cell eradication. After its instillation in the bladder (A), BCG (B) accumulates near the bladder wall, followed by adherence and passage through the GAG layer of the bladder wall (C). Bacillus Calmette-Guérin is internalised and processed by professional antigen-presenting cells (APCs) and (high-grade) tumour cells (D), and BCG antigens are presented to CD4+ T cells (E). Depending on various conditions, this results in the local synthesis of a particular set of cytokines, known as the Th1-type or cell-mediated immune response (F, G). The Th1 cytokine profile enables recruitment and maturation of cytotoxic effector cells. No definite statements can be made yet about the actual effector cell(s), but a key role for NK cells in tumour cell killing has been proposed (H).
Adjuvant agents were introduced to reduce the risk for recurrence and progression of TCC. Recent, comparative trials indicate that immunotherapy with Bacillus Calmette-Guérin (BCG; Figure 1B) is superior to chemotherapy in patients with intermediate to high risk for recurrence. In these patients, the benefits of treatment outweigh the burden of side effects. Moreover, BCG seems to exert a better antitumour effect in CIS and high-grade cancer (Melekos et al, 1993; Kurth et al, 2000).
Bacillus Calmette-Guérin, an attenuated strain of Mycobacterium bovis, was developed by Calmette and Guérin with the intention to generate a vaccine against tuberculosis. The clinical efficacy of the commercially available strains of BCG, such as Connaught and Tice, appears to be comparable (Witjes et al, 1996; Champetier et al, 2000).
Since the first report of intravesical use of BCG in 1976, investigators try to understand the working mechanism of BCG as an antitumour modality. Both BCG treatment regimen and dose are historically determined. Arbitrarily, BCG therapy consists of a single course of six weekly intravesical instillations. Extension of BCG treatment (maintenance immunotherapy) is used to increase efficacy. Despite its success, 30–50% of patients either fail to respond or relapse within the first 5 years of treatment. Unfortunately, BCG, a viable, living organism, can cause infections resulting in side effects ranging from bothersome cystitis in the majority of patients to sepsis eventually leading to death in rare cases.
In order to reduce the side effects of BCG and to improve efficacy, interesting approaches are developed, such as the application of a cell wall–DNA complex (MCC) of Mycobacterium phlei, which was effective in patients who failed BCG therapy (Morales et al, 2001), genetically engineered BCG secreting relevant cytokines such as human lL-2 (Yamada et al, 2000), or treatment schedules consisting of three times viable BCG and three subsequent instillations with killed BCG (De Boer et al, 2003).
This review presents an overview of the current state of ‘BCG research’. Special attention will be paid to the role of the urothelial cells in the cascade of events leading to BCG-associated tumour cell clearance. As an underdeveloped field of research, the significance of an interaction between potentially hostile bacteria and epithelial cells is a relatively unknown aspect of the host immune-defense system. Better knowledge about this interaction might open new avenues for improvement of the BCG immunotherapy.
For an actual overview on the practical, clinical aspects of the immunotherapy for TCC with BCG, the reader is referred to various, recently published papers (Kurth et al, 2000).
DETAILS OF THE MODE OF ACTION OF BCG
Nowadays, it is generally assumed that the BCG-induced antitumour activity is critically dominated by a local nonspecific immunological reaction reflecting the activity of immunocompetent cells (Alexandroff et al, 1999). It should be realised however that preceding the activation of the local immune system, some possibly rate-limiting events need to be executed. The sequential order of these initial events has been addressed only to a limited extent.
Interaction of BCG with the bladder wall
Interaction of BCG with the luminal surface of the bladder is the first step for BCG to accomplish. Accumulation of BCG near the bladder wall and adherence may be limiting processes for an adequate, clinical response. Suboptimal binding of BCG may explain the absence of clinical response in a subgroup of patients (Ratliff et al, 1987; Schamhart et al, 1994). Experimental modulation of BCG attachment affects BCG-induced response in animal models (Hudson et al, 1991). Systematic analysis of the interaction of BCG with the bladder wall has not been accomplished, probably due to poor recognition of the biological and physicochemical processes involved. The process of interaction should be divided into nonspecific, physicochemical and specific, receptor–ligand-mediated events.
