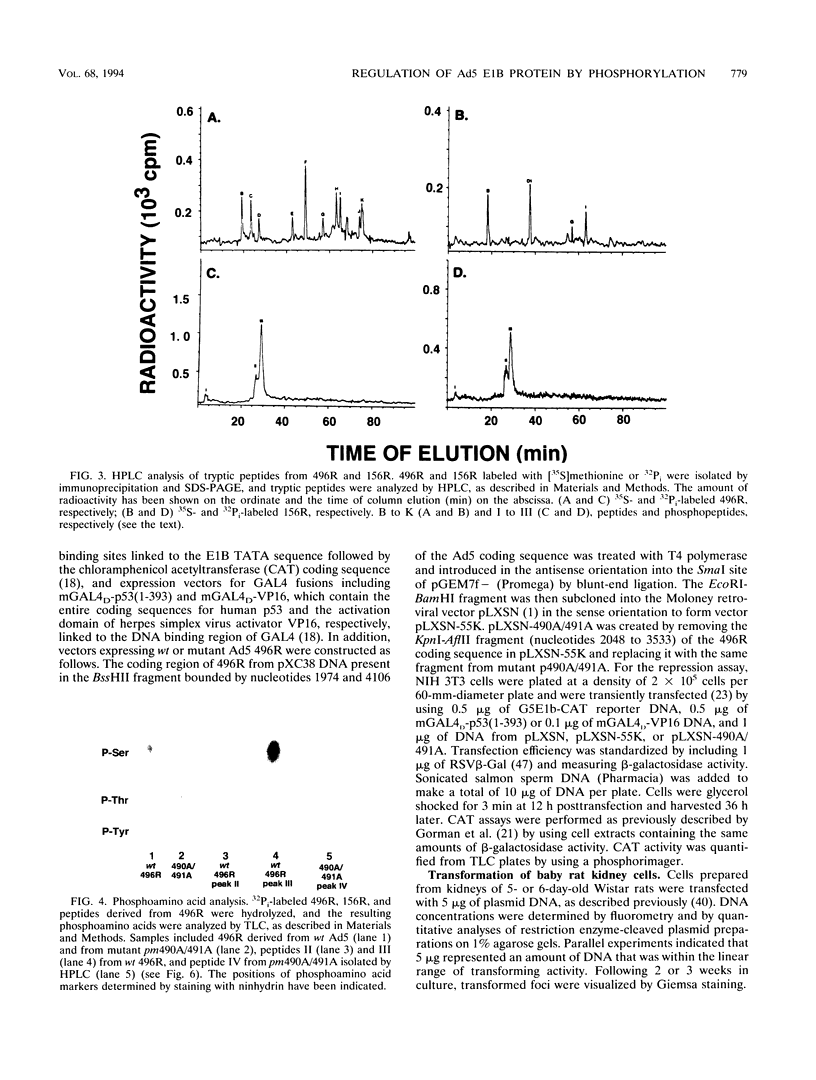
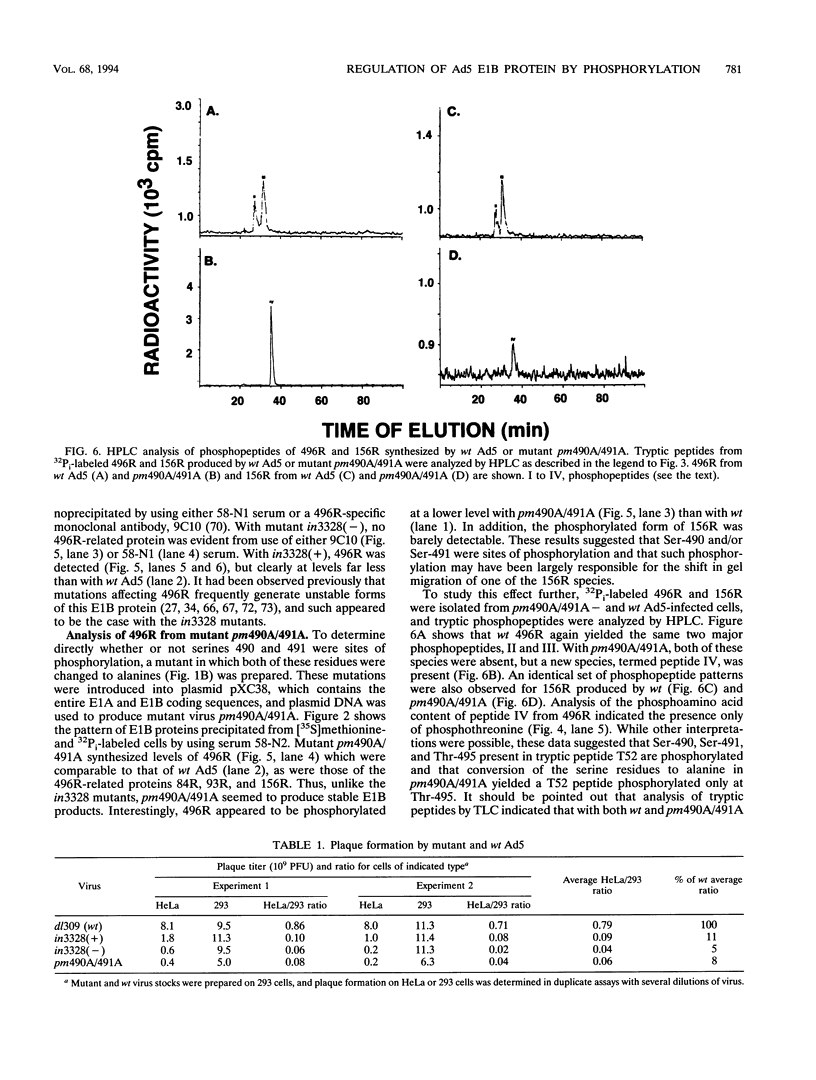
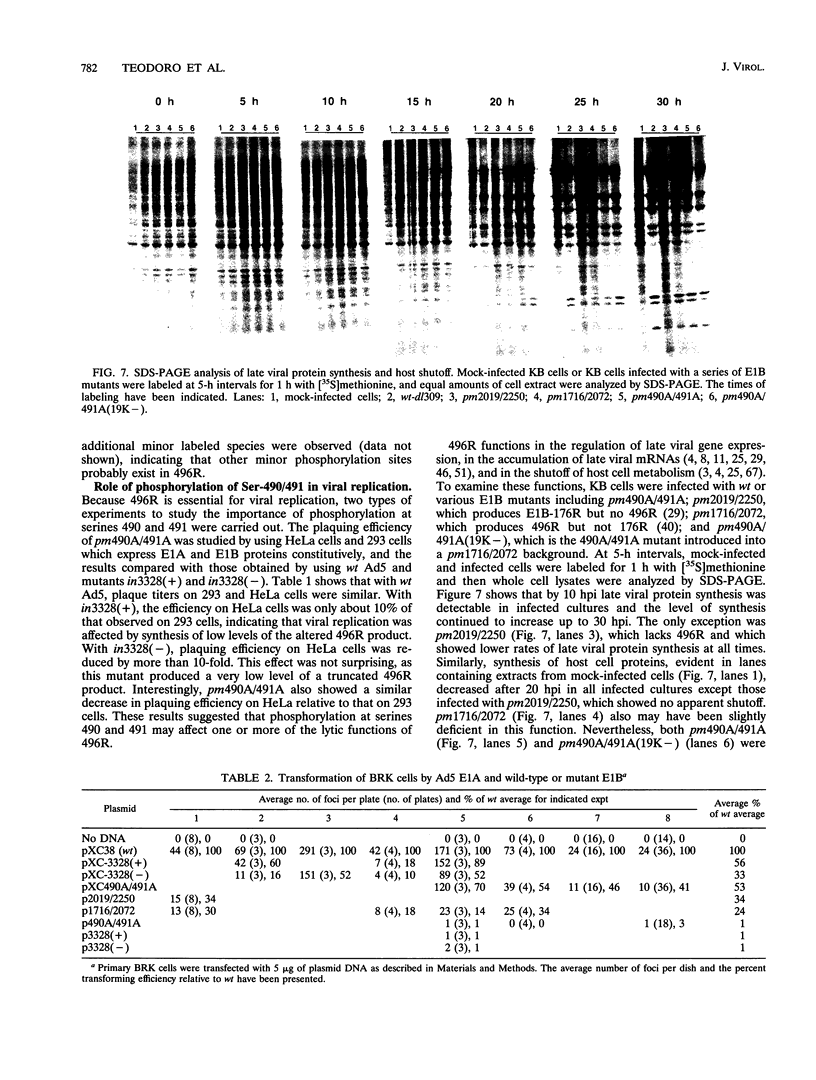
Abstract
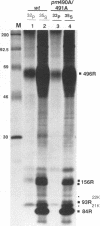
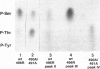
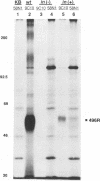
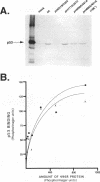
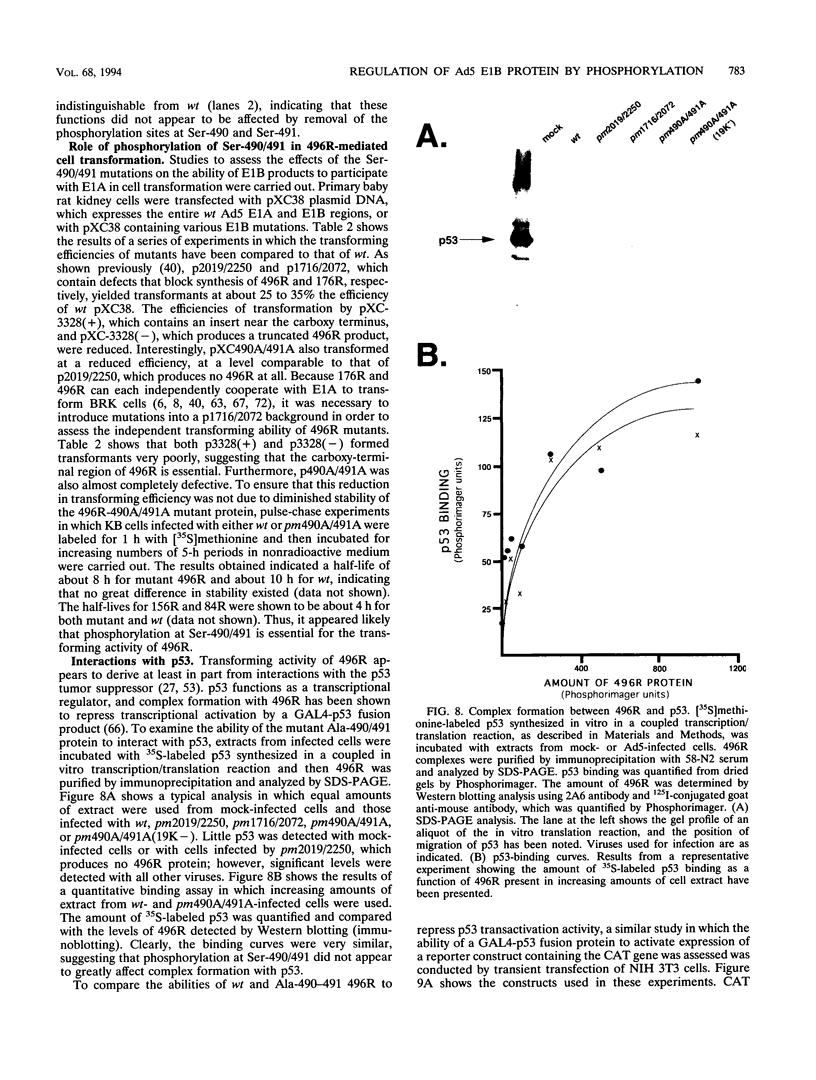
The 55-kDa product of early region 1B (E1B) of human adenoviruses is required for viral replication and participates in cell transformation through complex formation with and inactivation of the cellular tumor suppressor p53. We have used both biochemical and genetic approaches to show that this 496-residue (496R) protein of adenovirus type 5 is phosphorylated at serine and threonine residues near the carboxy terminus within sequences characteristic of substrates of casein kinase II. Mutations which converted serines 490 and 491 to alanine residues decreased viral replication and greatly reduced the efficiency of transformation of primary baby rat kidney cells. Such mutant 496R proteins interacted with p53 at efficiencies similar to those of wild-type 496R but only partially inhibited p53 transactivation activity. These results indicated that phosphorylation at these carboxy-terminal sites either regulates the inhibition of p53 or regulates some other 496R function required for cell transformation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Miceli M. C., Parnes J. R., Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991 Mar 7;350(6313):62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Schmitt R. C., Smart J. E., Lewis J. B. Early region 1B of adenovirus 2 encodes two coterminal proteins of 495 and 155 amino acid residues. J Virol. 1984 May;50(2):387–396. doi: 10.1128/jvi.50.2.387-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. D., Berk A. J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987 Jan;156(1):107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- Bautista D. S., Graham F. L. Insertional mutagenesis using a synthetic lac operator. Gene. 1989 Oct 30;82(2):201–208. doi: 10.1016/0378-1119(89)90045-0. [DOI] [PubMed] [Google Scholar]
