Abstract
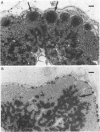
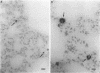
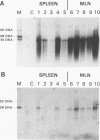
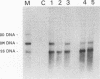
We suppressed the B-cell development and antibody response in mink by using treatment with polyclonal anti-immunoglobulin M (anti-IgM) to study the effects of antiviral antibodies on development of Aleutian mink disease parvovirus (ADV)-induced disease in more detail. Newborn mink kits were injected intraperitoneally with 1 mg of either anti-IgM or a control preparation three times a week for 30 to 34 days. At 21 days after birth, groups of mink kits were infected with the highly virulent United isolate of ADV. At selected time points, i.e., postinfection days 9, 13, 29, and 200, randomly chosen mink kits were sacrificed, and blood and tissues were collected for analyses. The efficacy of immunosuppressive treatment was monitored by electrophoretic techniques and flow cytometry. Effects of treatment on viral replication, on viral mRNA levels, and on development of acute or chronic disease were determined by histopathological, immunoelectrophoretic, and molecular hybridization techniques. Several interesting findings emerged from these studies. First, antiviral antibodies decreased ADV mRNA levels more than DNA replication. Second, suppression of B-cell development and antibody response in mink kits infected at 21 days of age resulted in production of viral inclusion bodies in alveolar type II cells. Some of these kits showed mild clinical signs of respiratory disease, and one kit died of respiratory distress; however, clinical signs were seen only after release of immunosuppression, suggesting that the production of antiviral antibodies, in combination with the massive amounts of free viral antigen present, somehow is involved in the induction of respiratory distress. It is suggested that the antiviral antibody response observed in mink older than approximately 14 days primarily, by a yet unknown mechanism, decreases ADV mRNA levels which, if severe enough, results in restricted levels of DNA replication and virion production. Furthermore, such a restricted ADV infection at low levels paves the way for a persistent infection leading to immunologically mediated disease. The potential mechanisms of antibody-mediated restriction of viral mRNA levels and mechanisms of disease induction are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B., Blixenkrone-Møller M., Larsen E. B., Bielefeldt Ohmann H., Simesen R. B., Uttenthal A. Reactivity of eleven anti-human leucocyte monoclonal antibodies with lymphocytes from several domestic animals. Vet Immunol Immunopathol. 1988 Jul;19(1):31–38. doi: 10.1016/0165-2427(88)90044-x. [DOI] [PubMed] [Google Scholar]
- Aasted B. Mink infected with Aleutian disease virus have an elevated level of CD8-positive T-lymphocytes. Vet Immunol Immunopathol. 1989 Mar;20(4):375–385. doi: 10.1016/0165-2427(89)90082-2. [DOI] [PubMed] [Google Scholar]
