Abstract
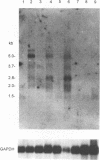
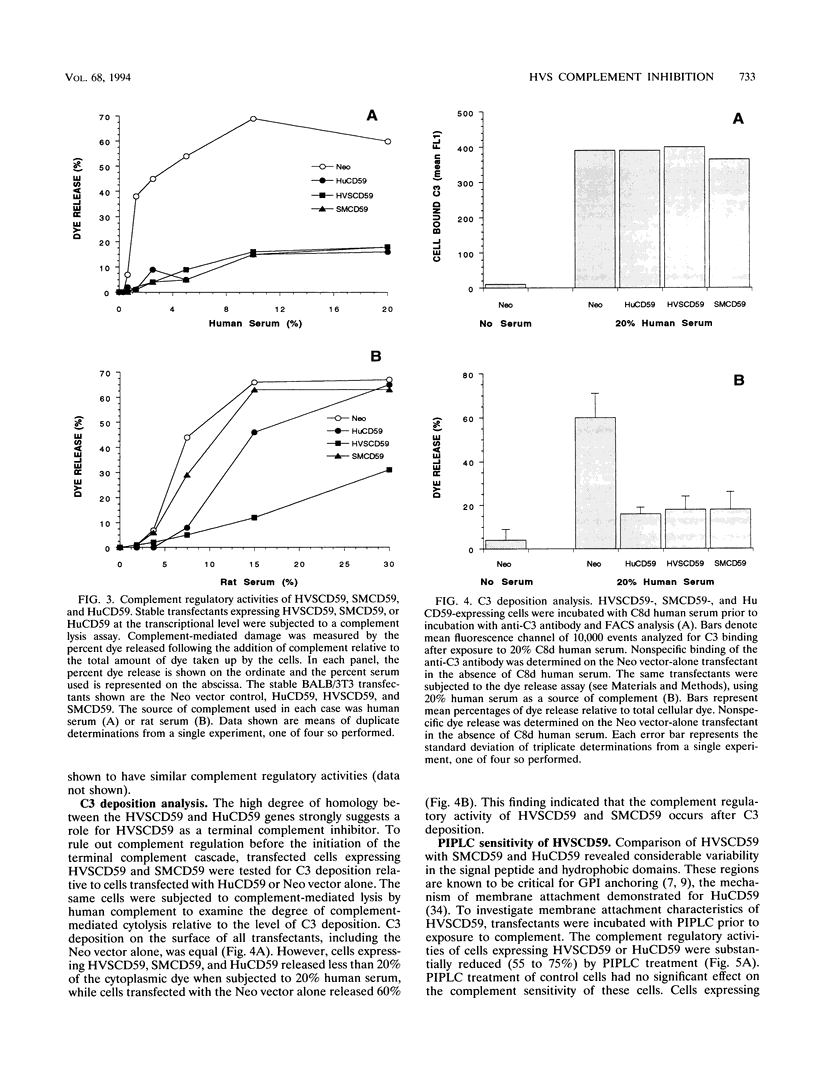
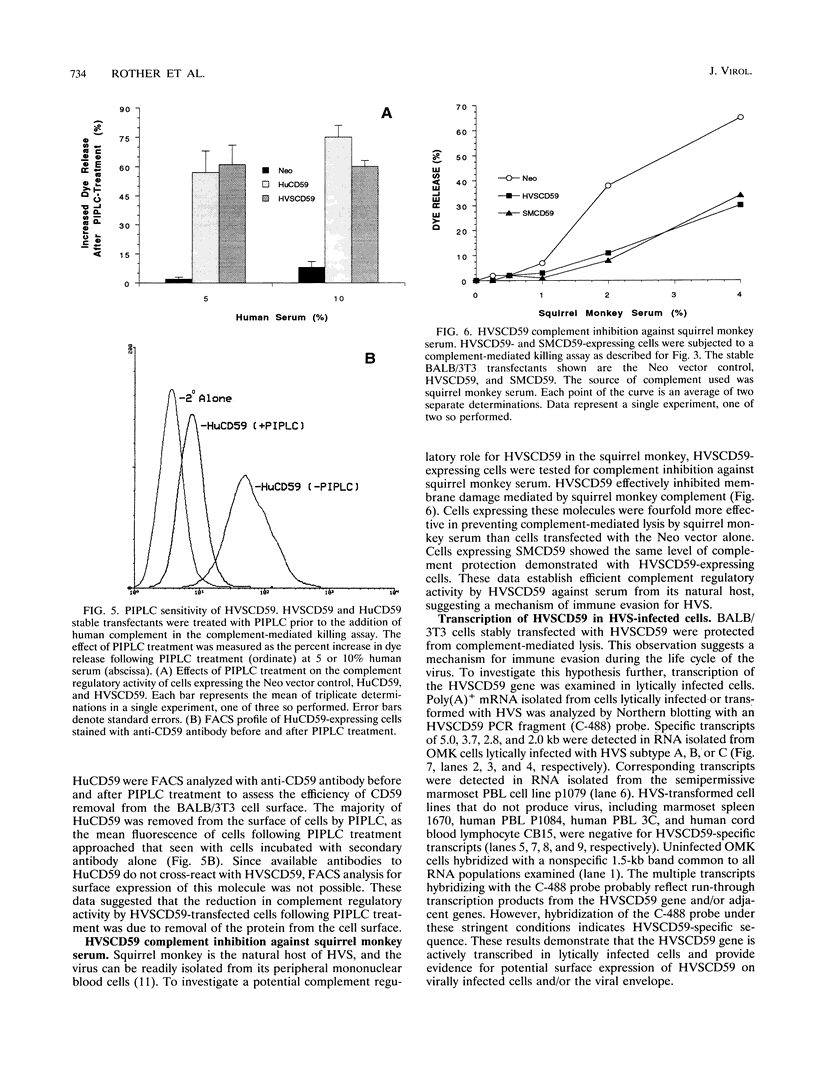
Herpesvirus saimiri (HVS) is a lymphotropic herpesvirus that induces T-cell transformation in vitro and causes lymphomas and leukemias in New World primates other than its natural host, the squirrel monkey. Nucleotide sequence analysis of the HVS genome revealed two open reading frames with significant homology to genes for human complement regulatory molecules. One of these genes encodes a predicted protein (designated HVSCD59) with 48% amino acid sequence identity to the human terminal complement regulatory protein CD59 (HuCD59). The CD59 homolog from squirrel monkey (SMCD59) was cloned, and the corresponding amino acid sequence showed 69% identity with HVSCD59. BALB/3T3 cells stably expressing HVSCD59, SMCD59, or HuCD59 were equally protected from complement-mediated lysis by human serum. However, only HVSCD59-expressing cells were effectively protected from complement-mediated lysis when challenged with rat serum, suggesting that HVSCD59 was less species restrictive. The complement regulatory activity of HVSCD59 and SMCD59 occurred after C3b deposition, indicating terminal complement inhibition. Treatment of BALB/3T3 stable transfectants with phosphatidylinositol-specific phospholipase C prior to complement attack decreased the complement regulatory function of HVSCD59, suggesting cell surface attachment via a glycosyl-phosphatidylinositol anchor. Cells expressing HVSCD59 effectively inhibited complement-mediated lysis by squirrel monkey serum in comparison with SMCD59-expressing cells. Finally HVSCD59-specific transcripts were detected in owl monkey cells permissive for lytic HVS replication but not in T cells transformed by HVS, which failed to produce virions. These data are the first to demonstrate a functional, virally encoded terminal complement inhibitor and suggest that HVSCD59 represents a humoral immune evasion mechanism supporting the lytic life cycle of HVS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J Virol. 1992 Jun;66(6):3937–3940. doi: 10.1128/jvi.66.6.3937-3940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J. C., Nicholas J., Biller D., Cameron K. R., Biesinger B., Newman C., Wittmann S., Craxton M. A., Coleman H., Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J Virol. 