- Bernards R., de Leeuw M. G., Houweling A., van der Eb A. J. Role of the adenovirus early region 1B tumor antigens in transformation and lytic infection. Virology. 1986 Apr 15;150(1):126–139. doi: 10.1016/0042-6822(86)90272-2. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Breiding D. E., Edbauer C. A., Tong J. Y., Byrd P., Grand R. J., Gallimore P. H., Williams J. Isolation and characterization of adenovirus type 12 E1 host-range mutants defective for growth in nontransformed human cells. Virology. 1988 Jun;164(2):390–402. doi: 10.1016/0042-6822(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Bridge E., Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990 Feb;174(2):345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993 Apr;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Branton P. E. Phosphorylation of adenovirus E1A proteins by the p34cdc2 protein kinase. Virology. 1992 Jul;189(1):111–120. doi: 10.1016/0042-6822(92)90686-j. [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Tremblay M. L., Branton P. E. Phosphorylation at serine 89 induces a shift in gel mobility but has little effect on the function of adenovirus type 5 E1A proteins. J Virol. 1989 Feb;63(2):987–991. doi: 10.1128/jvi.63.2.987-991.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Ginsberg D., Mechta F., Yaniv M., Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Cartas M. A., Matsuo T. Identification and purification of a protein encoded by the human adenovirus type 2 transforming region. J Virol. 1982 Apr;42(1):30–41. doi: 10.1128/jvi.42.1.30-41.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kao C. C., Yew P. R., Berk A. J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55K proteins. Virology. 1990 Dec;179(2):806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Leppard K. N., Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989 Aug;8(8):2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W. Identification of adenovirus type 2 early region 1B proteins that share the same amino terminus as do the 495R and 155R proteins. J Virol. 1987 Dec;61(12):3879–3888. doi: 10.1128/jvi.61.12.3879-3888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Brackmann K. H., Symington J. S., Green M. Antibody directed to a synthetic peptide encoding the NH2-terminal 16 amino acids of the adenovirus type 2 E1B-53K tumor antigen recognizes the E1B-20K tumor antigen. Virology. 1984 Jan 15;132(1):217–221. doi: 10.1016/0042-6822(84)90106-5. [DOI] [PubMed] [Google Scholar]
