Abstract
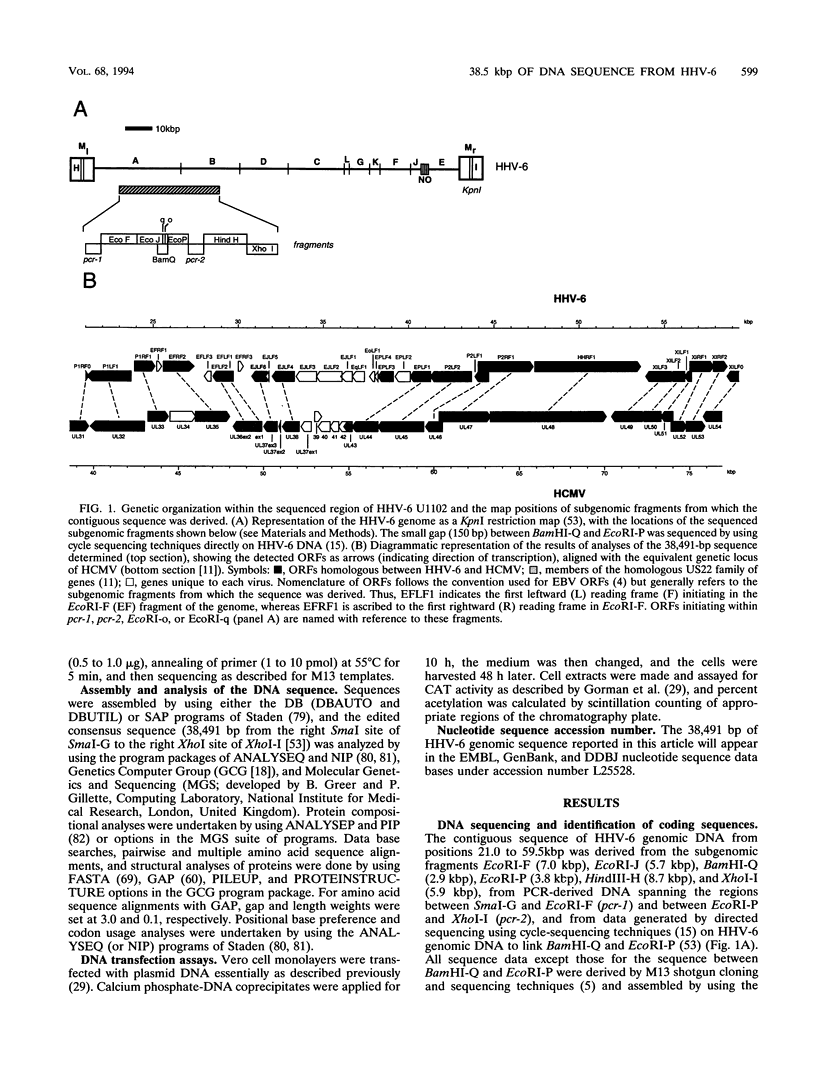
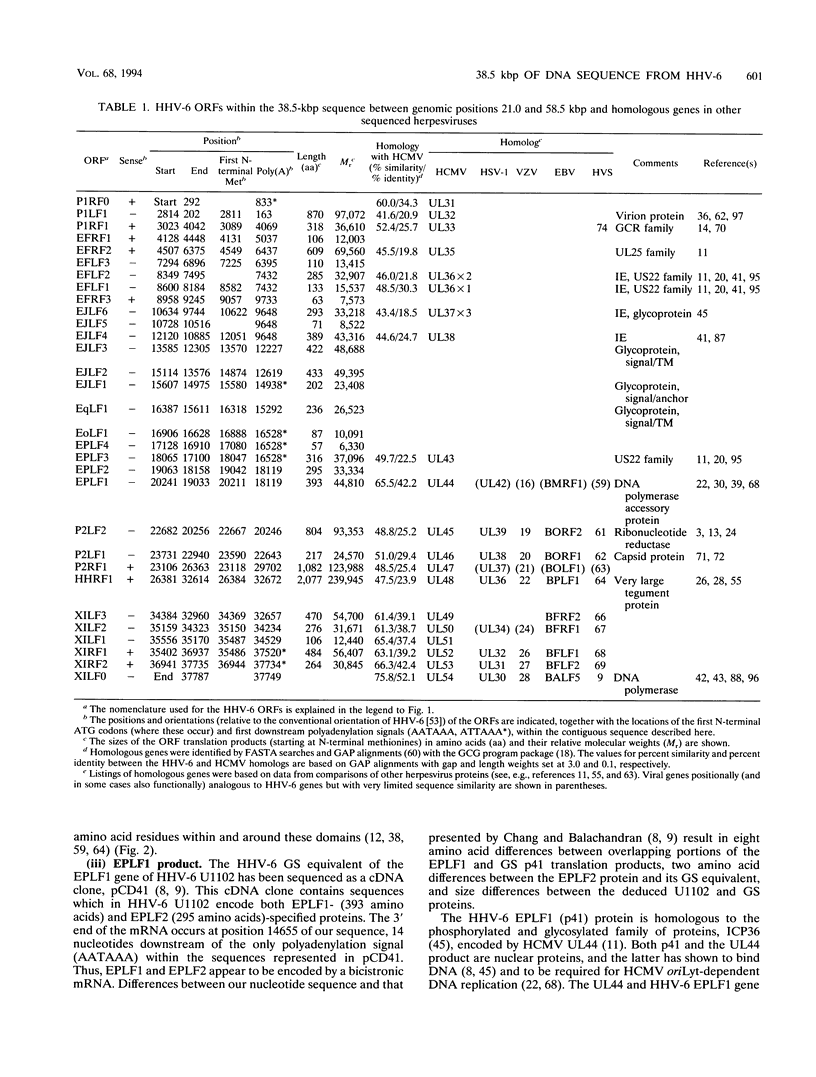
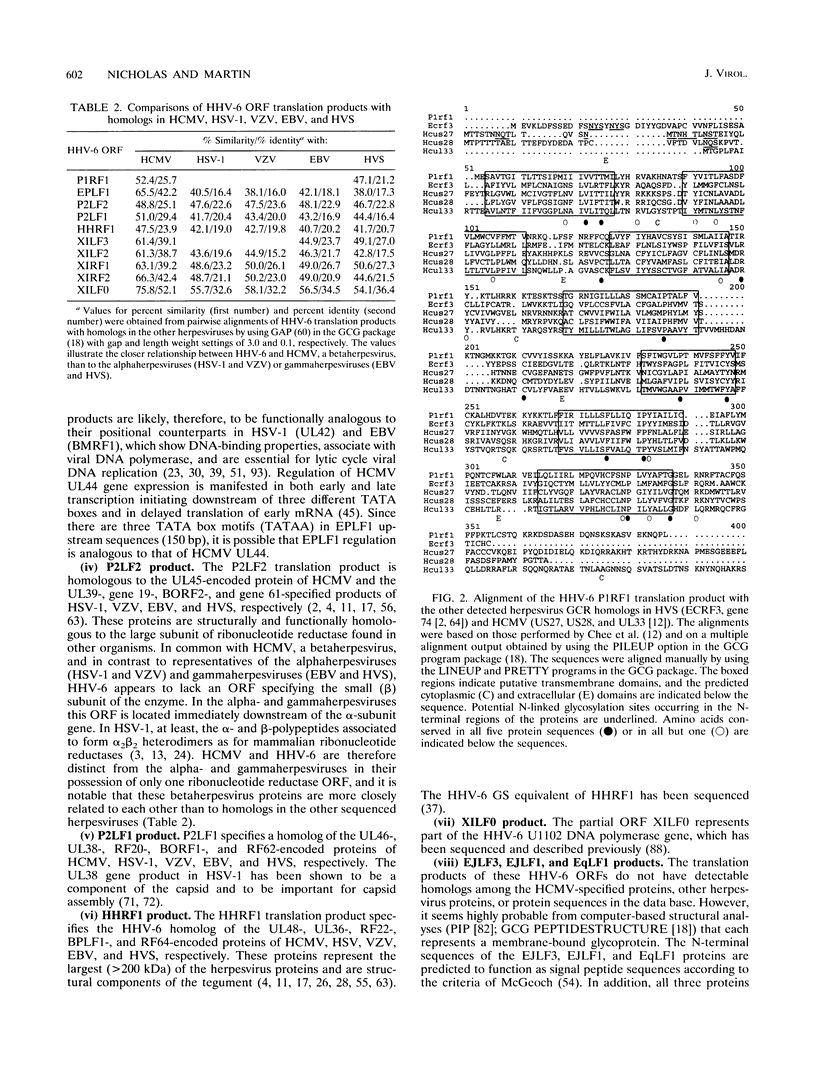
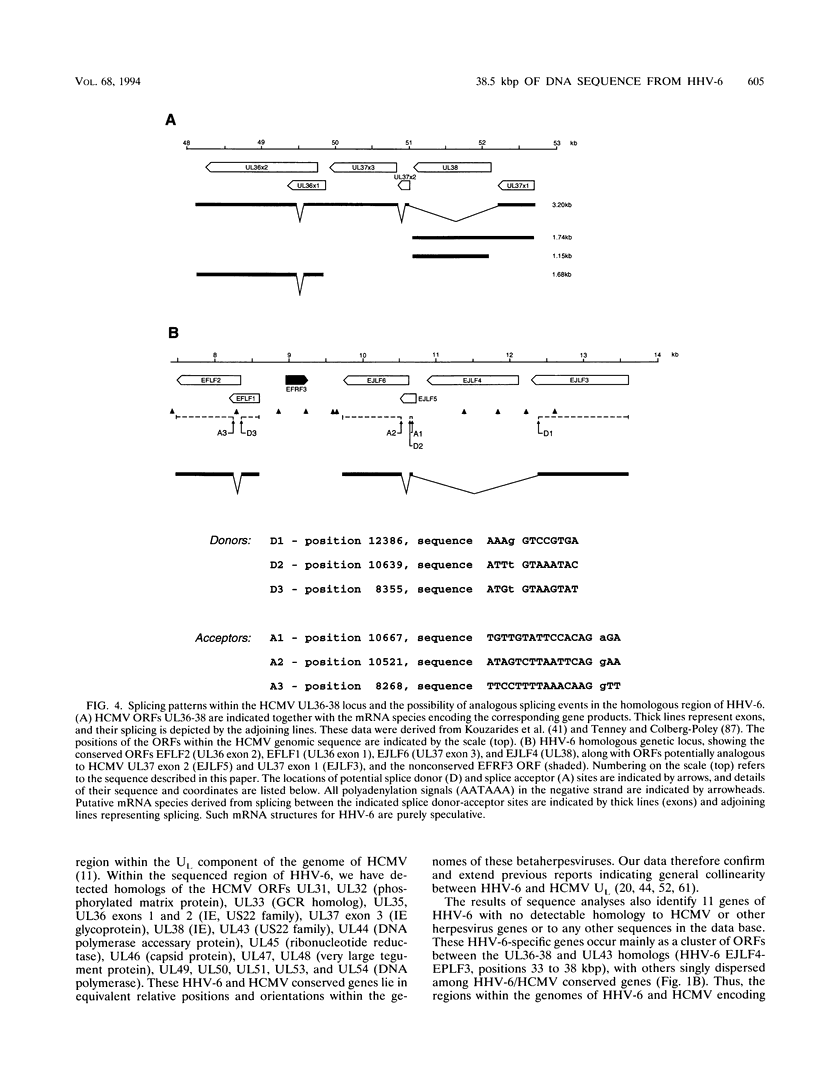
Human herpesvirus 6 (HHV-6) is prevalent in the human population, with primary infection occurring early in life. Its predominant CD4+ T-lymphocyte tropism, its ability to activate human immunodeficiency virus type 1 (HIV-1) gene expression in vitro, and its upregulation of CD4 expression has led to speculation that HHV-6 may act as a positive cofactor in the progression of HIV infection to AIDS in individuals infected with both viruses. Previous sequencing studies of restricted regions of the 161.5-kbp genome of HHV-6 have demonstrated unequivocally that it is a member of the betaherpesvirus subgroup and have indicated that the HHV-6 genome is generally collinear with the unique long (UL) component of human cytomegalovirus (HCMV). In the work described in this report we have extended these sequencing studies by determining the primary structure of 38.5-kbp of the HHV-6 genome (genomic position 21.0 to 59.5 kbp). Within the sequenced region lie 31 open reading frames, 20 of which are homologous to positional counterparts in HCMV. Of particular significance is the identification of homologs of the HCMV UL36-38 and US22-type genes, which have been shown to encode transactivating proteins. We show that DNA sequences encoding these HHV-6 homologs were able to transactivate HIV-1 long terminal repeat-directed chloramphenicol acetyltransferase expression in cotransfection assays, thus demonstrating functional as well as structural conservation of these betaherpesvirus-specific gene products. Our data therefore confirm the close relationship between HHV-6 and HCMV and identify putative immediate-early regulatory genes of HHV-6 likely to play key roles in lytic replication and possibly also in the interactions between HHV-6 and HIV in dually infected cells.
Full text
PDF





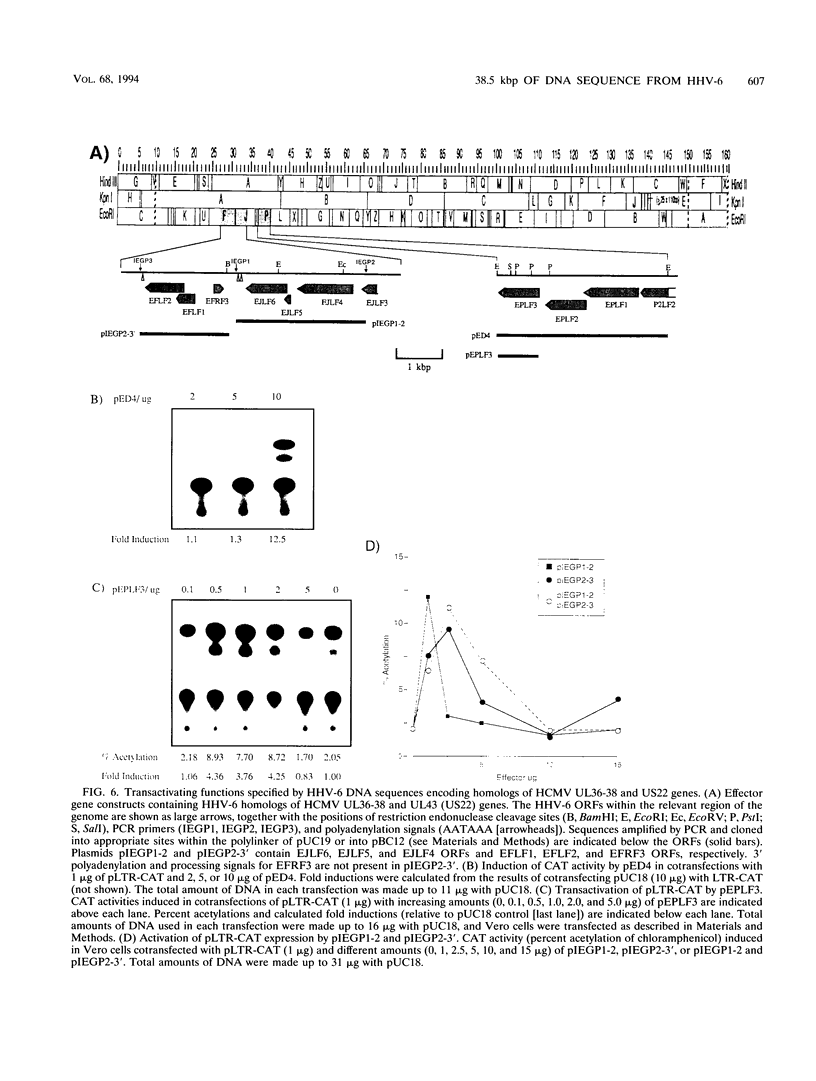