- Aasted B., Race R. E., Bloom M. E. Aleutian disease virus, a parvovirus, is proteolytically degraded during in vivo infection in mink. J Virol. 1984 Jul;51(1):7–13. doi: 10.1128/jvi.51.1.7-13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasted B., Tierney G. S., Bloom M. E. Analysis of the quantity of antiviral antibodies from mink infected with different Aleutian disease virus strains. Scand J Immunol. 1984 May;19(5):395–402. doi: 10.1111/j.1365-3083.1984.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Aasted B. Restricted heterogeneity of the early antibody response to Aleutian disease virus in mink kits. Acta Pathol Microbiol Immunol Scand C. 1986 Aug;94(4):137–143. doi: 10.1111/j.1699-0463.1986.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Alexandersen S. Acute interstitial pneumonia in mink kits: experimental reproduction of the disease. Vet Pathol. 1986 Sep;23(5):579–588. doi: 10.1177/030098588602300506. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987 Jan;61(1):81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
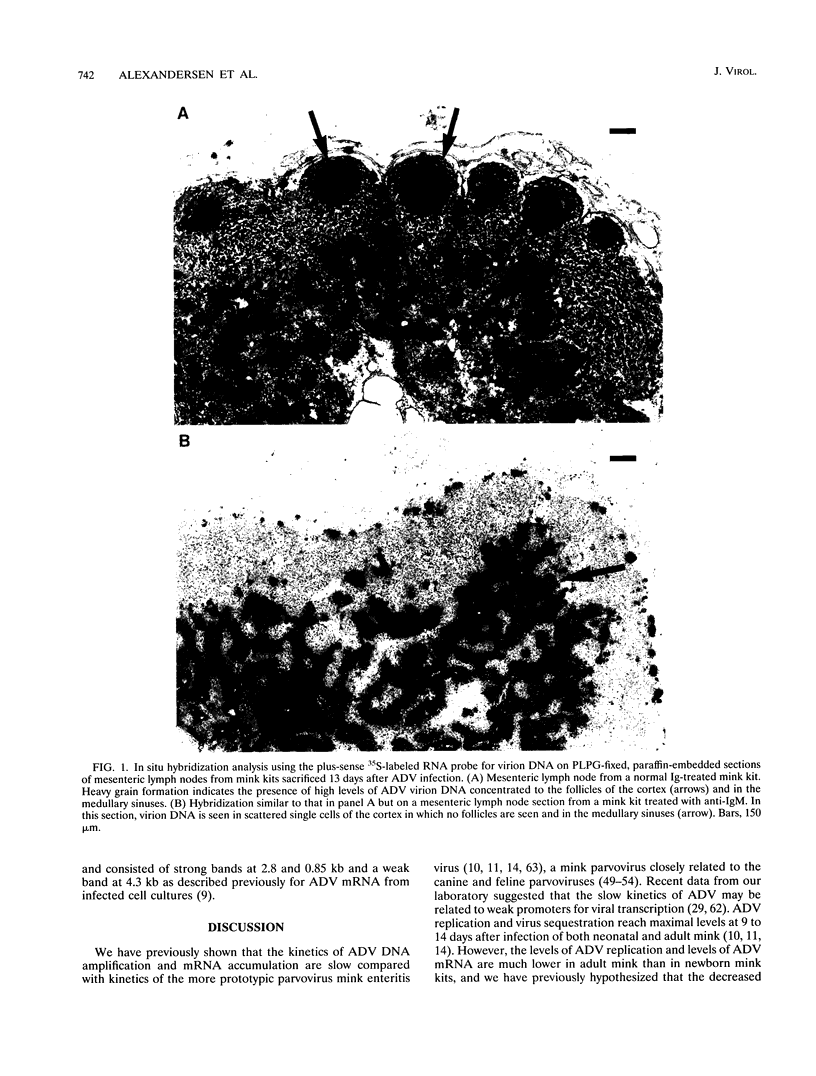
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Hau J. Rocket line immunoelectrophoresis: an improved assay for simultaneous quantification of a mink parvovirus (Aleutian disease virus) antigen and antibody. J Virol Methods. 1985 Feb;10(2):145–151. doi: 10.1016/0166-0934(85)90100-4. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Larsen S., Cohn A., Uttenthal A., Race R. E., Aasted B., Hansen M., Bloom M. E. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J Virol. 1989 Jan;63(1):9–17. doi: 10.1128/jvi.63.1.9-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S. Pathogenesis of disease caused by Aleutian mink disease parvovirus. APMIS Suppl. 1990;14:1–32. [PubMed] [Google Scholar]
- Alexandersen S., Uttenthal-Jensen A., Aasted B. Demonstration of non-degraded Aleutian disease virus (ADV) proteins in lung tissue from experimentally infected mink kits. Brief report. Arch Virol. 1986;87(1-2):127–133. doi: 10.1007/BF01310549. [DOI] [PubMed] [Google Scholar]
- Astry C. L., Jakab G. J. Influenza virus-induced immune complexes suppress alveolar macrophage phagocytosis. J Virol. 1984 May;50(2):287–292. doi: 10.1128/jvi.50.2.287-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Ball-Goodrich L. J., Tattersall P. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J Virol. 1992 Jun;66(6):3415–3423. doi: 10.1128/jvi.66.6.3415-3423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I. Parvovirus replication. Microbiol Rev. 1990 Sep;54(3):316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Mori S., Wolfinbarger J. B. Analysis of parvovirus infections using strand-specific hybridization probes. Virus Res. 1989 Sep;14(1):1–25. doi: 10.1016/0168-1702(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Cerny A., Heusser C., Sutter S., Huegin A. W., Bazin H., Hengartner H., Zinkernagel R. M. Generation of agammaglobulinaemic mice by prenatal and postnatal exposure to polyclonal or monoclonal anti-IgM antibodies. Scand J Immunol. 1986 Oct;24(4):437–445. doi: 10.1111/j.1365-3083.1986.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Cerny A., Hügin A. W., Sutter S., Heusser C. H., Bos N., Izui S., Hengartner H., Zinkernagel R. M. Suppression of B cell development and antibody responses in mice with polyclonal rabbit and monoclonal rat anti-IgM antibodies. I. Characterization of the suppressed state. Exp Cell Biol. 1985;53(6):301–313. doi: 10.1159/000163327. [DOI] [PubMed] [Google Scholar]
- Cerny A., Sutter S., Bazin H., Hengartner H., Zinkernagel R. M. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell-deprived mice. J Virol. 1988 May;62(5):1803–1807. doi: 10.1128/jvi.62.5.1803-1807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Hadlow W., Race R. Purification and ultrastructure of Aleutian disease virus of mink. Nature. 1975 Apr 3;254(5499):456–457. doi: 10.1038/254456a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Ingram D. G. Antigen and antibody in Aleutian disease in mink. I. Precipitation reaction by agar-gel electrophoresis. J Immunol. 1972 Feb;108(2):555–557. [PubMed] [Google Scholar]
