Bloody diarrhoea is an uncommon symptom in children, and it may indicate the presence of serious disease. This review focuses on children presenting in a primary care setting. The non-specialist should be aware of the likely causes, initial management, and indications for specialist referral. The emphasis is on children in the developed world, although traveller’s diarrhoea is also considered. The epidemiology and management of this condition are different in the developing world, where infectious causes predominate. In recent years the reported incidence of inflammatory bowel disease increased greatly in the developed world and important advances have been made in its management. This diagnosis should always be considered carefully.
Sources and selection criteria
I used the Medline database to search for evidence from the literature. Randomised controlled trials, meta-analyses, and Cochrane reviews were used when relevant and available. Other sources of evidence included large case series and cohort studies. I obtained information on the incidence of specific pathogens from the UK Health Protection Agency’s Centre for Infections
What are the most likely causes of bloody diarrhoea in children?
The likely diagnoses vary depending on age (box). At every age intestinal bacterial infections are an important cause. Inflammatory bowel disease may occur at any age but is more likely in older children (>1 year). In young infants non-specific (perhaps allergic) colitis is most likely. Other conditions are rarer but should be considered as they can be serious and even life threatening.
Causes of bloody diarrhoea (real or apparent) in infants and children
Infants aged <1 year
Common causes
Intestinal infection
-
Infant colitis
Non-specific colitis
Breast milk colitis
Cow’s milk colitis
Less common or rare causes
-
Intestinal ischaemia
Intussusception
Malrotation and volvulus
Necrotising enterocolitis
Hirschsprung’s disease
-
Inflammatory bowel disease
Crohn’s colitis
Ulcerative colitis
Systemic vasculitis
Factitious illness
Infants aged >1 year
Common causes
Intestinal infection
-
Inflammatory bowel disease
Crohn’s colitis
Ulcerative colitis
Juvenile polyp
Less common or rare causes
-
Intestinal ischaemia
Intussusception
Malrotation and volvulus
Mucosal prolapse syndrome
Henoch-Schönlein purpura or other forms of systemic vasculitis
Factitious illness
How common is infection compared with inflammatory bowel disease?
This is an important question because if it is assumed that bloody diarrhoea is caused by infection then inflammatory bowel disease will be missed. Because of this common assumption, children with inflammatory bowel disease often experience a delay in diagnosis.1
Summary points
Bloody diarrhoea in infancy and childhood often indicates serious gastrointestinal disease
Intestinal bacterial infection is the most common cause—Campylobacter, Salmonella, and Yersinia are important in the developed world
Bacterial gastroenteritis is usually self limiting—antibiotics are needed only in selected cases
Crohn’s disease and ulcerative colitis often present with bloody diarrhoea and should be considered in all ages
Children with severe bloody diarrhoea or signs of systemic illness need urgent specialist referral, as these symptoms may indicate a life threatening condition
In the United Kingdom, the most likely causative infective agents are species of Campylobacter, Salmonella, and Yersinia. Much less often, Shigella, types of Escherichia coli that produce shiga toxin (such as E coli 0157:H7), and other organisms are responsible. In the developing world other disorders including bacterial (Shigella) and amoebic (Entamoeba histolytica) dysentery are important. This should be considered in those who have recently been overseas.
Determining the incidence of infection as a cause of bloody diarrhoea is not simple, but an estimate can be made. A prospective cohort study in the UK found that 1:30 people (adults and children) presented to their general practitioner annually with gastroenteritis.2 Bacterial gastroenteritis was confirmed in a minority of cases. The annual incidences of specific bacterial isolates (per 1000 population) were Campylobacter 4.14 (95% confidence interval 3.34 to 5.13), Salmonella 1.57 (1.19 to 2.06), Yersinia 0.58 (0.42 to 0.88), Shigella 0.27 (0.16 to 0.47), and E coli 0157:H7 0.03 (0.01 to 0.11). Reports to the UK Health Protection Agency’s centre for infections suggest that about 15% of Campylobacter, 30% of Salmonella, and 50% of Yersinia infections are in children (www.hpa.org.uk). Thus, in a primary care setting the annual incidence of these bacterial infections in children may be around 1.5 per 1000. About 50-75 per 100 000 of children will develop bloody diarrhoea with these infections.
