Abstract
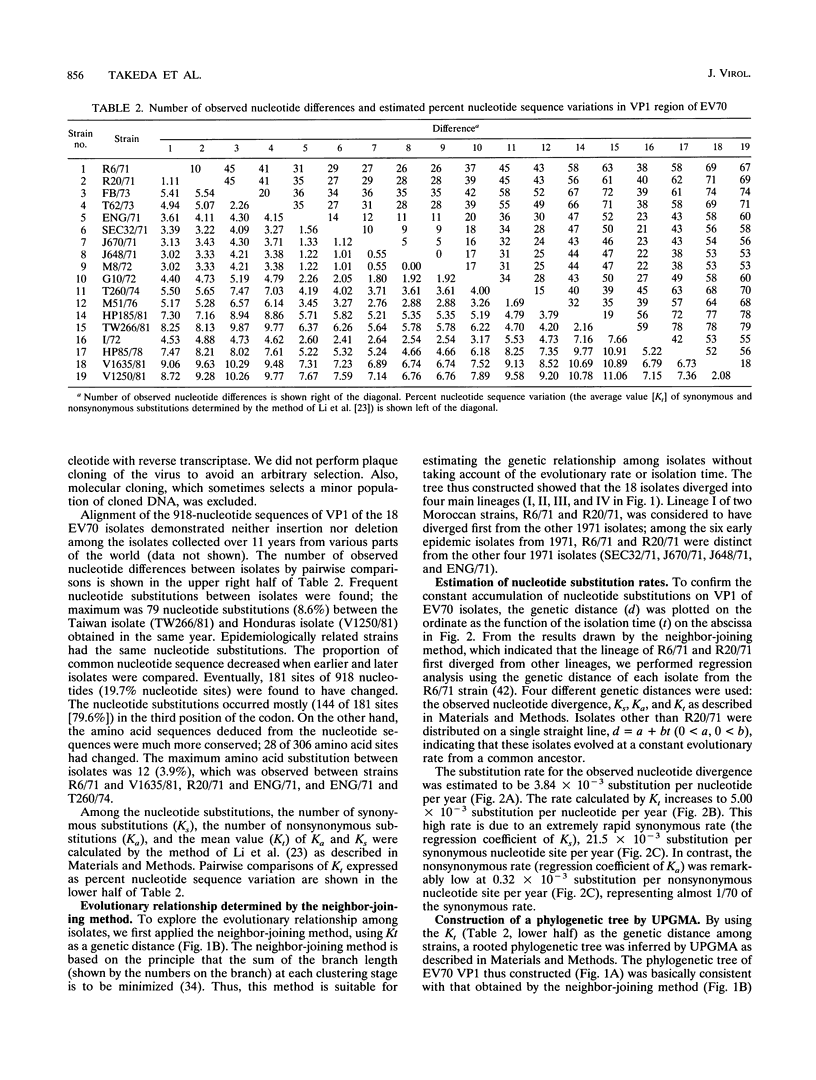
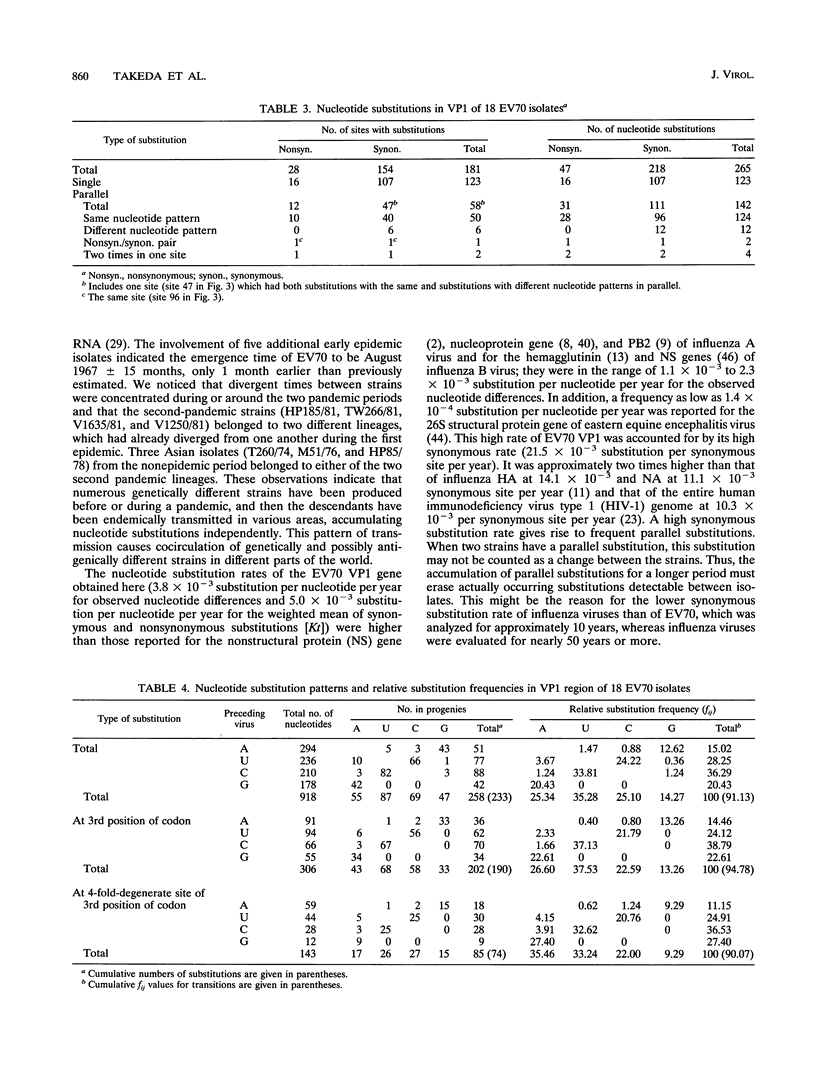
Nucleotide sequences of the genome RNA encoding capsid protein VP1 (918 nucleotides) of 18 enterovirus 70 (EV70) isolates collected from various parts of the world in 1971 to 1981 were determined, and nucleotide substitutions among them were studied. The genetic distances between isolates were calculated by the pairwise comparison of nucleotide difference. Regression analysis of the genetic distances against time of isolation of the strains showed that the synonymous substitution rate was very high at 21.53 x 10(-3) substitution per nucleotide per year, while the nonsynonymous rate was extremely low at 0.32 x 10(-3) substitution per nucleotide per year. The rate estimated by the average value of synonymous and nonsynonymous substitutions (W.-H. Li, C.-C. Wu, and C.-C. Luo, Mol. Biol. Evol. 2:150-174, 1985) was 5.00 x 10(-3) substitution per nucleotide per year. Taking the average value of synonymous and nonsynonymous substitutions as genetic distances between isolates, the phylogenetic tree was inferred by the unweighted pairwise grouping method of arithmetic average and by the neighbor-joining method. The tree indicated that the virus had evolved from one focal place, and the time of emergence was estimated to be August 1967 +/- 15 months, 2 years before first recognition of the pandemic of acute hemorrhagic conjunctivitis. By superimposing every nucleotide substitution on the branches of the phylogenetic tree, we analyzed nucleotide substitution patterns of EV70 genome