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Wilkinson G. W., Oram J. D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985 Mar;2(2):107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Albrecht J. C., Nicholas J., Biller D., Cameron K. R., Biesinger B., Newman C., Wittmann S., Craxton M. A., Coleman H., Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J Virol. 1992 Aug;66(8):5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Evelegh M. J., Muirhead B. Identification and separation of the two subunits of the herpes simplex virus ribonucleotide reductase. J Virol. 1986 Mar;57(3):1177–1181. doi: 10.1128/jvi.57.3.1177-1181.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Bublot M., Lomonte P., Lequarre A. S., Albrecht J. C., Nicholas J., Fleckenstein B., Pastoret P. P., Thiry E. Genetic relationships between bovine herpesvirus 4 and the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. Virology. 1992 Oct;190(2):654–665. doi: 10.1016/0042-6822(92)90903-3. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K., Tapper M. A. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J Infect Dis. 1990 Oct;162(4):844–851. doi: 10.1093/infdis/162.4.844. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Balachandran N. Identification, characterization, and sequence analysis of a cDNA encoding a phosphoprotein of human herpesvirus 6. J Virol. 1991 Dec;65(12):7085–7085. doi: 10.1128/jvi.65.12.7085-.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. K., Balachandran N. Identification, characterization, and sequence analysis of a cDNA encoding a phosphoprotein of human herpesvirus 6. J Virol. 1991 Jun;65(6):2884–2894. doi: 10.1128/jvi.65.6.2884-2894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. N., Dong D. L., Hayward G. S., Hayward S. D. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990 Jul;64(7):3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Satchwell S. C., Preddie E., Weston K. M., Barrell B. G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990 Apr 19;344(6268):774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Charron J., Perret J., Langelier Y. Herpes simplex virus ribonucleotide reductase induced in infected BHK-21/C13 cells: biochemical evidence for the existence of two non-identical subunits, H1 and H2. J Gen Virol. 1985 Apr;66(Pt 4):733–745. doi: 10.1099/0022-1317-66-4-733. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Santomenna L. D., Harlow P. P., Benfield P. A., Tenney D. J. Human cytomegalovirus US3 and UL36-38 immediate-early proteins regulate gene expression. J Virol. 1992 Jan;66(1):95–105. doi: 10.1128/jvi.66.1.95-105.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S., Ho Y. M., Hall S., Styles C. J., Scott S. D., Gompels U. A. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990 Jun;71(Pt 6):1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Lawrence G. L., Brown C. M., Barrell B. G. Identification of homologues to the human cytomegalovirus US22 gene family in human herpesvirus 6. J Gen Virol. 1992 Jul;73(Pt 7):1661–1671. doi: 10.1099/0022-1317-73-7-1661. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl P. F., Powell K. L. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J Virol. 1992 Jul;66(7):4126–4133. doi: 10.1128/jvi.66.7.4126-4133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixman E. D., Hayward G. S., Hayward S. D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992 Aug;66(8):5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., Marsden H. S., Dutia B. M. The ribonucleotide reductase induced by herpes simplex virus type 1 involves minimally a complex of two polypeptides (136K and 38K). J Gen Virol. 1985 Jul;66(Pt 7):1581–1587. doi: 10.1099/0022-1317-66-7-1581. [DOI] [PubMed] [Google Scholar]
- Geng Y. Q., Chandran B., Josephs S. F., Wood C. Identification and characterization of a human herpesvirus 6 gene segment that trans activates the human immunodeficiency virus type 1 promoter. J Virol. 1992 Mar;66(3):1564–1570. doi: 10.1128/jvi.66.3.1564-1570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Carss A. L., Sun N., Arrand J. R. Infectivity determinants encoded in a conserved gene block of human herpesvirus-6. DNA Seq. 1992;3(1):25–39. doi: 10.3109/10425179209039693. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J., Marcy A. I., Coen D. M., Challberg M. D. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol. 1990 Dec;64(12):5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Gompels U. A., Barrell B. G., Craxton M., Cameron K. R., Staden R., Chang Y. N., Hayward G. S. Deviations from expected frequencies of CpG dinucleotides in herpesvirus DNAs may be diagnostic of differences in the states of their latent genomes. J Gen Virol. 1989 Apr;70(Pt 4):837–855. doi: 10.1099/0022-1317-70-4-837. [DOI] [PubMed] [Google Scholar]
