Abstract
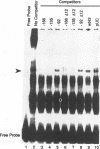
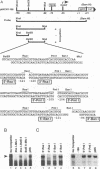
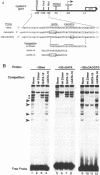
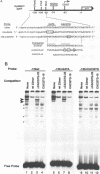
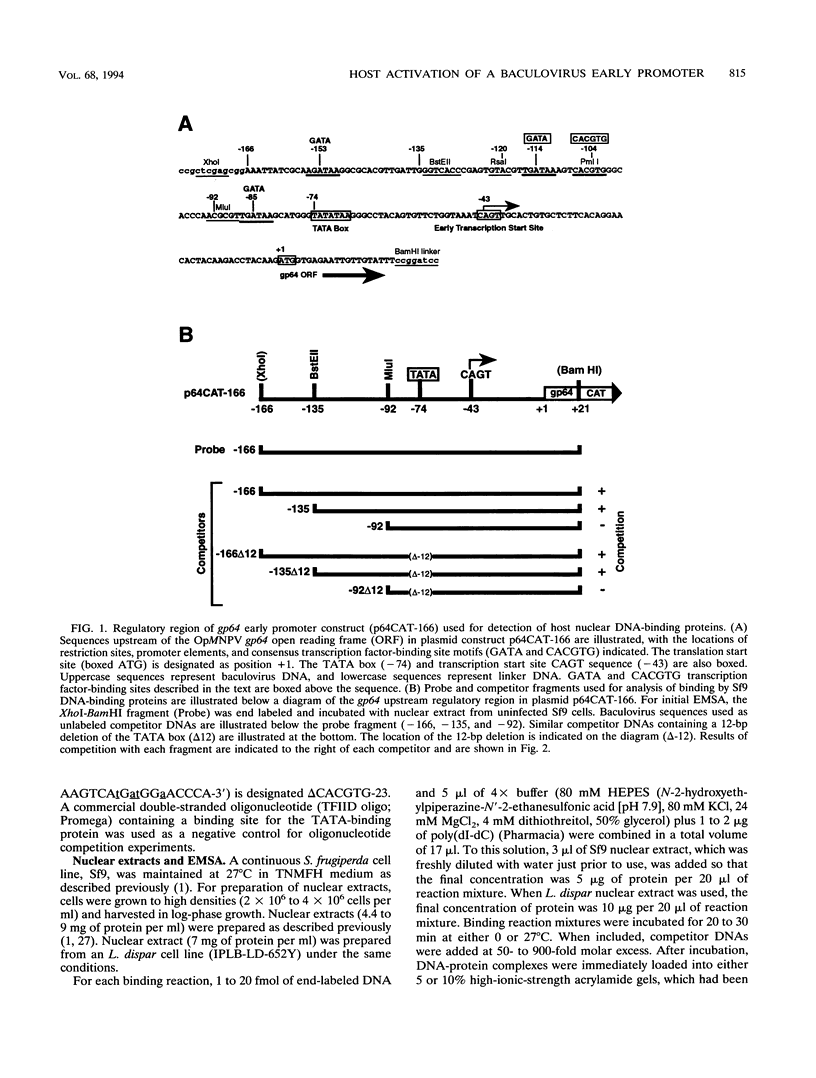
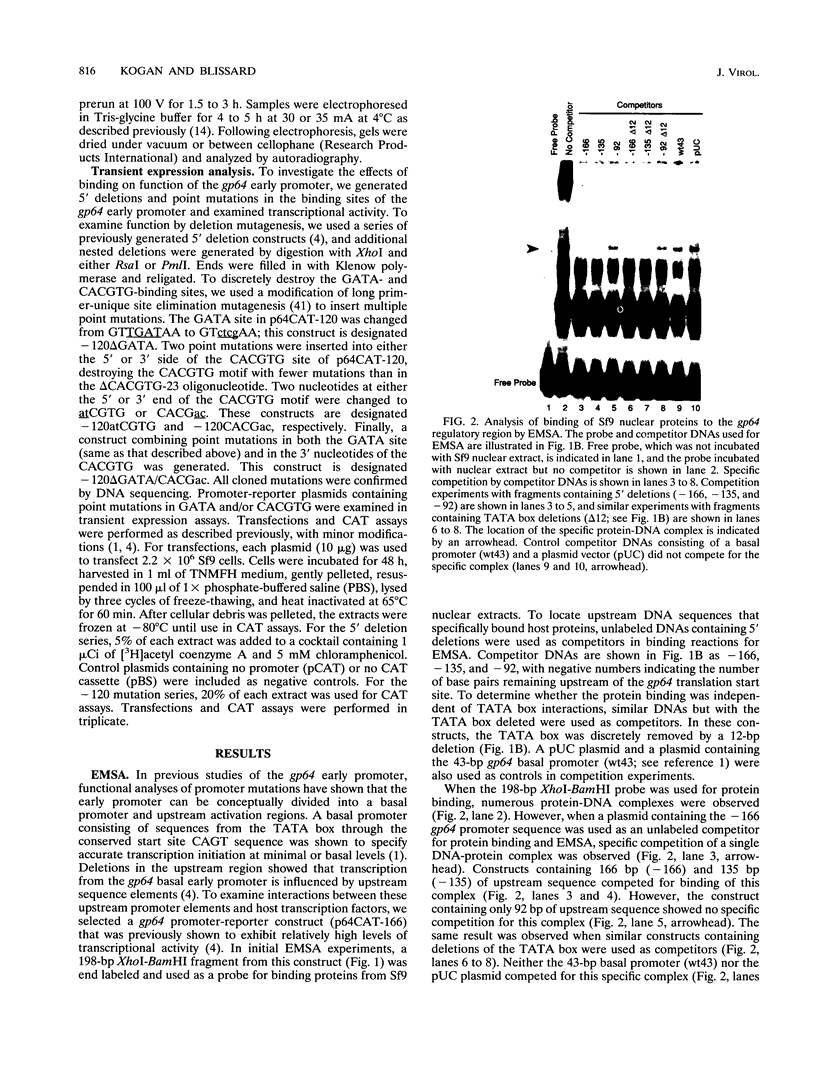
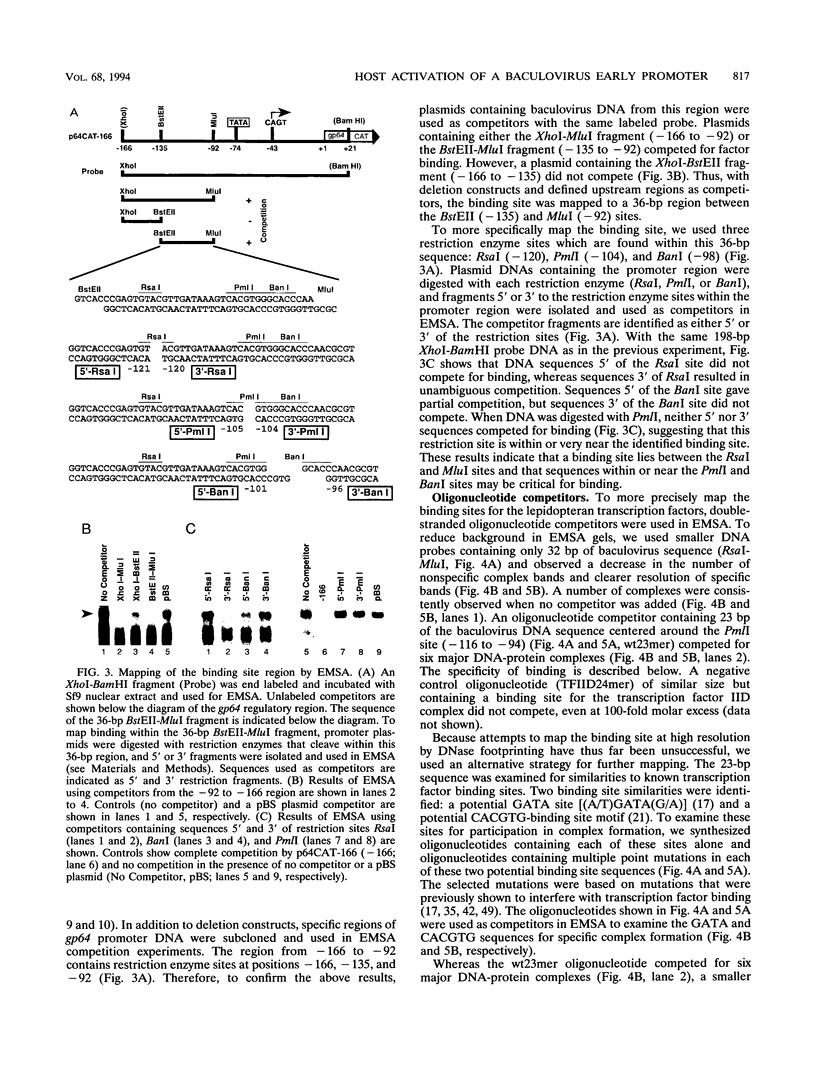
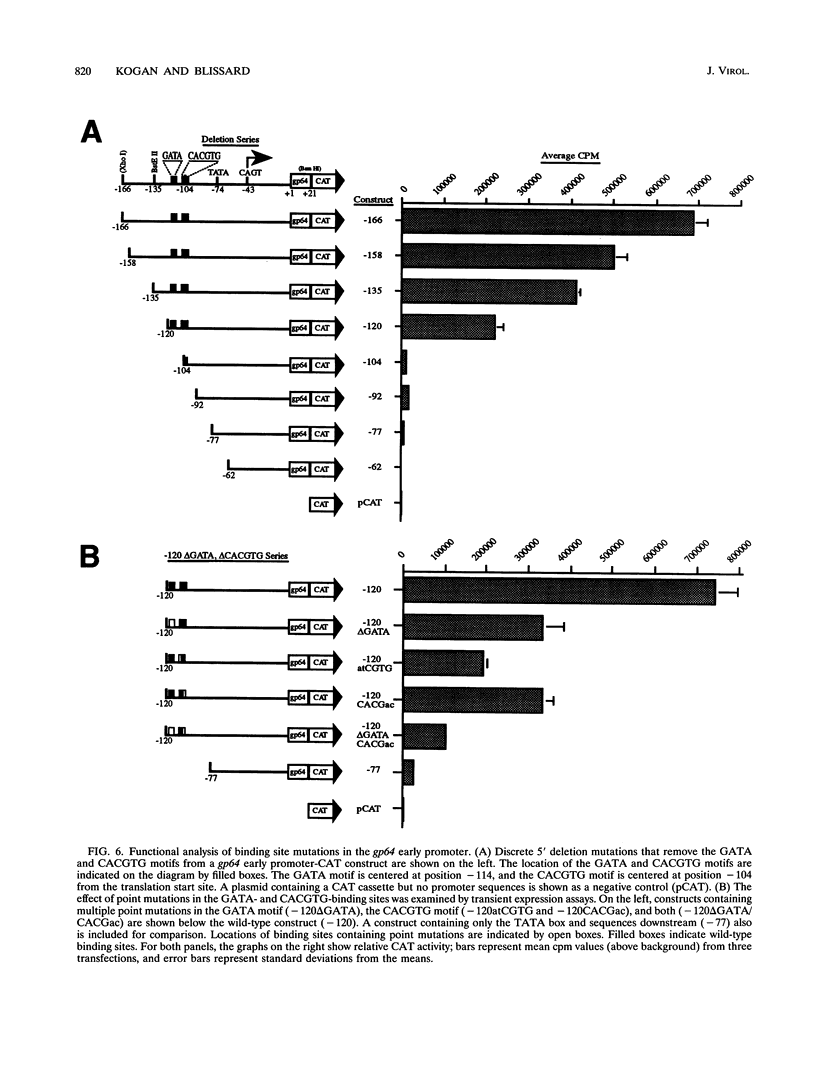
The early promoter of the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus gp64 gene is active when transfected into several insect cell lines and does not require viral gene products for transcription in uninfected cells. Because previous studies have shown that the gp64 early promoter is activated above basal levels in uninfected cells, host transcription factors are likely to play a role in gp64 activation at early times postinfection. By using nuclear extracts from uninfected Sf9 cells for electrophoretic mobility shift analysis of gp64 regulatory regions, host nuclear proteins were shown to bind specifically to the upstream regulatory region of the gp64 early promoter. Host factor binding was mapped to a 24-bp sequence centered approximately 35 bp upstream of the TATA box. Two consensus eukaryotic transcription factor-binding site motifs, GATA and CACGTG, were identified within the 24-bp sequence. Competition assays using oligonucleotides containing either a GATA or a CACGTG motif and similar oligonucleotides with point mutations in these sites showed that each site is required for binding host transcription factors. To investigate the functional significance of host factor binding to GATA and CACGTG motifs, constructs containing point mutations in these motifs were examined in transient expression assays. Mutations in either or both GATA and CACGTG sites decreased reporter activity in transient expression assays, suggesting that binding of host transcription factors to these motifs is important in transcriptional regulation of the gp64 early promoter.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blissard G. W., Kogan P. H., Wei R., Rohrmann G. F. A synthetic early promoter from a baculovirus: roles of the TATA box and conserved start site CAGT sequence in basal levels of transcription. Virology. 1992 Oct;190(2):783–793. doi: 10.1016/0042-6822(92)90916-d. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Quant-Russell R. L., Rohrmann G. F., Beaudreau G. S. Nucleotide sequence, transcriptional mapping, and temporal expression of the gene encoding p39, a major structural protein of the multicapsid nuclear polyhedrosis virus of Orgyia pseudotsugata. Virology. 1989 Feb;168(2):354–362. doi: 10.1016/0042-6822(89)90276-6. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Rohrmann G. F. Baculovirus gp64 gene expression: analysis of sequences modulating early transcription and transactivation by IE1. J Virol. 1991 Nov;65(11):5820–5827. doi: 10.1128/jvi.65.11.5820-5827.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard G. W., Rohrmann G. F. Location, sequence, transcriptional mapping, and temporal expression of the gp64 envelope glycoprotein gene of the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology. 1989 Jun;170(2):537–555. doi: 10.1016/0042-6822(89)90445-5. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Wenz J. R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992 Nov;66(11):6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. B., Blissard G. W., Rohrmann G. F. Characterization of the infection cycle of the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus in Lymantria dispar cells. J Gen Virol. 1990 Dec;71(Pt 12):2841–2846. doi: 10.1099/0022-1317-71-12-2841. [DOI] [PubMed] [Google Scholar]
- Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol. 1990 Apr 20;212(4):563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Guarino L. A., Summers M. D. Functional mapping of an AcNPV immediately early gene which augments expression of the IE-1 trans-activated 39K gene. Virology. 1988 Feb;162(2):444–451. doi: 10.1016/0042-6822(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Summers M. D., Guarino L. A. Molecular analysis of a baculovirus regulatory gene. Virology. 1991 May;182(1):279–286. doi: 10.1016/0042-6822(91)90671-w. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Summers M. D., Guarino L. A. Transient expression of the Autographa californica nuclear polyhedrosis virus immediate-early gene, IE-N, is regulated by three viral elements. J Virol. 1991 Feb;65(2):945–951. doi: 10.1128/jvi.65.2.945-951.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Cherbas L., Cherbas P. The arthropod initiator: the capsite consensus plays an important role in transcription. Insect Biochem Mol Biol. 1993 Jan;23(1):81–90. doi: 10.1016/0965-1748(93)90085-7. [DOI] [PubMed] [Google Scholar]
