Abstract
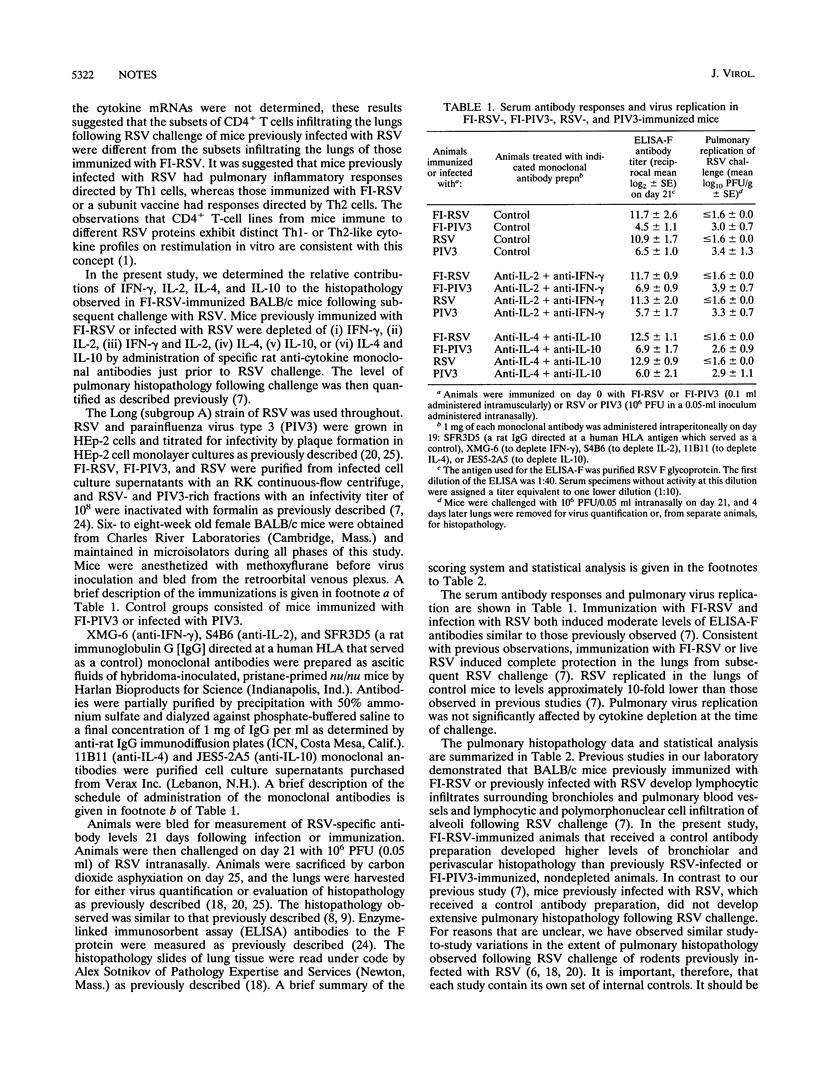
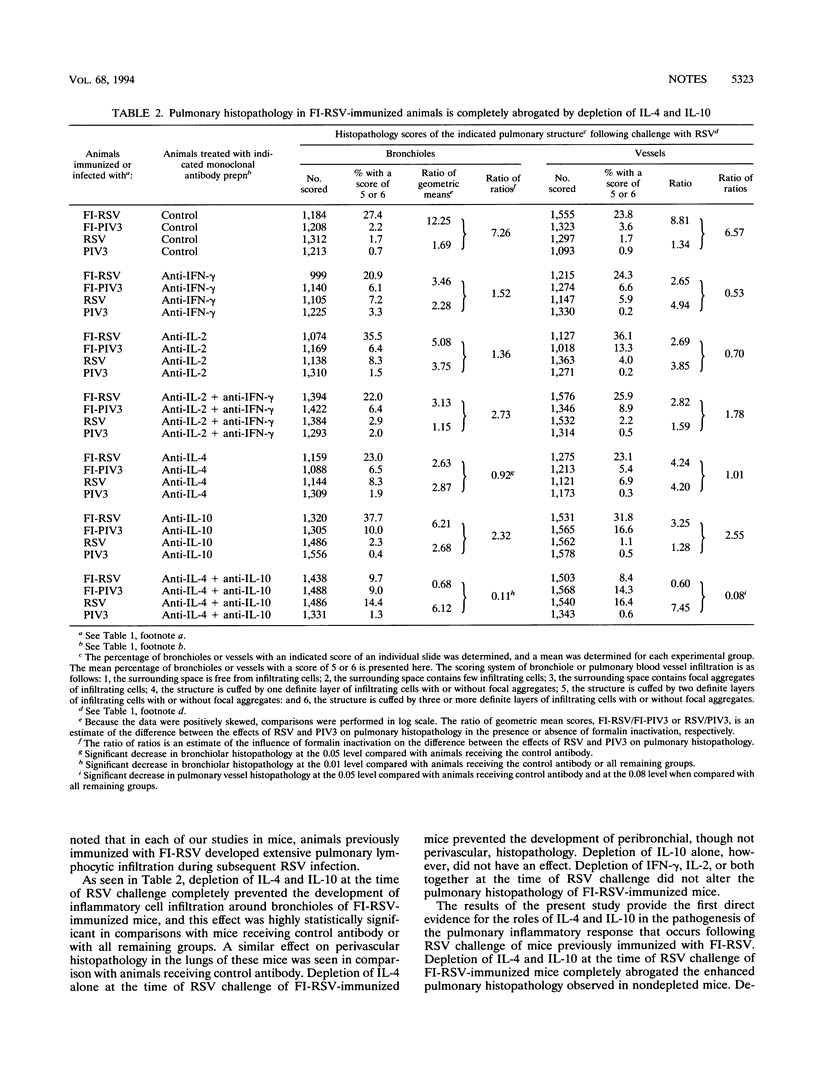
In previous studies, children immunized with a formalin-inactivated respiratory syncytial virus vaccine (FI-RSV) developed severe pulmonary disease with greater frequency than did controls during subsequent natural RSV infection. In earlier efforts to develop an animal model for this phenomenon, extensive pulmonary histopathology developed in FI-RSV-immunized cotton rats and mice subsequently challenged with RSV. In mice, depletion of CD4+ T cells at the time of RSV challenge completely abrogated this histopathology. Furthermore, the predominant cytokine mRNA present in lungs of FI-RSV-immunized mice during subsequent infection with RSV was that characteristically secreted by Th2 T cells, namely interleukin-4 (IL-4). In the present studies, we sought to determine the relative contributions of gamma interferon (IFN-gamma), IL-2, IL-4, and IL-10 to the lymphocytic infiltration into the lungs observed following RSV challenge of mice previously immunized with FI-RSV. Mice previously immunized with FI-RSV or infected with RSV were depleted of IFN-gamma, IL-2, IL-4, or IL-10 immediately before RSV challenge, and the magnitude of inflammatory cell infiltration around bronchioles and pulmonary blood vessels was quantified. The phenomenon of pulmonary-histopathology potentiation by FI-RSV was reproduced in the present study, thereby allowing us to investigate the effect of cytokine depletion on the process. Simultaneous depletion of both IL-4 and IL-10 completely abrogated pulmonary histopathology in FI-RSV-immunized mice. Depletion of IL-4 alone significantly reduced bronchiolar, though not perivascular, histopathology. Depletion of IL-10 alone had no effect. Depletion of IFN-gamma, IL-2, or both together had no effect on the observed histopathology. These data indicate that FI-RSV immunization primes for a Th2-, IL-4-, and IL-10-dependent inflammatory response to subsequent RSV infection. It is possible that this process played a role in enhanced disease observed in infants and children immunized with FI-RSV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwan W. H., Openshaw P. J. Distinct patterns of T- and B-cell immunity to respiratory syncytial virus induced by individual viral proteins. Vaccine. 1993;11(4):431–437. doi: 10.1016/0264-410x(93)90284-5. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Wathen M. W. A chimeric glycoprotein of human respiratory syncytial virus termed FG induces T-cell mediated immunity in mice. Vaccine. 1991 Dec;9(12):863–864. doi: 10.1016/0264-410x(91)90003-o. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Parrott R. H., Connors M., Collins P. L., Murphy B. R. Serious respiratory tract disease caused by respiratory syncytial virus: prospects for improved therapy and effective immunization. Pediatrics. 1992 Jul;90(1 Pt 2):137–143. [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Connors M., Collins P. L., Firestone C. Y., Sotnikov A. V., Waitze A., Davis A. R., Hung P. P., Chanock R. M., Murphy B. R. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia--RSV recombinants or RSV. Vaccine. 