Physicochemical interaction
The luminal side of the bladder is covered with a layer of hydrophilic, highly sulphated glycosaminoglycans (GAGs). This GAG layer protects the bladder from toxic compounds and microorganisms. Both the GAG layer and the BCG cell wall are highly negatively charged (the zeta potentials). These conditions prescribe that BCG bacteria accumulate without adherence, at a close docking distance (70–100 Å) to the bladder wall (Figure 1C) (Schamhart et al, 1994). In addition to this reversible adsorption, physicochemical considerations predict a low probability of irreversible adherence of BCG to the bladder wall, due to the high electrostatic, repellent force between the respective surfaces. The observed, in animals, low abundance of BCG adherence to the uninjured bladder wall and the dependency of BCG adherence to diluent properties (pH, salt concentration) seem to be in accord with these theoretical considerations (Ratliff et al, 1987; Hudson et al, 1991; Teppema et al, 1992). Damage of the GAG layer and urothelium may lower the negative charge of the bladder wall, leading to an increased BCG docking and adherence, as observed in a murine BCG model after electrocautery damage of the bladder (Ratliff et al, 1987).
Specific, receptor–ligand-mediated events
In addition to nonspecific interaction, more specific mechanisms seem to be involved in BCG adherence. A crucial binding of BCG to fibronectin (FN) in the bladder mucosa has been postulated (Kavoussi et al, 1990). Fibronectin is part of the extracellular matrix, is equally distributed on normal and malignant urothelium, and a soluble form can be found in urine. Binding of BCG to the murine bladder was impaired with anti-FN antibodies or addition of soluble FN (Kavoussi et al, 1990). Furthermore, BCG bacteria possess a receptor with high affinity for the collagen domain of FN, the fibronectin attachment protein (FAP). These data and the observation that the clinical effect of BCG therapy is related to the degree of FN expression on normal mucosa suggest a specific FN-mediated adherence of BCG to the bladder wall. However, these experimental, animal data were largely obtained with preinjured bladders, contradictory to the clinical situation, and a fortuitous relationship between the efficacy of BCG therapy and fibronectin has been suggested (Bevers et al, 1998, 2000).
Internalisation of BCG and phenotypical alterations of urothelial cells
Internalisation of BCG in urothelial cells
Several reports show that urothelial cells are capable of internalisation of BCG (Bevers et al, 1998, 2000; Durek et al, 1999). Bacillus Calmette-Guérin internalisation in vitro is time and dose dependent and can already be demonstrated after a 15-min incubation period. In a spheroid 3-D model, internalised BCG was found four cell layers deep, whereas normal urothelial cells in this system did not internalise BCG (Durek et al, 1999). Guinea pig studies showed no adherent or internalised BCG in normal urothelial cells (Teppema et al, 1992). In malignant cells, BCG internalisation appeared to be cell differentiation dependent. Contrary to well-differentiated (G1) bladder tumour cell lines, poorly differentiated (G3) cell lines exhibit significant internalisation of BCG (Bevers et al, 1998). Clinically, these data relate well to observations that show a better response to BCG treatment of high-grade compared to low-grade tumours (Table 1 ) (Melekos et al, 1993).
Table 1. Recurrence rate/100 patients-months in patients with superficial bladder cancer, following TUR with and without adjuvant intravesical BCG instillations.
| Treatment | Grade 1 | Grade 2 | Grade 3 | |||
|---|---|---|---|---|---|---|
| TUR | 1.5 | NS | 3.67 | P<0.01 | 20.83 | P<0.002 |
| TUR+BCG | 1.18 | 1.02 | 1.26 | |||
Data from Melekos et al (1993); NS=not significant.