Bloody diarrhoea is a presenting symptom in about 75% of children with ulcerative colitis and 25% with Crohn’s disease (table 1).1 3 A prospective study of paediatric inflammatory bowel disease in the UK and Ireland reported an annual incidence of 5.2 (4.8 to 5.6) per 100 000.3 Of these, 27% had ulcerative colitis and 60% Crohn’s disease. The incidence of children presenting with bloody diarrhoea as a result of inflammatory bowel disease is therefore 2-3 per 100 000 population.
Table 1.
Presenting symptoms and signs in inflammatory bowel disease1
| Symptoms | Crohn’s disease (%) | Ulcerative colitis (%) |
|---|---|---|
| Intestinal symptoms | ||
| Bloody diarrhoea | 22 | 75 |
| Non-bloody diarrhoea | 42 | 15 |
| Diarrhoea (overall) | 64 | 90 |
| Blood per rectum (no diarrhoea) | 1.6 | 10 |
| Abdominal pain | 83 | 83 |
| Anorexia and weight loss | 88 | 56 |
| Perianal disease | 45 | 0 |
| Constipation | 11 | 0 |
| Extraintestinal manifestations | ||
| Clubbing | 25 | 0 |
| Arthralgia | 8 | 4.7 |
| Erythema nodosum | 5 | 0 |
Thus, in the developed world bloody diarrhoea in children is 15-20 times more likely to be caused by intestinal infections than by inflammatory bowel disease. In the developing world, although the true incidence of inflammatory bowel disease is unknown, bacterial and amoebic dysentery are much more likely to be the cause.
How should I investigate and manage bloody diarrhoea in primary care?
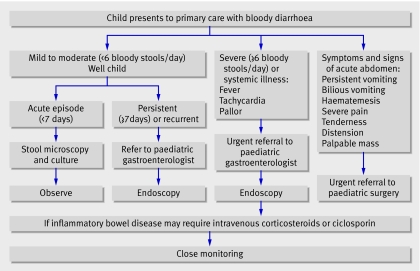
An appropriate strategy must take into account the severity and symptoms or signs of systemic illness or abdominal complications. Figure 1 outlines an approach to management, with suggested indications for specialist referral.
Fig 1 Strategy for initial evaluation, management, and referral of children presenting with bloody diarrhoea
How should I investigate and manage intestinal infections?
Table 2 summarises the sources, clinical presentation, diagnosis, and management of various types of intestinal bacterial infections. Antibiotics are usually contraindicated. With Campylobacter, a meta-analysis of 11 randomised controlled trials reported that early antibiotics shortened the illness slightly,4 but treatment is usually reserved for those with severe symptoms or impaired immunity. For Salmonella, a Cochrane review of 12 randomised controlled trials concluded that antibiotics provided no benefit and that treatment may prolong carriage.5 Antibiotics are usually reserved for young infants and children with suspected bacteraemia, extraintestinal spread, or impaired immunity. Patients with Shigella require antibiotic treatment with, for example, ciprofloxacin. For this reason routine antibiotic treatment is given to patients with bloody diarrhoea in developing countries.
Table 2.