- Chisholm G. E., Henner D. J. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE-1 gene. J Virol. 1988 Sep;62(9):3193–3200. doi: 10.1128/jvi.62.9.3193-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Dolde C., Gillison M. L., Kato G. J. Discrimination between related DNA sites by a single amino acid residue of Myc-related basic-helix-loop-helix proteins. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):599–602. doi: 10.1073/pnas.89.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson J. A., Friesen P. D. Identification of upstream promoter elements mediating early transcription from the 35,000-molecular-weight protein gene of Autographa californica nuclear polyhedrosis virus. J Virol. 1991 Aug;65(8):4006–4016. doi: 10.1128/jvi.65.8.4006-4016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher F., Goding C. R. Single amino acid substitutions alter helix-loop-helix protein specificity for bases flanking the core CANNTG motif. EMBO J. 1992 Nov;11(11):4103–4109. doi: 10.1002/j.1460-2075.1992.tb05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Miller L. K. Temporal regulation of baculovirus RNA: overlapping early and late transcripts. J Virol. 1985 May;54(2):392–400. doi: 10.1128/jvi.54.2.392-400.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs L. Y., Woods M. S., Weaver R. F. Viral Transcription During Autographa californica Nuclear Polyhedrosis Virus Infection: a Novel RNA Polymerase Induced in Infected Spodoptera frugiperda Cells. J Virol. 1983 Dec;48(3):641–646. doi: 10.1128/jvi.48.3.641-646.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacca M., Gutierrez M. I., Menzo S., d'Adda di Fagagna F., Falaschi A. A human binding site for transcription factor USF/MLTF mimics the negative regulatory element of human immunodeficiency virus type 1. Virology. 1992 Jan;186(1):133–147. doi: 10.1016/0042-6822(92)90067-y. [DOI] [PubMed] [Google Scholar]