Knowledge about the mechanism of BCG internalisation is scarce. Fibronectin is suggested to act as a bridging molecule, binding both to urothelial cells and BCG. Urothelial cells express an integrin (α5β1 receptor) with affinity for FN (Zhao et al, 2000). Pretreatment of T24 cells, a human TCC cell line, with anti-FN or anti-α5β1-receptor antibodies resulted in an impaired BCG attachment to and internalisation in these cells. However, other investigators could not inhibit BCG internalisation with anti-FN antibodies (Schneider et al, 1994; Bevers et al, 2000). The current available data do not suggest a mandatory role of FN in BCG internalisation. It seems that BCG internalisation is mediated by additional molecule(s), possibly coexpressed with FN, such as heparan sulphate-containing proteoglycans interacting with the mycobacterial heparin-binding haemagglutinin adhesin (HBHA) (Schneider et al, 1994; Bevers et al, 2000).
Regardless of the exact mechanism of internalisation, intracellular BCG is transported within phagosomes. These fuse with lysosomes to form phagolysosomes. Recent evidence shows that intracellular, live BCG can interfere with this phagolysosome formation (Maksymowych and Kane, 2000). However, only a small fraction of commercially available BCG preparations consists of live BCG. Therefore, most BCG particles will be degraded, and mycobacterial glycoproteins and lipoproteins are transported to the cell surface (Neyrolles et al, 2001).
Patient studies, analysing bladder washings after BCG instillations, revealed vigorous phagocytosis of BCG by leucocytes. However, concerning internalised BCG in urothelial cells, conflicting results are reported (Teppema et al, 1992). Accepting the inability of normal urothelial cells to internalise BCG, these conflicting observations may be explained by the presence or absence of residual urinary tumour cells in the limited number of patients included in both studies.
In summary, BCG particles can be internalised and processed by residual, especially high-grade tumour cells. As a consequence, the possibility that BCG introduces phenotypical alterations of TCC cells that affect tumoricidal effector mechanisms has been a subject of study. Several effects, ranging from a direct antiproliferative/cytotoxic effect of BCG, to a role in the initiation and/or modulation of the host immune response, and to an increase of susceptibility of tumour cells, have been proposed.
Cytotoxic effects of BCG on BCG-internalising urothelial cells
In vitro studies with human TCC cell lines show that BCG exerts cytolytic, antiproliferative and antimotility effects. The inhibitory effects on cell proliferation were most pronounced in highly dedifferentiated cell lines. Today, the causal mechanisms are unknown. Internalised BCG increased the production of cytotoxic nitric oxide (NO) in TCC cells. Patients treated with BCG showed an augmented bladder NO production and an upregulation of urothelial-associated nitric oxide synthase (Jansson et al, 1998). NO, at high concentration, may cause DNA damage, and cytostatic and cytotoxic effects. Possible accumulation of DNA damage may be related to the observation that, contrary to nontreated patients, urothelial cells of BCG-treated patients express regulatory genes related to DNA repair, knowingly wild-type p53 and P21waf1/cip1 (Fontana et al, 1999). Whether these observations represent an indirect or direct cytotoxic effect of BCG in situ remains unclear.
Bacillus Calmette-Guérin-internalising urothelial cells and the initiation/modulation of the immune response
Activation of the host immune system has been considered an exclusive characteristic of professional antigen-presenting cells (APCs), like dendritic cells and macrophages. New insights support a role for the interaction of (airway)epithelial cells and bacteria in the initiation of the immunological cascade (Levine, 1995; Neyrolles et al, 2001). Bacillus Calmette-Guérin-treated urothelial tumour cells are now considered as active participants in the cytokine-mediated initiation and/or regulation of the early immune response (Alexandroff et al, 1999).
The initial immune response to BCG
The local immune response to bacteria, including live BCG, is complex, but the presentation of bacterial antigens by APC to T-helper cells is the pivotal interaction (Alexandroff et al, 1999). Derived from an extensive series of papers, it appears that, after internalisation and processing by professional APC, processed BCG antigen(s) become associated with MHC class II molecules (Figure 1D). The antigen–MHC class II complex is expressed at the cell surface to be recognised by CD4+ T-helper lymphocytes via the T-cell receptor (TCR) molecule (Figure 1E). Along with this binding, the interaction between the APC and CD4+ T cell is only fully accomplished by an additional series of costimulatory, but essential, interacting molecules. Among others, binding of CD4 to MHC class II, lymphocyte function-associated antigen-3 (LFA-3) to intercellular adhesion molecule-1 (ICAM-1) and CD28 (CD4+ cell) to B7 enhances the conjugation of the cells and promotes T-cell activation signals. Soluble cytokines, such as IL-2, IL-6 and IFN-γ, provide generation of APC and T cells and ‘fine-tuning’.