Summary of bacterial intestinal infections that cause bloody diarrhoea
| Pathogen | Sources | Incubation period | Clinical presentation | Diagnosis | Treatment |
|---|---|---|---|---|---|
| Campylobacter jejuni | Mainly from farm and domestic animals and animal food products, especially undercooked chicken | 1-3 days (occasionally up to 10 days) | Fever and diarrhoea; bloody diarrhoea in up to 50%; usually lasts less than 1 week, with relapses in up to 25% | Stool culture; selective growth medium needed | Antibiotics reserved for those with severe symptoms or impaired immunity4 |
| Salmonella species | Mainly food borne—can cause large outbreaks; farm animals (especially undercooked poultry); pets (including reptiles); person to person transmission less frequent; infants (3-5 months) especially vulnerable | 6-48 hours (occasionally longer) | Gastroenteritis-like illness, often with fever lasting 3-4 days; bloody mucoid diarrhoea may follow as colitis develops; colitis may persist for 1-12 weeks | Stool culture; may stay positive for weeks | Antibiotics not usually beneficial and may prolong bacterial carriage5; antibiotics reserved for young infants, those with suspected bacteraemia or with extraintestinal spread, and those with impaired immunity |
| Yersinia enterocolitica | Farm and domestic animals; epidemics related to contaminated milk and ice cream | 3-7 days | Usually presents with fever, abdominal pain, and diarrhoea; blood present in the stool in about 30%; illness usually lasts 1-3 weeks | Stool culture; organism easily missed so the laboratory should be advised of suspicion | No evidence of benefit with antibiotics, and diagnosis is often late; antibiotics reserved for those with impaired immunity or extraintestinal spread |
| Shiga toxin producing Escherichia coli (such as O157 H7) | Often caused by foods contaminated with bovine faeces, such as undercooked minced (ground) beef; large outbreaks may occur | 3-9 days | Often presents with watery diarrhoea, which progresses to bloody diarrhoea; typically lasts 3-8 days; haemolytic uraemic syndrome can develop after 3-16 days | Specialised diagnostic techniques needed | Antibiotics seem to have no clinical benefit and may increase the risk of haemolytic uraemic syndrome6 |
| Shigella species | Highly contagious; usually person to person transmission; occasional outbreaks from contamination of food or water; most common between 6 months and 5 years of age; more severe in adults | 1-4 days | A few patients present with gastroenteritis-like illness; most experience lower abdominal pain, bloody mucoid stools, and fever; illness may be life threatening, with septicaemia | Stool microscopy shows pus cells and red cells; the organism is fastidious and requires prompt inoculation into appropriate medium for culture | Treated with antibiotics (for example, ciprofloxacin); during outbreaks or in high prevalence areas in the developing world antibiotics are given presumptively |
Salmonella infections generally present with diarrhoea and fever, and it usually settles within days. It is readily cultured from stool. In some patients, typically after a period of persistent diarrhoea, bloody mucoid diarrhoea develops as a result of colitis. If the organism is no longer detectable and the symptoms of colitis persist it may be difficult to distinguish from inflammatory bowel disease (fig 2). Yersinia is most common in children under 5 years. It may cause pain and ulceration in the terminal ileum and Crohn’s disease may be wrongly suspected.
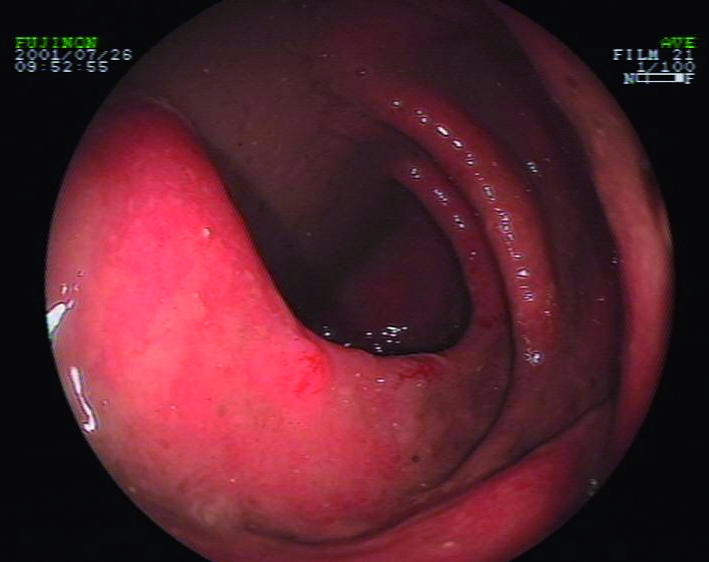
Fig 2 Ulcerative colitis resulting in mucosal inflammation with spontaneous bleeding. Patients with haemorrhagic colitis caused by Salmonella or other infections may present with an identical appearance
Haemolytic uraemic syndrome is a rare and life threatening condition with sudden onset of microangiopathic haemolytic anaemia, thrombocytopenia, and renal insufficiency. Most (80%) patients will have had bloody diarrhoea for three to 16 days previously. It is usually caused by shiga toxin producing E coli, often 0157:H7.