- Honess R. W. Herpes simplex and 'the herpes complex': diverse observations and a unifying hypothesis. The eighth Fleming lecture. J Gen Virol. 1984 Dec;65(Pt 12):2077–2107. doi: 10.1099/0022-1317-65-12-2077. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Balachandran N. Transactivation of human immunodeficiency virus promoter by human herpesvirus 6. J Virol. 1989 Feb;63(2):970–973. doi: 10.1128/jvi.63.2.970-973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Josephs S. F., Balachandran N. Transactivation of the human immunodeficiency virus promoter by human herpesvirus 6 (HHV-6) strains GS and Z-29 in primary human T lymphocytes and identification of transactivating HHV-6(GS) gene fragments. J Virol. 1991 Jun;65(6):2895–2902. doi: 10.1128/jvi.65.6.2895-2902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Kouzarides T., Mach M., Scholl B. C., Plachter B., Traupe B., Preddie E., Satchwell S. C., Fleckenstein B., Barrell B. G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987 May;61(5):1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Ablashi D. V., Salahuddin S. Z., Jagodzinski L. L., Wong-Staal F., Gallo R. C. Identification of the human herpesvirus 6 glycoprotein H and putative large tegument protein genes. J Virol. 1991 Oct;65(10):5597–5604. doi: 10.1128/jvi.65.10.5597-5604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlavage A. R., Fraser C. M., Chung F. Z., Venter J. C. Molecular structure and evolution of adrenergic and cholinergic receptors. Proteins. 1986 Dec;1(4):287–301. doi: 10.1002/prot.340010403. [DOI] [PubMed] [Google Scholar]
- Kiehl A., Dorsky D. I. Cooperation of EBV DNA polymerase and EA-D(BMRF1) in vitro and colocalization in nuclei of infected cells. Virology. 1991 Sep;184(1):330–340. doi: 10.1016/0042-6822(91)90849-7. [DOI] [PubMed] [Google Scholar]