- Grula M. A., Buller P. L., Weaver R. F. alpha-Amanitin-Resistant Viral RNA Synthesis in Nuclei Isolated from Nuclear Polyhedrosis Virus-Infected Heliothis zea Larvae and Spodoptera frugiperda Cells. J Virol. 1981 Jun;38(3):916–921. doi: 10.1128/jvi.38.3.916-921.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Gonzalez M. A., Summers M. D. Complete Sequence and Enhancer Function of the Homologous DNA Regions of Autographa californica Nuclear Polyhedrosis Virus. J Virol. 1986 Oct;60(1):224–229. doi: 10.1128/jvi.60.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Smith M. Regulation of delayed-early gene transcription by dual TATA boxes. J Virol. 1992 Jun;66(6):3733–3739. doi: 10.1128/jvi.66.6.3733-3739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J Virol. 1986 Feb;57(2):563–571. doi: 10.1128/jvi.57.2.563-571.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J Virol. 1987 Jul;61(7):2091–2099. doi: 10.1128/jvi.61.7.2091-2099.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes R. R., Jr, Rohrmann G. F. In vitro transcription of baculovirus immediate early genes: accurate mRNA initiation by nuclear extracts from both insect and human cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4513–4517. doi: 10.1073/pnas.88.10.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh N. E., Weaver R. F. Identifying the RNA polymerases that synthesize specific transcripts of the Autographa californica nuclear polyhedrosis virus. J Gen Virol. 1990 Jan;71(Pt 1):195–201. doi: 10.1099/0022-1317-71-1-195. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Klucher K. M., Spector D. H. The human cytomegalovirus 2.7-kilobase RNA promoter contains a functional binding site for the adenovirus major late transcription factor. J Virol. 1990 Sep;64(9):4189–4198. doi: 10.1128/jvi.64.9.4189-4198.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G. R., Choi J., Guarino L. A., Summers M. D. Functional dissection of the Autographa californica nuclear polyhedrosis virus immediate-early 1 transcriptional regulatory protein. J Virol. 1992 Dec;66(12):7429–7437. doi: 10.1128/jvi.66.12.7429-7437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G. R., Guarino L. A., Summers M. D. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J Virol. 1991 Oct;65(10):5281–5288. doi: 10.1128/jvi.65.10.5281-5288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappa R., Behn-Krappa A., Jahnel F., Doerfler W., Knebel-Mörsdorf D. Differential factor binding at the promoter of early baculovirus gene PE38 during viral infection: GATA motif is recognized by an insect protein. J Virol. 