When should I suspect inflammatory bowel disease?
Inflammatory bowel disease is uncommon in children, but prompt diagnosis is important. The interval from onset to diagnosis is often prolonged, and this can result in avoidable morbidity.1 3 In a study of children presenting to a tertiary centre, even though 75% of those with ulcerative colitis had persistent or recurrent bloody diarrhoea, the mean time to diagnosis was 20 weeks (table 3).1
Table 3.
Time interval from first symptoms to diagnosis of inflammatory bowel disease1
| Diagnosis | Crohn’s disease (weeks) | Ulcerative colitis |
|---|---|---|
| Crohn’s disease | 47 | 4 weeks to 7 years |
| With diarrhoea | 28 | 4 weeks to 5 years |
| Without diarrhoea | 66 | 27 weeks to 7 years |
| Ulcerative colitis | 20 | 2 weeks to 3 years |
| Indeterminate colitis* | 45 | 2 weeks to 2 years |
*Colitis caused by inflammatory bowel disease in which it is not possible to distinguish between ulcerative colitis and Crohn’s disease.
Although infection may be the first consideration, the possibility of inflammatory bowel disease should not be dismissed even in young children. Around half of children with inflammatory bowel disease present before 11 years of age, and the disease may occur even in the first year of life. If any symptoms suggest chronic gastrointestinal disease, inflammatory bowel disease should be considered (table 2). Persistent (more than seven days) or recurrent bloody diarrhoea are indications for referral to a paediatric gastroenterologist. Other important signs include impaired growth, weight loss, finger clubbing, and—in Crohn’s disease—oral or perianal abnormalities. Perianal disease occurs in up to 45% of people with Crohn’s disease.1 3
Unanswered questions
How can the incidence of bacterial intestinal infection be reduced?
Given the increased incidence of inflammatory bowel disease in the developed world, what environmental factors are responsible?
Does bacterial gastroenteritis cause irritable bowel syndrome in children, as is reported in adults?
Are antibiotics advisable for patients with haemorrhagic colitis caused by Escherichia coli that produce shiga toxin?
What are the aetiology and pathogenesis of infant colitis?
How should I screen for inflammatory bowel disease?
Screening blood tests can be helpful, but in the context of bloody diarrhoea their role is limited. In a study of children presenting to a specialist paediatric gastroenterology clinic with suspected inflammatory bowel disease the simple combination of haemoglobin and platelet count was useful.7 Using “one or both tests abnormal” as a positive outcome gave a sensitivity of 92%, a specificity of 80%, and positive and negative predictive values of 77% and 93% for ulcerative colitis. However, another study found that haemoglobin, albumin, erythrocyte sedimentation rate, and C reactive protein were normal in 19% of children presenting with clinically mild ulcerative colitis.8 Normal blood tests do not rule out inflammatory bowel disease in children with bloody diarrhoea. Moreover, abnormal results may be found in children with bacterial gastroenteritis. In the absence of an identified stool pathogen, endoscopic evaluation is required in children with severe or persistent symptoms (fig 1).
How can I recognise and manage severe colitis?
Severe bloody diarrhoea (more than five bloody stools daily) requires urgent referral to a paediatric gastroenterologist. Severe colitis is associated with an increased risk of non-response to medical treatment, progression to toxic megacolon, and colonic perforation. Early referral may reduce these risks. Intravenous corticosteroids or ciclosporin are often effective in severe disease, and children need expert monitoring for signs of deterioration. In some cases emergency colectomy may be life saving.
Which diagnoses are most likely in infants?