- Knowles W. A., Gardner S. D. High prevalence of antibody to human herpesvirus-6 and seroconversion associated with rash in two infants. Lancet. 1988 Oct 15;2(8616):912–913. doi: 10.1016/s0140-6736(88)92522-6. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Preddy E., Barrell B. G. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology. 1988 Jul;165(1):151–164. doi: 10.1016/0042-6822(88)90668-x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late-gene expression: differential use of three start sites in the transcriptional activation of ICP36 gene expression. J Virol. 1989 Apr;63(4):1783–1791. doi: 10.1128/jvi.63.4.1783-1791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Sample J., Hayward G. S., Hayward S. D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990 Mar;64(3):1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., De Maria A., Malnati M., Lori F., DeRocco S. E., Baseler M., Gallo R. C. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991 Feb 7;349(6309):533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Malnati M. S., Garzino-Demo A., Crowley R. W., Long E. O., Gallo R. C. Infection of natural killer cells by human herpesvirus 6. Nature. 1993 Apr 1;362(6419):458–462. doi: 10.1038/362458a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Markham P. D., Tschachler E., di Marzo Veronese F., Salahuddin S. Z., Ablashi D. V., Pahwa S., Krohn K., Gallo R. C. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J Exp Med. 1988 May 1;167(5):1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Campbell M. E., Haarr L., Frame M. C., Parris D. S., Murphy M., Hope R. G., Muller M. T., Preston C. M. The 65,000-Mr DNA-binding and virion trans-inducing proteins of herpes simplex virus type 1. J Virol. 1987 Aug;61(8):2428–2437. doi: 10.1128/jvi.61.8.2428-2437.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Nicholas J., Thomson B. J., Newman C., Honess R. W. Identification of a transactivating function mapping to the putative immediate-early locus of human herpesvirus 6. J Virol. 1991 Oct;65(10):5381–5390. doi: 10.1128/jvi.65.10.5381-5390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Thomson B. J., Honess R. W., Craxton M. A., Gompels U. A., Liu M. Y., Littler E., Arrand J. R., Teo I., Jones M. D. The genome of human herpesvirus 6: maps of unit-length and concatemeric genomes for nine restriction endonucleases. J Gen Virol. 