Depending on many nonspecific factors, including antigen dose, type of APC and the expression of the mentioned membrane-bound costimulatory signals of the T-helper cells, a so-called Th1-type, and to some degree a Th2-type, response develops during BCG treatment. The Th1 or cell-mediated immune response and the Th2 or humoral immune response are characterised by the patterns of cytokines, secreted by the CD4+ T-helper cells following antigen-specific stimulation. In mouse models, the Th1 response is primarily characterised by IL-2, IL-12, IFN-γ and TNF-β, and the Th2 response by IL-4, IL-5, IL-6 and IL-10. The recognition that this description of two types of responses reflects the human immune response and that they are regulated in a reciprocal fashion, critically regulated by the cytokines, represent a major advance in the field of antitumour cytotoxicity mechanisms.
Knowledge concerning the APC–T-helper cell interaction and development of Th1 and Th2 responses has been used to study the BCG-induced role of the urothelium in antigen presentation and initiation of the immune response.
Uroepithelial tumour cells and antigen-presenting properties
Immunocompetence of the host is essential for BCG therapy. Dendritic cells, macrophages and CD4+ T lymphocytes play a crucial role in the antitumour effect. In addition, evidence exists for an important role of epithelial, TCC cancer cells. Mouse bladder tumour cells present BCG antigen to CD4+ T cells, via MHC class II molecules (Lattime et al, 1992). The initial observations were extended for another murine cell line and a panel of human cell lines. Constitutive and BCG-induced expression of MHC class II and the major costimulatory molecules ICAM-1 and B7-1 was observed. The antigen presentation factors are enhanced in high-grade TCC cell lines only (Figure 1D) (Ikeda et al, 2002). Quantitative immunohistochemistry has confirmed the in vitro findings. Serial bladder biopsies and urinary cytospins, taken before and after BCG therapy, revealed an upregulation of MHC class II and ICAM-1 expression of urothelial tumour cells (Jackson et al, 1994).
Interestingly and likely of uttermost importance, recently non-MHC-encoded, CD1-restricted presentation of (glyco)lipid antigens has been recognised (Maksymowych and Kane, 2000). Although for BCG no data are available yet, CD4+ CD1-restricted T cells were observed in patients suffering from Mycobacterium leprae (Sieling et al, 2000). These cells produce IFN-γ, but not IL-4. Bacillus Calmette-Guérin therapy-related research has revealed an increased CD1 expression of TCC cell lines in the presence of live BCG (Ikeda et al, 2002) and a virtual absence of urinary IL-4 during BCG treatment (Nadler et al, 2003). Accordingly, it is tempting to hypothesise that CD1-facilitated BCG antigen presentation contributes to the development of BCG immunity.
In summary, epithelial TCC cells gain the phenotypical characteristics and functioning of APCs in the presence of BCG. These functions strongly suggest that the BCG–high-grade tumour cell interaction acts in cohort with the BCG–professional APC interaction. Clinical observations seem to confirm this conclusion, since BCG therapy seems to exert a better antitumour effect in high-grade bladder cancer (Table 1).
Secretion of cytokines by urothelial tumour cells
Multiple studies have reported on cytokine production as a result of BCG therapy. Following intravesical BCG instillations, there is an increase in the urinary level of several cytokines, such as IL-1, IL-2, IL-6, IL-8, IL-12, IL-18, TNF-α and IFN-γ. Undoubtedly, the major cell sources are the immunocompetent cells, but urothelial cells contribute to a significant degree. In vitro studies with human TCC cells revealed a BCG-induced upregulation of the cytokines IL-6, IL-8, IL-10, GM-CSF, TNF-α and IFN-α, but not IFN-γ, IL-2, IL-4 and IL-12 (Figure 1F and G) (Boer de et al, 1997; Bevers et al, 1998). Of all cytokines produced by TCC cell lines, the cytokines IL-6 and IL-8 are highly prominent and their role will be discussed here in some detail.