Infant colitis
Colonoscopy often shows mucosal inflammation and ulceration in infants who present with bloody diarrhoea. Although cows’ milk allergy is usually suspected, the aetiology is often uncertain. Allergy is probably overdiagnosed.9 10 Many infants with bloody diarrhoea are breast fed and have “breast milk colitis.” In these cases, it has been proposed that small but immunologically relevant amounts of intact maternal dietary antigens might be transferred to breast milk via the mother’s bloodstream. However, this hypothesis has not been confirmed. A recent study of 40 infants presenting with blood in the stool provided a useful insight.10 The mean age at presentation was 3 months (range 1-6). The stools were watery in 38% and mucoid in 73%. Colonoscopy showed mucosal aphthae (33%), microscopic inflammation (33%), and focal eosinophilic infiltration (23%). The infants were randomly allocated to a cows’ milk-free diet (n=19) or a normal diet (n=21), with breastfeeding mothers in the first group adopting a cows’ milk elimination diet. They were reviewed at one and 12 months. During follow-up, bleeding was often intermittent, with an average time to the final episode of 24 days (range 1-85). All of the infants thrived. Cows’ milk elimination did not affect the duration of bleeding, and re-challenge supported a diagnosis of cows’ milk allergy in only 18%. The authors concluded that infant colitis is usually a benign self limiting disorder.
Necrotising enterocolitis
Necrotising enterocolitis is a serious disorder, rarely seen in primary care. It is characterised by diffuse or focal ulceration and necrosis in the small intestine and colon, and it may present with rectal bleeding or bloody diarrhoea. Other common features include abdominal distension, bilious vomiting, and signs of septicaemia. It mainly occurs in premature infants in the neonatal unit, although it can develop at any time up to 10 weeks of age. Moreover, up to 10% of cases are in full term infants.11 In such cases, predisposing factors such as cardiac disease may be present.11 12 When necrotising enterocolitis does occur in full term infants, the onset is usually within the first week of life.11 13 If it is suspected then urgent hospital referral is necessary. Abdominal radiography may show features to support the diagnosis.
Hirschsprung’s disease
Hirschsprung’s disease (congenital absence of ganglion cells in the colon) occurs in 1:5000 live births. About 80% of affected children present in the first year of life. In more than 90% of affected infants the passage of meconium is delayed beyond the first 24 hours. The classic presentation is with constipation. However, 25% of infants present with enterocolitis causing abdominal distension, and severe watery and sometimes bloody diarrhoea.14 This may cause hypovolaemic shock and colonic perforation, and mortality is 33% in these patients.15 Early diagnosis is therefore essential.
Tips for non-specialists
Children with fewer than six stools daily may be managed in primary care if they are not systemically unwell and do not have an acute abdomen
Evidence of systemic illness includes fever, tachycardia, pallor, and shock
Evidence of an abdominal surgical emergency includes severe pain, persistent or bilious vomiting, haematemesis, distension, tenderness, a palpable mass, and signs of septicaemia or shock
Consider inflammatory bowel disease in children with evidence of chronic disease—persistent or recurrent bloody diarrhoea or other gastrointestinal symptoms, weight loss, or poor growth
Severe colitis—associated with severe bloody diarrhoea—is life threatening and requires immediate referral to a paediatric gastroenterologist
What other disorders should I consider?
Any disorder that leads to mucosal ischaemia can cause bloody diarrhoea.