1991 Jan;72(Pt 1):157–168. doi: 10.1099/0022-1317-72-1-157. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. On the predictive recognition of signal peptide sequences. Virus Res. 1985 Oct;3(3):271–286. doi: 10.1016/0168-1702(85)90051-6. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. The genomes of the human herpesviruses: contents, relationships, and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- Messerle M., Bühler B., Keil G. M., Koszinowski U. H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992 Jan;66(1):27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J. Molecular biology of visual pigments. Annu Rev Neurosci. 1987;10:163–194. doi: 10.1146/annurev.ne.10.030187.001115. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Neipel F., Ellinger K., Fleckenstein B. Gene for the major antigenic structural protein (p100) of human herpesvirus 6. J Virol. 1992 Jun;66(6):3918–3924. doi: 10.1128/jvi.66.6.3918-3924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F., Ellinger K., Fleckenstein B. The unique region of the human herpesvirus 6 genome is essentially collinear with the UL segment of human cytomegalovirus. J Gen Virol. 1991 Sep;72(Pt 9):2293–2297. doi: 10.1099/0022-1317-72-9-2293. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Cameron K. R., Coleman H., Newman C., Honess R. W. Analysis of nucleotide sequence of the rightmost 43 kbp of herpesvirus saimiri (HVS) L-DNA: general conservation of genetic organization between HVS and Epstein-Barr virus. Virology. 1992 May;188(1):296–310. doi: 10.1016/0042-6822(92)90759-i. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Cameron K. R., Honess R. W. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature. 1992 Jan 23;355(6358):362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Coles L. S., Newman C., Honess R. W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991 May;65(5):2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J., Smith E. P., Coles L., Honess R. Gene expression in cells infected with gammaherpesvirus saimiri: properties of transcripts from two immediate-early genes. Virology. 1990 Nov;179(1):189–200. doi: 10.1016/0042-6822(90)90288-3. [DOI] [PubMed] [Google Scholar]