Interleukin-6
Within the challenged immune system, several cell types including APC produce IL-6. This multifunctional cytokine is critically involved in the acute phase response, T-cell proliferation, B-cell maturation, macrophage maturation and cytotoxic T-cell differentiation. In combination with IL-1, IL-2 and IFN-γ, IL-6 induces expression of the IL-2 receptor (IL-2R), thus participating in the activation of resting T cells (Lauta, 2003). Furthermore, Il-6 seems to contribute to MHC-nonrestricted cytotoxic activity by inducing natural killer (NK) cell proliferation. Nowadays, NK cells are considered as essential, cytotoxic effector cells during BCG therapy (Figure 1H) (Brandau et al, 2001).
High-grade TCC cell lines show a high constitutive and BCG-induced production of IL-6. However, the importance of IL-6 produced by bladder cancer cells in the BCG-induced immune response remains to be established. In vitro studies revealed that IL-6 mRNA upregulation and IL-6 production depend on BCG dose, incubation period, BCG internalisation and TCC cell grade (Bevers et al, 1998). Interleukin-6 production requires a minimal BCG exposure time of 0.5–1 h, which is in accordance with clinical practice.
Interleukin-6 is considered a major source of early Th1/Th2 control during CD4+ T-cell activation as it attributes to the promotion of the Th2 response and simultaneously inhibition of Th1 polarisation (Figure 1G). Interleukin-6 activates the production of IL-4 by CD4+ T cells and their differentiation into Th2 effector cells. Moreover, it inhibits Th1 differentiation by interference with IFN-γ signalling and the development of Th1 cells (Diehl and Rincon, 2002). The abundant IL-6 response during BCG therapy and virtually the absence of urinary IL-4 seem to be however in conflict with the recognition that the presence of BCG primarily induces a Th1 response. The absence of IL-4 and a high production of IFN-γ may prevent or contradict the Th2-promoting effect of IL-6. Moreover, this finding suggests an important role of the CD1-restricted presentation of (glyco)lipid antigens of live BCG , since CD4+ CD1-restricted T cells produce IFN-γ but not IL-4 (Sieling et al, 2000; Ikeda et al, 2002).
Interleukin-8
Interleukin-8 is a proinflammatory cytokine with strong chemotactic properties, attracting T lymphocytes and neutrophils. Interleukin-8 is induced rapidly, already after the first instillation, during BCG therapy (Boer de et al, 1997). Dendritic cells, macrophages and a number of other cells, including TCC cell lines, produce IL-8. The early urinary IL-8 production in vivo may indicate the significance of an interaction of BCG bacilli with (residual) bladder cancer cells in the initiation of the host immune response.
Cell-mediated antitumour effects: the effector cells
The final step in the eradication of tumour cells consists of mobilisation and activation of cytotoxic effector cells (Figure 1H). Several in vitro studies show evidence for several nonspecific cytolytic cells like NK cells, BCG-activated killer cells (BAKs), macrophage-activated killer cells (MAKs), lymphokine-activated killer cells (LAKs) and cytotoxic T lymphocytes (Kawashima et al, 2003).