- Mak I., Mak S., Benchimol S. Expression of the cellular p53 protein in cells transformed by adenovirus 12 and viral DNA fragments. Virology. 1988 Mar;163(1):201–204. doi: 10.1016/0042-6822(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Mak I., Mak S. Separate regions of an adenovirus E1B protein critical for different biological functions. Virology. 1990 Jun;176(2):553–562. doi: 10.1016/0042-6822(90)90026-n. [DOI] [PubMed] [Google Scholar]
- Malette P., Yee S. P., Branton P. E. Studies on the phosphorylation of the 58000 dalton early region 1B protein of human adenovirus type 5. J Gen Virol. 1983 May;64(Pt 5):1069–1078. doi: 10.1099/0022-1317-64-5-1069. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Samuels M., Sharp P. A. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- McGlade C. J., Tremblay M. L., Yee S. P., Ross R., Branton P. E. Acylation of the 176R (19-kilodalton) early region 1B protein of human adenovirus type 5. J Virol. 1987 Oct;61(10):3227–3234. doi: 10.1128/jvi.61.10.3227-3234.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- McLorie W., McGlade C. J., Takayesu D., Branton P. E. Individual adenovirus E1B proteins induce transformation independently but by additive pathways. J Gen Virol. 1991 Jun;72(Pt 6):1467–1471. doi: 10.1099/0022-1317-72-6-1467. [DOI] [PubMed] [Google Scholar]
- O'Rourke R. W., Miller C. W., Kato G. J., Simon K. J., Chen D. L., Dang C. V., Koeffler H. P. A potential transcriptional activation element in the p53 protein. Oncogene. 1990 Dec;5(12):1829–1832. [PubMed] [Google Scholar]
- Oliner J. D., Pietenpol J. A., Thiagalingam S., Gyuris J., Kinzler K. W., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993 Apr 29;362(6423):857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Münger K., Yee C. L., Barnes J. A., Howley P. M. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992 Apr;66(4):2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Moore M., Logan J., Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986 Feb;6(2):470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöpperl H., Featherstone M. S. An autoregulatory element of the murine Hox-4.2 gene. EMBO J. 1992 Oct;11(10):3673–3680. doi: 10.1002/j.1460-2075.1992.tb05452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L., Branton P. E. Intracellular localization of adenovirus type 5 tumor antigens in productively infected cells. Virology. 