1992 Jun;66(6):3494–3503. doi: 10.1128/jvi.66.6.3494-3503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappa R., Knebel-Mörsdorf D. Identification of the very early transcribed baculovirus gene PE-38. J Virol. 1991 Feb;65(2):805–812. doi: 10.1128/jvi.65.2.805-812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. F., Concino M. F., Weinmann R. Genetic profile of the transcriptional signals from the adenovirus major late promoter. Virology. 1988 Jul;165(1):51–56. doi: 10.1016/0042-6822(88)90657-5. [DOI] [PubMed] [Google Scholar]
- Leikina E., Onaran H. O., Zimmerberg J. Acidic pH induces fusion of cells infected with baculovirus to form syncytia. FEBS Lett. 1992 Jun 15;304(2-3):221–224. doi: 10.1016/0014-5793(92)80623-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen M. S., Friesen P. D. Molecular analysis of the transcriptional regulatory region of an early baculovirus gene. J Virol. 1989 Feb;63(2):493–503. doi: 10.1128/jvi.63.2.493-503.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omichinski J. G., Clore G. M., Schaad O., Felsenfeld G., Trainor C., Appella E., Stahl S. J., Gronenborn A. M. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993 Jul 23;261(5120):438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992 Aug 1;80(3):575–581. [PubMed] [Google Scholar]
- Passarelli A. L., Miller L. K. Three baculovirus genes involved in late and very late gene expression: ie-1, ie-n, and lef-2. J Virol. 1993 Apr;67(4):2149–2158. doi: 10.1128/jvi.67.4.2149-2158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray F. A., Nickoloff J. A. Site-specific mutagenesis of almost any plasmid using a PCR-based version of unique site elimination. Biotechniques. 1992 Sep;13(3):342–348. [PubMed] [Google Scholar]
- Reach M., Babiss L. E., Young C. S. The upstream factor-binding site is not essential for activation of transcription from the adenovirus major late promoter. J Virol. 1990 Dec;64(12):5851–5860. doi: 10.1128/jvi.64.12.5851-5860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. C., Miller L. K. Baculovirus transcription in the presence of inhibitors and in nonpermissive Drosophila cells. Virus Res. 1986 Nov;6(2):155–172. doi: 10.1016/0168-1702(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Rohel D. Z., Faulkner P. Time Course Analysis and Mapping of Autographa californica Nuclear Polyhedrosis Virus Transcripts. J Virol. 