1992 Aug;66(8):5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J. C., Nicholas J., Cameron K. R., Newman C., Fleckenstein B., Honess R. W. Herpesvirus saimiri has a gene specifying a homologue of the cellular membrane glycoprotein CD59. Virology. 1992 Sep;190(1):527–530. doi: 10.1016/0042-6822(92)91247-r. [DOI] [PubMed] [Google Scholar]
- Bell S., Cranage M., Borysiewicz L., Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990 May;64(5):2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesinger B., Müller-Fleckenstein I., Simmer B., Lang G., Wittmann S., Platzer E., Desrosiers R. C., Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras I. W. An internally positioned signal can direct attachment of a glycophospholipid membrane anchor. J Cell Biol. 1991 Apr;113(1):77–85. doi: 10.1083/jcb.113.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N. Signal peptide for protein secretion directing glycophospholipid membrane anchor attachment. Science. 1989 Mar 3;243(4895):1196–1198. doi: 10.1126/science.2466338. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Friedman H. M., Cohen G. H., Eisenberg R. J., Hammer C. H., Frank M. M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986 Sep 1;137(5):1636–1641. [PubMed] [Google Scholar]
- Fujita T., Inoue T., Ogawa K., Iida K., Tamura N. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J Exp Med. 1987 Nov 1;166(5):1221–1228. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Hahn W. C., Menu E., Bothwell A. L., Sims P. J., Bierer B. E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science. 1992 Jun 26;256(5065):1805–1807. doi: 10.1126/science.1377404. [DOI] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Isaacs S. N., Kotwal G. J., Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Liang X., Tang M., Zamb T. J., Babiuk L. A., Kowalski J., Tykocinski M. L. Expression of glycoprotein gIII-human decay-accelerating factor chimera on the bovine herpesvirus 1 virion via a glycosyl phosphatidylinositol-based membrane anchor. J Virol. 1993 Aug;67(8):4896–4904. doi: 10.1128/jvi.67.8.4896-4904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. E. Site-specific mutations in the COOH-terminus of placental alkaline phosphatase: a single amino acid change converts a phosphatidylinositol-glycan-anchored protein to a secreted protein. J Cell Biol. 1992 Feb;116(3):799–807. doi: 10.1083/jcb.116.