A key role is supposed for NK cells (Brandau et al, 2001). NK cells, a special population of mononuclear cells, recognise ‘self’-peptides presented by MHC class I molecules on the surface of cells. A cell not displaying these peptides in a correct way is attacked and killed by NK cells (Kärre, 1995). In untreated bladder cancer patients, a loss or alteration of MHC class I expression is seen in tumour cells (Saint et al, 2001). Bacillus Calmette-Guérin-infected cells present BCG glycoprotein and lipoprotein antigens on their MHC class I molecule (Neyrolles et al, 2001). This may be a trigger for NK cells to attack BCG-infected urothelial tumour cells. Bacillus Calmette-Guérin therapy in the murine model, using NK cell-deficient mice, is ineffective (Brandau et al, 2001). However, in studies regarding the presence of NK cells after BCG therapy, relatively few NK cells were seen 3 weeks after the last instillation of a 6-week course (Lattime et al, 1992; Saint et al, 2001). It would be interesting to know if NK cells are more abundant earlier, during the BCG course. Strong direct evidence for NK cell or other effector cell(s) is still lacking. The recent acknowledgement of effector cells that recognise mycobacterial (glyco)lipid antigens through nonpolymorphic MHC molecules, such as CD1, may provide new insights into the true nature of the cytolytic effector cells involved in tumour cell eradication during BCG treatment (Maksymowych and Kane, 2000; Kawashima et al, 2003).
SUMMARY AND CONCLUSIONS
Current insight of the mode of action of BCG, ranging from its introduction into the bladder to killing of residual tumour cells, has revealed a complex sequence of processes. Bacillus Calmette-Guérin accumulates near, and adheres to, the bladder wall. After passage through the GAG layer, BCG is internalised and processed by professional APC and tumour cells. The modified gene expression of these cells accumulates in the secretion of particular cytokines and presentation of BCG antigens. Bacillus Calmette-Guérin antigens are presented via MHC class II molecules to CD4+ T cells and via MHC class I molecules to CD8+ T cells. Lipid and glycolipid BCG antigens can be presented to CD4+ and CD8+ T cells in a non-MHC-restricted, CD1-restricted fashion. Production of chemokines, such as IL-8, secreted partly by BCG-internalised tumour cells, contributes to the local activation of the immune system. Consequently, activated leucocytes and mononuclear cells invade the bladder wall. These developments provide the condition for a Th1 response, associated with particular cytokines (IFN-γ, IL-2, IL-12 and TNF-β). This cytokines profile promotes delayed-type hypersensitivity response, cytotoxic cell response, and macrophage activation or cellular immune inflammatory reaction. Depending on bacterial and host components, an upregulation of the Th2 response, associated with cytokines IL-6 and IL-10, may occur to some degree and adversely affect the functioning of the Th1 response. The Th1 cytokine profile enables recruitment and maturation of cytotoxic effector cells.
No definite statements can be made yet about the actual effector cell(s), but a crucial, cytotoxic role of NK cells has been proposed. In addition, some of the cytokines, and BCG itself, may exhibit a direct cytotoxic effect on tumour cells.
In 28 years of major research efforts, understanding of the mode of action underlying BCG therapy for bladder carcinoma is obviously much improved. Yet the jigsaw is not complete and many details wait unraveling. However, if successful, the reward might be a better, evidence-based BCG immunotherapy with optimal clinical efficacy and minimal occurrence of side effects in the form of an optimal BCG dose and treatment schedule, genetically engineered BCG, or particular antigenic molecule(s) that trigger immunological antitumour activity in a well-controlled manner.