1984 Jun;50(3):739–747. doi: 10.1128/jvi.50.3.739-747.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G. F. Baculovirus structural proteins. J Gen Virol. 1992 Apr;73(Pt 4):749–761. doi: 10.1099/0022-1317-73-4-749. [DOI] [PubMed] [Google Scholar]
- Sawadogo M. Multiple forms of the human gene-specific transcription factor USF. II. DNA binding properties and transcriptional activity of the purified HeLa USF. J Biol Chem. 1988 Aug 25;263(24):11994–12001. [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W., Gregor P. D., Roeder R. G. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from HeLa cell nuclei. J Biol Chem. 1988 Aug 25;263(24):11985–11993. [PubMed] [Google Scholar]
- Skeiky Y. A., Iatrou K. Synergistic interactions of silkmoth chorion promoter-binding factors. Mol Cell Biol. 1991 Apr;11(4):1954–1964. doi: 10.1128/mcb.11.4.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilmann D. A., Stewart S. Identification and characterization of the IE-1 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology. 1991 Feb;180(2):492–508. doi: 10.1016/0042-6822(91)90063-h. [DOI] [PubMed] [Google Scholar]
- Theilmann D. A., Stewart S. Molecular analysis of the trans-activating IE-2 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology. 1992 Mar;187(1):84–96. doi: 10.1016/0042-6822(92)90297-3. [DOI] [PubMed] [Google Scholar]
- Theilmann D. A., Stewart S. Tandemly repeated sequence at the 3' end of the IE-2 gene of the baculovirus Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus is an enhancer element. Virology. 1992 Mar;187(1):97–106. doi: 10.1016/0042-6822(92)90298-4. [DOI] [PubMed] [Google Scholar]
- Volkman L. E. The 64K envelope protein of budded Autographa californica nuclear polyhedrosis virus. Curr Top Microbiol Immunol. 1986;131:103–118. doi: 10.1007/978-3-642-71589-1_6. [DOI] [PubMed] [Google Scholar]
- Whitford M., Stewart S., Kuzio J., Faulkner P. Identification and sequence analysis of a gene encoding gp67, an abundant envelope glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1989 Mar;63(3):1393–1399. doi: 10.1128/jvi.63.3.1393-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]