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczynska B., Falk L., Wolfe L., Deinhardt F. Transplantation and cytogenetic studies of Herpesvirus saimiri-induced disease in Marmoset monkeys. J Natl Cancer Inst. 1973 Feb;50(2):331–337. doi: 10.1093/jnci/50.2.331. [DOI] [PubMed] [Google Scholar]
- McKenzie R., Kotwal G. J., Moss B., Hammer C. H., Frank M. M. Regulation of complement activity by vaccinia virus complement-control protein. J Infect Dis. 1992 Dec;166(6):1245–1250. doi: 10.1093/infdis/166.6.1245. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medveczky P., Szomolanyi E., Desrosiers R. C., Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984 Dec;52(3):938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Caras I. W. A nonfunctional sequence converted to a signal for glycophosphatidylinositol membrane anchor attachment. J Cell Biol. 1991 Oct;115(2):329–336. doi: 10.1083/jcb.115.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Raab H., Kohr W. J., Caras I. W. Glycophospholipid membrane anchor attachment. Molecular analysis of the cleavage/attachment site. J Biol Chem. 1991 Jan 15;266(2):1250–1257. [PubMed] [Google Scholar]
- Ninomiya H., Stewart B. H., Rollins S. A., Zhao J., Bothwell A. L., Sims P. J. Contribution of the N-linked carbohydrate of erythrocyte antigen CD59 to its complement-inhibitory activity. J Biol Chem. 1992 Apr 25;267(12):8404–8410. [PubMed] [Google Scholar]
- Petranka J. G., Fleenor D. E., Sykes K., Kaufman R. E., Rosse W. F. Structure of the CD59-encoding gene: further evidence of a relationship to murine lymphocyte antigen Ly-6 protein. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7876–7879. doi: 10.1073/pnas.89.17.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins S. A., Sims P. J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990 May 1;144(9):3478–3483. [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Zhao J., Rollins S. A., Maher S. E., Bothwell A. L., Sims P. J. Amplified gene expression in CD59-transfected Chinese hamster ovary cells confers protection against the membrane attack complex of human complement. J Biol Chem. 1991 Jul 15;266(20):13418–13422. [PubMed] [Google Scholar]