Footnotes
This review is devoted to the role of the urothelial cell in the mechanism of BCG action
References
- Alexandroff AB, Jackson AM, O'Donnell MA, James K (1999) BCG immunotherapy of bladder cancer: 20 years on. Lancet 353: 1689–1694 [DOI] [PubMed] [Google Scholar]
- Bevers RFM, de Boer EC, Kurth K-H, Schamhart DHJ (1998) BCG-induced interleukin-6 upregulation and BCG internalization in well and poorly differentiated human bladder cancer cell lines. Eur Cytokine Network 9: 181–186 [PubMed] [Google Scholar]
- Bevers RFM, de Boer EC, Kurth K-H, Schamhart DHJ (2000) BCG internalization in human bladder cancer cell lines, especially with regard to cell surface-expressed fibronectin. Aktuelle Urol 31(Suppl 1): 31–34 [Google Scholar]
- Boer de EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH, Schamhart DH (1997) Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res 25: 31–34 [DOI] [PubMed] [Google Scholar]
- Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, Jocham D, Ratliff TL, Böhle A (2001) NK cells are essential for the effective BCG immunotherapy. Int J Cancer 92: 697–702 [DOI] [PubMed] [Google Scholar]
- Champetier D, Valignat C, Lopez JG, Ruffion A, Devonec M, Perrin P (2000) Intravesical BCG-therapy: comparison of side effects of Connaught (Toronto) and Pasteur (Paris) strains [Article in French]. Prog Urol 10: 542–547 [PubMed] [Google Scholar]
- De Boer EC, Rooijakkers SJ, Schamhart DH, Kurth KH (2003) Cytokine gene expression in a mouse model: the first instillations with viable bacillus Calmette-Guerin determine the succeeding Th1 response. J Urol 170: 2004–2008 [DOI] [PubMed] [Google Scholar]
- Diehl S, Rincon M (2002) The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 39: 531–536 [DOI] [PubMed] [Google Scholar]
- Durek C, Brandau S, Ulmer AJ, Flad HD, Jocham D, Böhle A (1999) Bacillus-Calmette-Guérin (BCG) and 3D tumors: an in vitro model for the study of adhesion and invasion. J Urol 162: 600–605 [DOI] [PubMed] [Google Scholar]
- Fontana D, Bellina M, Galietti F, Scoffone C, Cagnazzi E, Guercio S, Cappia S, Pozzi E (1999) Intravesical Bacillus Calmette-Guérin (BCG) as inducer of tumor suppressing proteins p53 and p21Waf1-Cip1 during treatment of superficial bladder cancer. J Urol 162: 225–230 [DOI] [PubMed] [Google Scholar]
- Hudson MA, Brown EJ, Ritchey JK, Ratliff TL (1991) Modulation of fibronectin-mediated Bacillus Calmette-Guérin attachment to murine bladder mucosa by drugs influencing the coagulation pathways. Cancer Res 51: 3726–3732 [PubMed] [Google Scholar]
- Ikeda N, Toida I, Iwasaki A, Kawai K, Akaza H (2002) Surface antigen expression on bladder tumor cells induced by bacillus Calmette-Guérin (BCG): a role of BCG internalization into tumor cells. Int J Urol 9: 29–35 [DOI] [PubMed] [Google Scholar]
- Jackson AM, Alexandroff AB, McIntyre M, Esuvaranathan K, James K, Chisholm GD (1994) Induction of ICAM 1 expression on bladder tumors by BCG immunotherapy. J Clin Pathol 47: 309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson OT, Morcos E, Brundin L, Lundberg JO, Adolfsson J, Soderhall M, Wiklund NP (1998) The role of nitric oxide in bacillus Calmette-Guérin mediated anti-tumor effects in human bladder cancer. Br J Cancer 78: 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K (1995) Express yourself or die: Peptides, MHC molecules and NK cells. Science 267: 978–979 [DOI] [PubMed] [Google Scholar]
- Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL (1990) Fibronectin-mediated Calmette-Guérin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest 85: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Norose Y, Watanabe Y, Enomoto Y, Narazaki H, Watari E, Tanaka S, Takahashi H, Yano I, Brenner MB, Sugita M (2003) Cutting edge: major CD8 T cell response to live bacillus Calmette-Guèrin is mediated by CD1 molecules. J Immunol 170: 5345–5348 [DOI] [PubMed] [Google Scholar]