- Christensen J., Storgaard T., Viuff B., Aasted B., Alexandersen S. Comparison of promoter activity in Aleutian mink disease parvovirus, minute virus of mice, and canine parvovirus: possible role of weak promoters in the pathogenesis of Aleutian mink disease parvovirus infection. J Virol. 1993 Apr;67(4):1877–1886. doi: 10.1128/jvi.67.4.1877-1886.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Linnekin D. Regulation of protein kinases and gene expression by immunocytokines. Ann N Y Acad Sci. 1990;594:240–252. doi: 10.1111/j.1749-6632.1990.tb40484.x. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Gardiner E. M., Tattersall P. Evidence that developmentally regulated control of gene expression by a parvoviral allotropic determinant is particle mediated. J Virol. 1988 May;62(5):1713–1722. doi: 10.1128/jvi.62.5.1713-1722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Turpin J., Kalter D. C., Hansen B., Orenstein J. M., Dieffenbach C. W., Friedman R. M., Meltzer M. S. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol. 1990 Oct 15;145(8):2669–2676. [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature. 1986 May 15;321(6067):244–246. doi: 10.1038/321244a0. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Gantz D., Eble B., Walker D., Stowring L., Ventura P., Blum H., Wietgrefe S., Zupancic M., Tourtellotte W. Natural history of restricted synthesis and expression of measles virus genes in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1985 May;82(9):3020–3024. doi: 10.1073/pnas.82.9.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N., Castranova V. Interferon production in rat type II pneumocytes and alveolar macrophages. Exp Lung Res. 1989 May;15(3):429–445. doi: 10.3109/01902148909087869. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Agy M. B. Regulation of viral and cellular RNA turnover in cells infected by eukaryotic viruses including HIV-1. Enzyme. 1990;44(1-4):332–346. doi: 10.1159/000468769. [DOI] [PubMed] [Google Scholar]
- Kauffman S. L., Burri P. H., Weibel E. R. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec. 1974 Sep;180(1):63–76. doi: 10.1002/ar.1091800108. [DOI] [PubMed] [Google Scholar]
- Kauffman S. L. Cell proliferation in the mammalian lung. Int Rev Exp Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- Liebert U. G., Schneider-Schaulies S., Baczko K., ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990 Feb;64(2):706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macé K., Duc Dodon M., Gazzolo L. Restriction of HIV-1 replication in promonocytic cells: a role for IFN-alpha. Virology. 1989 Feb;168(2):399–405. doi: 10.1016/0042-6822(89)90282-1. [DOI] [PubMed] [Google Scholar]
- Mandel B. The interaction of neutralized poliovirus with HeLa cells. I. Adsorption. Virology. 1967 Feb;31(2):238–247. doi: 10.1016/0042-6822(67)90167-5. [DOI] [PubMed] [Google Scholar]
- Millar A. B., Foley N. M., Singer M., Johnson N. M., Meager A., Rook G. A. Tumour necrosis factor in bronchopulmonary secretions of patients with adult respiratory distress syndrome. Lancet. 1989 Sep 23;2(8665):712–714. doi: 10.1016/s0140-6736(89)90772-1. [DOI] [PubMed] [Google Scholar]
- Oka T., Ohtsuki Y., Sonobe H., Furihata M., Miyoshi I. Suppressive effects of interferons on the production and release of human T-lymphotropic virus type-I (HTLV-I). Arch Virol. 1990;115(1-2):63–73. doi: 10.1007/BF01310623. [DOI] [PubMed] [Google Scholar]