Intestinal infarction—a surgical emergency
Bloody diarrhoea can indicate a major surgical emergency. Intussusception occurs most often but not exclusively in the first year of life. The classic presentation is with episodic abdominal pain, vomiting, and the passage of blood and mucous. In some cases bloody diarrhoea is reported.16 In infants and children with congenital gut malrotation, midgut volvulus may result in extensive intestinal gangrene. This catastrophic event typically presents with symptoms of obstruction including bilious vomiting. Again, however, bloody diarrhoea may be reported.17
Additional educational resources
Resources for healthcare professionals
Health Protection Agency, Centre for Infections (www.hpa.org.uk)—Provides up to date surveillance information on the epidemiology of gastrointestinal infections in the UK
Resources for parents
National Association for Colitis and Crohn’s disease (www.nacc.org.uk)—Provides support and information for patients with ulcerative colitis and Crohn’s disease
Crohn’s in Childhood Research Association (www.cicra.org)—Provides support for patients with inflammatory bowel disease, particularly children and young adults, and raises funds to support medical research into the disease
Henoch-Schönlein purpura
Henoch-Schönlein purpura is a common form of idiopathic systemic vasculitis in children. It is associated with a characteristic rash (easily overlooked), abdominal pain, arthralgia, and overt or microscopic haematuria.18 Overt gastrointestinal bleeding occurs in 25% of patients and bloody diarrhoea is sometimes seen.19
Is it really bloody diarrhoea?
Juvenile polyps
Juvenile polyps (inflammatory polyps) occur in about 1% of children (fig 3). They are usually associated with the passage of blood and mucous, but diarrhoea may be reported.20 21 Colonoscopy is required for diagnosis.
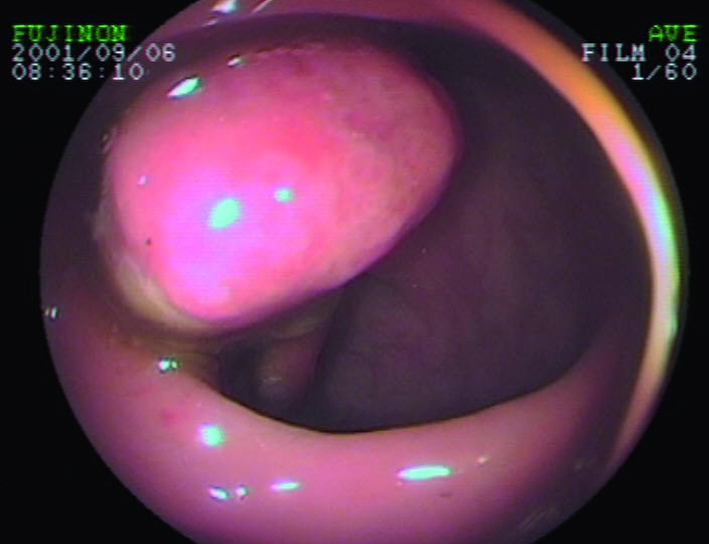
Fig 3 Typical appearance of pedunculated juvenile polyp identified at colonoscopy in the sigmoid colon. This child was said to have bloody mucoid “diarrhoea”
Mucosal prolapse syndrome and solitary rectal ulcer syndrome
Children with mucosal prolapse syndrome may report bloody diarrhoea.22 However, the true symptom may be tenesmus—the frequent urge to defecate with just the passage of blood and mucous. In this disorder the anterior rectal mucosa is prone to prolapse, although this is often not reported by the child. The prolapse leads to mucosal injury. In some cases it is associated with the development of inflammatory cloacogenic polyps at the anorectal junction. The polyps may be detected on digital examination and are seen at endoscopy; they have a characteristic histological appearance.23
Solitary rectal ulcer syndrome presents with similar symptoms. The pathogenesis of this condition is uncertain, but it is probably also caused by mucosal prolapse. At endoscopy, anterior rectal ulceration is seen several centimetres above the anal canal.
Factitious illness and illness induction
Very rarely, diarrhoea and gastrointestinal bleeding may be falsely reported either by young people or by carers.24 25 This possibility should be considered if the clinical circumstances are bizarre or if there are other reasons for concern.