- Kurth KH, Bouffioux C, Sylvester R, van der Meijden AP, Oosterlinck W, Brausi M (2000) Treatment of superficial bladder tumors: achievements and needs The EORTC Genitourinary Group. Eur Urol 37(Suppl 3): 1–9 [DOI] [PubMed] [Google Scholar]
- Lattime EC, Gomella LG, McCue P (1992) Murine bladder carcinoma cells present antigen to BCG-specific CD4+ T-cells. Cancer Res 52: 4286–4290 [PubMed] [Google Scholar]
- Lauta VM (2003) A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer 97: 2440–2452 [DOI] [PubMed] [Google Scholar]
- Levine SJ (1995) Bronchial epithelial cell–cytokine interactions in airway inflammation. J Invest Med 43: 241–249 [PubMed] [Google Scholar]
- Maksymowych WP, Kane KP (2000) Bacterial modulation of antigen processing and presentation. Microb Infect 2: 199–211 [DOI] [PubMed] [Google Scholar]
- Melekos MD, Chionis H, Pantazakos A, Fokaefs E, Paranychianakis G, Dauaher H (1993) Intravesical bacillus Calmette-Guérin immunoprophylaxis of superficial bladder cancer: results of a controlled prospective trial with modified treatment schedule. J Urol 149: 744–748 [DOI] [PubMed] [Google Scholar]
- Morales A, Chin JL, Ramsey EW (2001) Mycobacterial cell wall extract for the treatment of carcinoma in situ of the bladder. J Urol 166: 1633–1638 [PubMed] [Google Scholar]
- Nadler R, Luo Y, Zhao W, Ritchey JK, Austin JC, Cohen MB, O'Donnell MA, Ratliff TL (2003) Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guérin (BCG) mediated antitumor activity. Clin Exp Immunol 131: 206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrolles O, Gould K, Gares MP, Brett S, Janssen R, O'Gaora P, Herrmann JL, Prevost MC, Perret E, Thole JE, Young D (2001) Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J Immunol 166: 447–457 [DOI] [PubMed] [Google Scholar]
- Ratliff TL, Palmer JO, McGarr JA, Brown EJ (1987) Intravesical Bacillus Calmette-Guérin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guérin. Cancer Res 47: 1762–1766 [PubMed] [Google Scholar]
- Saint F, Patard JJ, Groux Muscatelli B, Lefefre Belda MA, Gil Diez de Medina S, Abbou CC, Chopin DK (2001) Evaluation of cellular tumor rejection mechanisms in the peritumoral bladder wall after bacillus Calmette-Guérin treatment. BJU Int 88: 602–610 [DOI] [PubMed] [Google Scholar]
- Schamhart DH, De Boer EC, Vleeming R, Kurth KH (1994) Theoretical and experimental evidence on the use of glycosaminoglycans in BCG-mediated immunotherapy of superficial bladder cancer. Semin Thromb Hemost 20: 301–309 [DOI] [PubMed] [Google Scholar]
- Schneider B, Thanhauser A, Jocham D, Loppnow H, Vollmer E, Galle J, Flad HD, Ulmer AJ, Böhle A (1994) Specific binding of bacillus Calmette-Guérin to urothelial tumor cells in vitro. World J Urol 12: 337–344 [DOI] [PubMed] [Google Scholar]
- Sieling PA, Ochoa MT, Jullien D, Leslie DS, Sabet S, Rosat JP, Burdick AE, Rea TH, Brenner MB, Porcelli SA, Modlin RL (2000) Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J Immunol 164: 4790–4796 [DOI] [PubMed] [Google Scholar]
- Teppema JS, de Boer EC, Steerenberg PA, van der Meijden AP (1992) Morphological aspects of the interaction of Bacillus Calmette-Guérin with urothelial bladder cells in vivo and in vitro: relevance for antitumor activity? Urol Res 20: 219–228 [DOI] [PubMed] [Google Scholar]
- Witjes WP, Witjes JA, Oosterhof GO, Debruyne MJ (1996) Update on the Dutch Cooperative Trial: mitomycin versus bacillus Calmette-Tice-Tice versus bacillus Calmette-Guérin RIVM in the treatment of patients with pTA-pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Semin Urol Oncol 14(Suppl 1): 10–16 [PubMed] [Google Scholar]
- Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U (2000) Murine IL-2 secreting recombinant Bacillus Calmette-Guèrin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol 164: 526–531 [PubMed] [Google Scholar]
- Zhao W, Schorey JS, Bong-Mastek M, Ritchey J, Brown EJ, Ratliff TL (2000) Role of a bacillus Calmette-Guérin fibronectin attachment protein in BCG-induced antitumor activity. Int J Cancer 86: 83–88 [DOI] [PubMed] [Google Scholar]