- Nikas I., McLauchlan J., Davison A. J., Taylor W. R., Clements J. B. Structural features of ribonucleotide reductase. Proteins. 1986 Dec;1(4):376–384. doi: 10.1002/prot.340010411. [DOI] [PubMed] [Google Scholar]
- Pari G. S., Kacica M. A., Anders D. G. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J Virol. 1993 May;67(5):2575–2582. doi: 10.1128/jvi.67.5.2575-2582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Pertuiset B., Boccara M., Cebrian J., Berthelot N., Chousterman S., Puvion-Dutilleul F., Sisman J., Sheldrick P. Physical mapping and nucleotide sequence of a herpes simplex virus type 1 gene required for capsid assembly. J Virol. 1989 May;63(5):2169–2179. doi: 10.1128/jvi.63.5.2169-2179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Davison M. D., Davison A. J. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990 May;71(Pt 5):1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- Roizmann B., Desrosiers R. C., Fleckenstein B., Lopez C., Minson A. C., Studdert M. J. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol. 1992;123(3-4):425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- Rooney C. M., Rowe D. T., Ragot T., Farrell P. J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989 Jul;63(7):3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford G. R., Ho K., Burns W. H. Characterization of the major locus of immediate-early genes of rat cytomegalovirus. J Virol. 1993 Jul;67(7):4093–4103. doi: 10.1128/jvi.67.7.4093-4103.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira T. J., Bozeman L. H., Sanderlin K. C., Warfield D. T., Feorino P. M., Holman R. C., Kaplan J. E., Fishbein D. B., Lopez C. Lack of correlation between human herpesvirus-6 infection and the course of human immunodeficiency virus infection. J Infect Dis. 1990 Mar;161(3):567–570. doi: 10.1093/infdis/161.3.567. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak P. C., Mocarski E. S. Transactivation of the cytomegalovirus ICP36 gene promoter requires the alpha gene product TRS1 in addition to IE1 and IE2. J Virol. 