1983 Sep;129(2):456–468. doi: 10.1016/0042-6822(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Sandler A. B., Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989 Feb;63(2):624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam U., Ray A., Sehgal P. B. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Sullivan C. A., Levine A. J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982 Jul 30;120(2):510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiio Y., Yamamoto T., Yamaguchi N. Negative regulation of Rb expression by the p53 gene product. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5206–5210. doi: 10.1073/pnas.89.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Ohshima K., Fukui Y., Ariga H. The adenovirus type 12 early-region 1B 58,000-Mr gene product is required for viral DNA synthesis and for initiation of cell transformation. J Virol. 1986 Mar;57(3):792–801. doi: 10.1128/jvi.57.3.792-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey A., Almond N., Osborn K., Crawford L. Mutations of the human papillomavirus type 16 E7 gene that affect transformation, transactivation and phosphorylation by the E7 protein. J Gen Virol. 1990 Apr;71(Pt 4):965–970. doi: 10.1099/0022-1317-71-4-965. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., Dumont D. J., Branton P. E. Analysis of phosphorylation sites in the exon 1 region of E1A proteins of human adenovirus type 5. Virology. 1989 Apr;169(2):397–407. doi: 10.1016/0042-6822(89)90165-7. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., McGlade C. J., Gerber G. E., Branton P. E. Identification of the phosphorylation sites in early region 1A proteins of adenovirus type 5 by amino acid sequencing of peptide fragments. J Biol Chem. 1988 May 5;263(13):6375–6383. [PubMed] [Google Scholar]
