Abstract
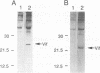
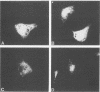
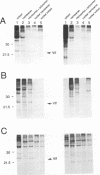
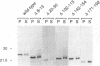
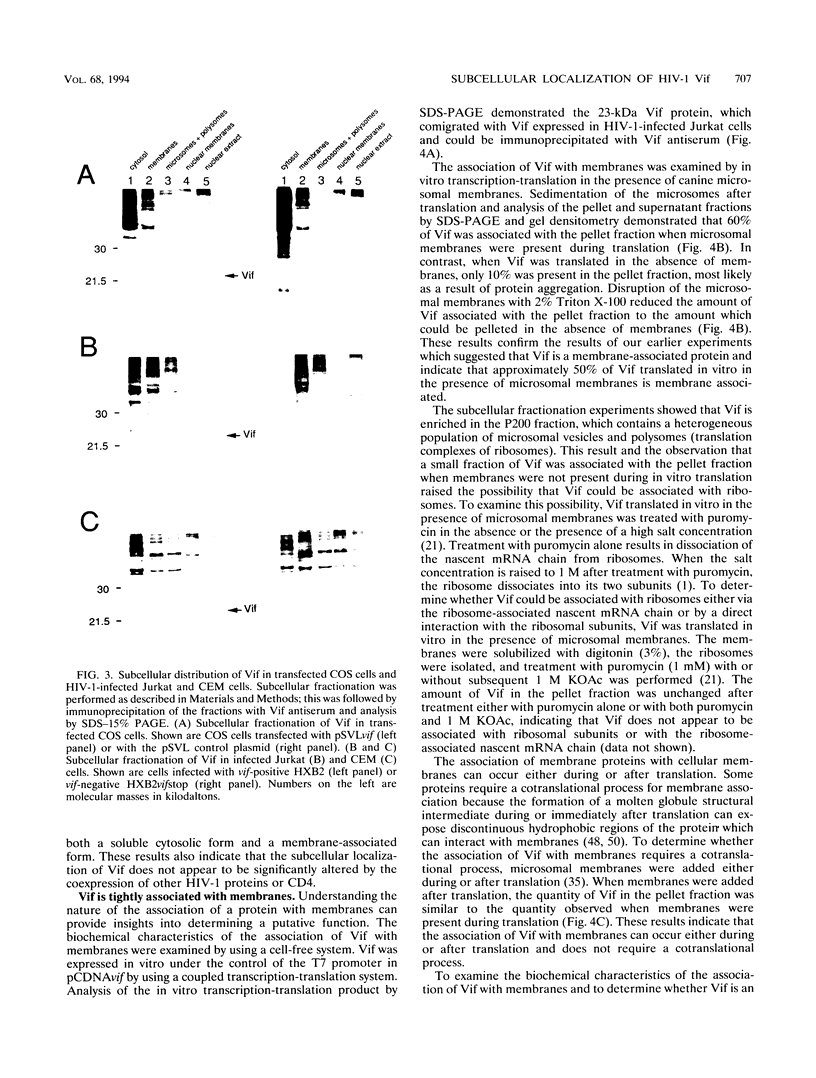
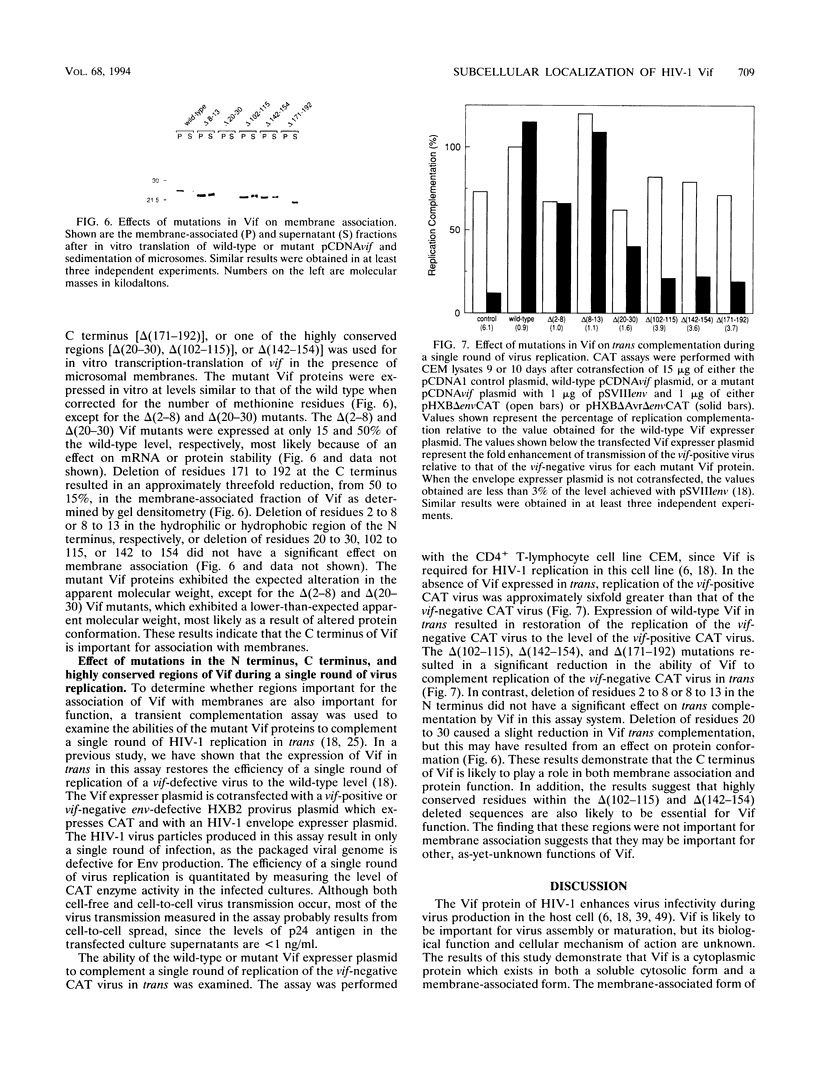
The Vif (viral infectivity factor) protein of human immunodeficiency virus type 1 (HIV-1) has been shown to dramatically enhance the infectivity of HIV-1 virus particles during virus production. The subcellular localization of Vif was examined to elucidate cellular pathways which may be important for Vif function. Indirect immunofluorescence staining of Vif demonstrated a diffuse cytoplasmic distribution and showed that most Vif was not associated with the Golgi complex, a proposed site of localization (B. Guy, M. Geist, K. Dott, D. Spehner, M.-P. Kieny, and J.-P. Lecocq, J. Virol. 65:1325-1331, 1991). Subcellular fractionation of transfected COS cells and HIV-1-infected Jurkat and CEM cells demonstrated that Vif is a cytoplasmic protein which exists in both a soluble cytosolic form and membrane-associated form. The membrane-associated form of Vif is a peripheral membrane protein which is tightly associated with the cytoplasmic side of cellular membranes. The C terminus of Vif was required for the stable association of Vif with membranes. The C terminus was also essential for Vif function, suggesting that the association of Vif with membranes is likely to be important for its biological activity. The highly conserved regions at residues 103 to 115 and 142 to 150 were important for Vif function but did not affect membrane association, indicating that these regions are likely to be important for other, as-yet-unknown functions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akari H., Sakuragi J., Takebe Y., Tomonaga K., Kawamura M., Fukasawa M., Miura T., Shinjo T., Hayami M. Biological characterization of human immunodeficiency virus type 1 and type 2 mutants in human peripheral blood mononuclear cells. Arch Virol. 1992;123(1-2):157–167. doi: 10.1007/BF01317146. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Gallo R. C. Three novel genes of human T-lymphotropic virus type III: immune reactivity of their products with sera from acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2209–2213. doi: 10.1073/pnas.83.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audoly G., Sauze N., Harkiss G., Vitu C., Russo P., Querat G., Suzan M., Vigne R. Identification and subcellular localization of the Q gene product of visna virus. Virology. 1992 Aug;189(2):734–739. doi: 10.1016/0042-6822(92)90596-h. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Blanc D., Patience C., Schulz T. F., Weiss R., Spire B. Transcomplementation of VIF- HIV-1 mutants in CEM cells suggests that VIF affects late steps of the viral life cycle. Virology. 1993 Mar;193(1):186–192. doi: 10.1006/viro.1993.1114. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chong L. D., Rose J. K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993 Jan;67(1):407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dedera D., Hu W., Vander Heyden N., Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989 Jul;63(7):3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Peden K. Cell-free transmission of Vif mutants of HIV-1. Virology. 1992 Sep;190(1):19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- Fath K. R., Burgess D. R. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J Cell Biol. 1993 Jan;120(1):117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992 Feb 7;68(3):545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Gabuzda D. H., Lawrence K., Langhoff E., Terwilliger E., Dorfman T., Haseltine W. A., Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992 Nov;66(11):6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. D., Tiley L. S., Cullen B. R. Rev activates expression of the human immunodeficiency virus type 1 vif and vpr gene products. J Virol. 1991 Mar;65(3):1653–1657. doi: 10.1128/jvi.65.3.1653-1657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy B., Geist M., Dott K., Spehner D., Kieny M. P., Lecocq J. P. A specific inhibitor of cysteine proteases impairs a Vif-dependent modification of human immunodeficiency virus type 1 Env protein. J Virol. 1991 Mar;65(3):1325–1331. doi: 10.1128/jvi.65.3.1325-1331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Paterson H., Marshall C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991 Dec;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Helseth E., Kowalski M., Gabuzda D., Olshevsky U., Haseltine W., Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990 May;64(5):2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Franchini G., Wong-Staal F., DuBois G. C., Robey W. G., Lautenberger J. A., Papas T. S. Identification of HTLV-III/LAV sor gene product and detection of antibodies in human sera. Science. 1986 Mar 28;231(4745):1553–1555. doi: 10.1126/science.3006245. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Garber E. A., Goldberg A. R. Differences in intracellular location of pp60src in rat and chicken cells transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4142–4146. doi: 10.1073/pnas.77.7.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Allan J. S., McLane M. F., Groopman J. E., Essex M. A new HTLV-III/LAV protein encoded by a gene found in cytopathic retroviruses. Science. 1986 Mar 28;231(4745):1546–1549. doi: 10.1126/science.3006243. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993 Mar 26;259(5103):1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- Oberste M. S., Gonda M. A. Conservation of amino-acid sequence motifs in lentivirus Vif proteins. Virus Genes. 1992 Jan;6(1):95–102. doi: 10.1007/BF01703760. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J. Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell. 1992 Oct 2;71(1):87–96. doi: 10.1016/0092-8674(92)90268-h. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Poiesz B., Ruscetti F. W., Gallo R. C. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981 Jul 15;112(1):355–360. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- Sakai H., Shibata R., Sakuragi J., Sakuragi S., Kawamura M., Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993 Mar;67(3):1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Ma X. Y., Gordienko I., Volsky D. J. Recombinational analysis of a natural noncytopathic human immunodeficiency virus type 1 (HIV-1) isolate: role of the vif gene in HIV-1 infection kinetics and cytopathicity. J Virol. 1991 Nov;65(11):5765–5773. doi: 10.1128/jvi.65.11.5765-5773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Pavlakis G. N. Expression of human immunodeficiency virus type 1 vif and vpr mRNAs is Rev-dependent and regulated by splicing. Virology. 1991 Aug;183(2):677–686. doi: 10.1016/0042-6822(91)90996-o. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Tartar A., Portetelle D., Burny A., Haseltine W. Replicative and cytopathic potential of HTLV-III/LAV with sor gene deletions. Science. 1986 Mar 28;231(4745):1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- Sova P., Volsky D. J. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with vif-negative human immunodeficiency virus type 1. J Virol. 1993 Oct;67(10):6322–6326. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Daugherty D., Clouse K., Cohen D., Folks T., Martin M. A. The HIV 'A' (sor) gene product is essential for virus infectivity. Nature. 1987 Aug 20;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- Terwilliger E. F., Godin B., Sodroski J. G., Haseltine W. A. Construction and use of a replication-competent human immunodeficiency virus (HIV-1) that expresses the chloramphenicol acetyltransferase enzyme. Proc Natl Acad Sci U S A. 1989 May;86(10):3857–3861. doi: 10.1073/pnas.86.10.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga K., Norimine J., Shin Y. S., Fukasawa M., Miyazawa T., Adachi A., Toyosaki T., Kawaguchi Y., Kai C., Mikami T. Identification of a feline immunodeficiency virus gene which is essential for cell-free virus infectivity. J Virol. 1992 Oct;66(10):6181–6185. doi: 10.1128/jvi.66.10.6181-6185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Yu G., Felsted R. L. Effect of myristoylation on p27 nef subcellular distribution and suppression of HIV-LTR transcription. Virology. 1992 Mar;187(1):46–55. doi: 10.1016/0042-6822(92)90293-x. [DOI] [PubMed] [Google Scholar]
- van der Goot F. G., González-Mañas J. M., Lakey J. H., Pattus F. A 'molten-globule' membrane-insertion intermediate of the pore-forming domain of colicin A. Nature. 1991 Dec 5;354(6352):408–410. doi: 10.1038/354408a0. [DOI] [PubMed] [Google Scholar]
- von Schwedler U., Song J., Aiken C., Trono D. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993 Aug;67(8):4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]