1992 Feb;66(2):1050–1058. doi: 10.1128/jvi.66.2.1050-1058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Higashi K., Kondo T., Takahashi H., Takahashi M., Yamanishi K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989 Jul;63(7):3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney D. J., Colberg-Poley A. M. Expression of the human cytomegalovirus UL36-38 immediate early region during permissive infection. Virology. 1991 May;182(1):199–210. doi: 10.1016/0042-6822(91)90663-v. [DOI] [PubMed] [Google Scholar]
- Teo I. A., Griffin B. E., Jones M. D. Characterization of the DNA polymerase gene of human herpesvirus 6. J Virol. 1991 Sep;65(9):4670–4680. doi: 10.1128/jvi.65.9.4670-4680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B. J., Honess R. W. The right end of the unique region of the genome of human herpesvirus 6 U1102 contains a candidate immediate early gene enhancer and a homologue of the human cytomegalovirus US22 gene family. J Gen Virol. 1992 Jul;73(Pt 7):1649–1660. doi: 10.1099/0022-1317-73-7-1649. [DOI] [PubMed] [Google Scholar]
- Vaughan P. J., Purifoy D. J., Powell K. L. DNA-binding protein associated with herpes simplex virus DNA polymerase. J Virol. 1985 Feb;53(2):501–508. doi: 10.1128/jvi.53.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988 Feb;162(2):406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- Weston K., Barrell B. G. Sequence of the short unique region, short repeats, and part of the long repeats of human cytomegalovirus. J Mol Biol. 1986 Nov 20;192(2):177–208. doi: 10.1016/0022-2836(86)90359-1. [DOI] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Black J. B., Stewart J. A., Lopez C., Pellett P. E. Identification of a nucleocapsid protein as a specific serological marker of human herpesvirus 6 infection. J Clin Microbiol. 1990 Sep;28(9):1957–1962. doi: 10.1128/jcm.28.9.1957-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- van Santen V. L. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. J Virol. 1993 Feb;67(2):773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen V. L. Characterization of the bovine herpesvirus 4 major immediate-early transcript. J Virol. 1991 Oct;65(10):5211–5224. doi: 10.1128/jvi.65.10.5211-5224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]