- Virtanen A., Pettersson U. Organization of early region 1B of human adenovirus type 2: identification of four differentially spliced mRNAs. J Virol. 1985 May;54(2):383–391. doi: 10.1128/jvi.54.2.383-391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R. Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol Cell Biol. 1990 Jan;10(1):120–130. doi: 10.1128/mcb.10.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R., Sabbatini P., Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991 Jun;65(6):2968–2978. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Rowe D. T., Tremblay M. L., McDermott M., Branton P. E. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. doi: 10.1128/jvi.46.3.1003-1013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew P. R., Berk A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992 May 7;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- Yew P. R., Kao C. C., Berk A. J. Dissection of functional domains in the adenovirus 2 early 1B 55K polypeptide by suppressor-linker insertional mutagenesis. Virology. 1990 Dec;179(2):795–805. doi: 10.1016/0042-6822(90)90147-j. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Bargonetti J., Walker K., Prives C., Levine A. J. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 1992 Jul;6(7):1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]
- Zantema A., Fransen J. A., Davis-Olivier A., Ramaekers F. C., Vooijs G. P., DeLeys B., Van der Eb A. J. Localization of the E1B proteins of adenovirus 5 in transformed cells, as revealed by interaction with monoclonal antibodies. Virology. 1985 Apr 15;142(1):44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]
- Zantema A., Schrier P. I., Davis-Olivier A., van Laar T., Vaessen R. T., van der EB A. J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985 Nov;5(11):3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Mak S., Branton P. E. Adenovirus type 12 early region 1B proteins and metabolism of early viral mRNAs. Virology. 1992 Dec;191(2):793–802. doi: 10.1016/0042-6822(92)90255-n. [DOI] [PubMed] [Google Scholar]
- Zhang S., Mak S., Branton P. E. Overexpression of the E1B 55-kilodalton (482R) protein of human adenovirus type 12 appears to permit efficient transformation of primary baby rat kidney cells in the absence of the E1B 19-kilodalton protein. J Virol. 1992 Apr;66(4):2302–2309. doi: 10.1128/jvi.66.4.2302-2309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]