- PORTER D. D. TRANSFER OF GAMMA GLOBULIN FROM MOTHER TO OFFSPRING IN MINK. Proc Soc Exp Biol Med. 1965 May;119:131–133. doi: 10.3181/00379727-119-30117. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Carmichael L. E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988 Oct;166(2):293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E., Antczak D. F. Antigenic relationships between canine parvovirus type 2, feline panleukopenia virus and mink enteritis virus using conventional antisera and monoclonal antibodies. Arch Virol. 1982;72(4):267–278. doi: 10.1007/BF01315223. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Antigenic structure and variation of canine parvovirus type-2, feline panleukopenia virus, and mink enteritis virus. Virology. 1983 Sep;129(2):401–414. doi: 10.1016/0042-6822(83)90179-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv Virus Res. 1990;38:403–450. doi: 10.1016/S0065-3527(08)60867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Gorham J. R., Schwartz T. M., Carmichael L. E. Characterization of antigenic variation among mink enteritis virus isolates. Am J Vet Res. 1984 Dec;45(12):2591–2599. [PubMed] [Google Scholar]
- Parrish C. R., Leathers C. W., Pearson R., Gorham J. R. Comparisons of feline panleukopenia virus, canine parvovirus, raccoon parvovirus, and mink enteritis virus and their pathogenicity for mink and ferrets. Am J Vet Res. 1987 Oct;48(10):1429–1435. [PubMed] [Google Scholar]
- Poli G., Fauci A. S. The role of monocyte/macrophages and cytokines in the pathogenesis of HIV infection. Pathobiology. 1992;60(4):246–251. doi: 10.1159/000163729. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Porter H. G., Suffin S. C., Larsen A. E. Immunoglobulin classes of Aleutian disease virus antibody. Infect Immun. 1984 Feb;43(2):463–466. doi: 10.1128/iai.43.2.463-466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgaard T., Christensen J., Aasted B., Alexandersen S. cis-acting sequences in the Aleutian mink disease parvovirus late promoter important for transcription: comparison to the canine parvovirus and minute virus of mice. J Virol. 1993 Apr;67(4):1887–1895. doi: 10.1128/jvi.67.4.1887-1895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu J., Misset B., Lebargy F., Carlet J., Bernaudin J. F. Expression of tumor necrosis factor-alpha gene in alveolar macrophages from patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1585–1589. doi: 10.1164/ajrccm/147.6_Pt_1.1585. [DOI] [PubMed] [Google Scholar]
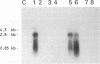
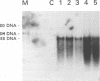
- Uttenthal A., Larsen S., Lund E., Bloom M. E., Storgård T., Alexandersen S. Analysis of experimental mink enteritis virus infection in mink: in situ hybridization, serology, and histopathology. J Virol. 1990 Jun;64(6):2768–2779. doi: 10.1128/jvi.64.6.2768-2779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Remick D. G., Kunkel S. L., Chensue S. W., Kunkel R. G., Johnson K. J., Ward P. A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989 Dec;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J. A., Clark J. C., Wispé J. R., Pryhuber G. S. Effects of TNF-alpha and phorbol ester on human surfactant protein and MnSOD gene transcription in vitro. Am J Physiol. 1992 Jun;262(6 Pt 1):L688–L693. doi: 10.1152/ajplung.1992.262.6.L688. [DOI] [PubMed] [Google Scholar]