Contributors: MSM is sole author. Olivier Fontaine, of the World Health Organization (Geneva), kindly reviewed this manuscript and contributed helpful comments and advice.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Spray C, Debelle GD, Murphy MS. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr 2001;90:400-5. [PubMed] [Google Scholar]
- 2.Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ 1999;318:1046-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child 2003;88:995-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007;44:696-700. [DOI] [PubMed] [Google Scholar]
- 5.Sirinavin S, Garner P. Antibiotics for treating salmonella gut infections. Cochrane Database Syst Rev 2000;(2):CD001167. [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 2000;342:1930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera-Abreu JC, Davies P, Matek Z, Murphy MS. Performance of blood tests in diagnosis of inflammatory bowel disease in a specialist clinic. Arch Dis Child 2004;89:69-71. [PMC free article] [PubMed] [Google Scholar]
- 8.Mack DR, Langton C, Markowitz J, LeLeiko N, Griffiths A, Bousvaros A, et al. Laboratory values for children with newly diagnosed inflammatory bowel disease. Pediatrics 2007;119:1113-9. [DOI] [PubMed] [Google Scholar]
- 9.Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr 2005;41:16-22. [DOI] [PubMed] [Google Scholar]
- 10.Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 2006;117:e760-8. [DOI] [PubMed] [Google Scholar]
- 11.Ostlie DJ, Spilde TL, St Peter SD, Sexton N, Miller KA, Sharp RJ, et al. Necrotizing enterocolitis in full-term infants. J Pediatr Surg 2003;38:1039-42. [DOI] [PubMed] [Google Scholar]
- 12.Siahanidou T, Mandyla H, Anagnostakis D, Papandreou E. Twenty-six full-term (FT) neonates with necrotizing enterocolitis (NEC). J Pediatr Surg 2004;39:791. [DOI] [PubMed] [Google Scholar]
- 13.Maayan-Metzger A, Itzchak A, Mazkereth R, Kuint J. Necrotizing enterocolitis in full-term infants: case-control study and review of the literature. J Perinatol 2004;24:494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swenson O, Sherman JO, Fisher JH. Diagnosis of congenital megacolon: an analysis of 501 patients. J Pediatr Surg 1973;8:587-94. [DOI] [PubMed] [Google Scholar]
- 15.Swenson O, Davidson FZ. Similarities of mechanical intestinal obstruction and aganglionic megacolon in the newborn infant: a review of 64 cases. N Engl J Med 1960;262:64-7. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald IA, Beattie TF. Intussusception presenting to a paediatric accident and emergency department. J Accid Emerg Med 1995;12:182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford EG, Senac MO, Jr, Srikanth MS, Weitzman JJ. Malrotation of the intestine in children. Ann Surg 1992;215:172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aalberse J, Dolman K, Ramnath G, Pereira RR, Davin JC. Henoch Schonlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann Rheum Dis 2007;66:1648-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uppal SS, Hussain MA, Al Raqum HA, Nampoory MR, Al Saeid K, Al Assousi A, et al. Henoch-Schonlein’s purpura in adults versus children/adolescents: a comparative study. Clin Exp Rheumatol 2006;24(2 Suppl 41):S26-30. [PubMed] [Google Scholar]
- 20.Ukarapol N, Singhavejakul J, Lertprasertsuk N, Wongsawasdi L. Juvenile polyp in Thai children—clinical and colonoscopic presentation. World J Surg 2007;31:395-8. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK, Fitzgerald JF, Croffie JM, Chong SK, Pfefferkorn MC, Davis MM, et al. Experience with juvenile polyps in North American children: the need for pancolonoscopy. Am J Gastroenterol 2001;96:1695-7. [DOI] [PubMed] [Google Scholar]
- 22.Du Boulay CE, Fairbrother J, Isaacson PG. Mucosal prolapse syndrome—a unifying concept for solitary ulcer syndrome and related disorders. J Clin Pathol 1983;36:1264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon KK, Mills S, Booth IW, Murphy MS. Inflammatory cloacogenic polyp: an unrecognized cause of hematochezia and tenesmus in childhood. J Pediatr 1997;130:327-9. [DOI] [PubMed] [Google Scholar]
- 24.Mills RW, Burke S. Gastrointestinal bleeding in a 15 month old male. A presentation of Munchausen’s syndrome by proxy. Clin Pediatr (Phila) 1990;29:474-7. [DOI] [PubMed] [Google Scholar]
- 25.Libow JA. Child and adolescent illness falsification. Pediatrics 2000;105:336-42. [DOI] [PubMed] [Google Scholar]