1992;10(7):475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- Connors M., Kulkarni A. B., Firestone C. Y., Holmes K. L., Morse H. C., 3rd, Sotnikov A. V., Murphy B. R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992 Dec;66(12):7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. S., Bunton L. A., Wright P. F., Karzon D. T. Reinfection of mice with respiratory syncytial virus. J Med Virol. 1991 May;34(1):7–13. doi: 10.1002/jmv.1890340103. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Bunton L. A., Wright P. F., Karzon D. T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991 Sep;88(3):1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. S., Henderson G. S., Tang Y. W., Lu X., Neuzil K. M., Colley D. G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993 Aug 15;151(4):2032–2040. [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Leikin S. L., Arrobio J., Brandt C. D., Chanock R. M., Parrott R. H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976 Jan;10(1):75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- Locksley R. M. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Braciale V. L., Braciale T. J. Antigen form influences induction and frequency of influenza-specific class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1988 Jul 15;141(2):363–368. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murphy B. R., Prince G. A., Walsh E. E., Kim H. W., Parrott R. H., Hemming V. G., Rodriguez W. J., Chanock R. M. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986 Aug;24(2):197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Sotnikov A. V., Lawrence L. A., Banks S. M., Prince G. A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990 Oct;8(5):497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Sotnikov A., Paradiso P. R., Hildreth S. W., Jenson A. B., Baggs R. B., Lawrence L., Zubak J. J., Chanock R. M., Beeler J. A. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989 Dec;7(6):533–540. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- Nicholas J. A., Rubino K. L., Levely M. E., Adams E. G., Collins P. L. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990 Sep;64(9):4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Nov;23(11):3043–3049. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Rockett K. A., Awburn M. M., Aggarwal B. B., Cowden W. B., Clark I. A. In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin, and interleukin-1: possible roles in malaria. Infect Immun. 1992 Sep;60(9):3725–3730. doi: 10.1128/iai.60.9.3725-3730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98(4):279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Romani L., Mocci S., Bietta C., Lanfaloni L., Puccetti P., Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991 Dec;59(12):4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux-Peretz F., Chapsal J. M., Meignier B. Comparison of the ability of formalin-inactivated respiratory syncytial virus, immunopurified F, G and N proteins and cell lysate to enhance pulmonary changes in Balb/c mice. Vaccine. 1992;10(2):113–118. doi: 10.1016/0264-410x(92)90027-h. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]