RNA. In synonymous substitutions, the proportion of transitions, i.e., C<==>U and G<==>A, was found to be extremely frequent in comparison with that reported on other viruses or pseudogenes. In addition, parallel substitutions (independent substitutions at the same nucleotide position on different branches, i.e., different isolates, of the tree) were frequently found in both synonymous and nonsynonymous substitutions. These frequent parallel substitutions and the low nonsynonymous substitution rate despite the very high synonymous substitution rate described above imply a strong restriction on nonsynonymous substitution sites of VP1, probably due to the requirement for maintaining the rigid icosahedral conformation of the virus.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Leibowitz J., Diamond D. C., Bonin J., Wimmer E. Recombinants of Mahoney and Sabin strain poliovirus type 1: analysis of in vitro phenotypic markers and evidence that resistance to guanidine maps in the nonstructural proteins. Virology. 1984 Aug;137(1):74–85. doi: 10.1016/0042-6822(84)90010-2. [DOI] [PubMed] [Google Scholar]
- Ghafoor A., Burney M. I., Iqbal J., Khan Z. Acute haemorrhagic conjunctivitis (AHC) epidemic of 1981. J Pak Med Assoc. 1984 Aug;34(8):245–246. [PubMed] [Google Scholar]
- Gojobori T., Li W. H., Graur D. Patterns of nucleotide substitution in pseudogenes and functional genes. J Mol Evol. 1982;18(5):360–369. doi: 10.1007/BF01733904. [DOI] [PubMed] [Google Scholar]
- Gorman O. T., Bean W. J., Kawaoka Y., Webster R. G. Evolution of the nucleoprotein gene of influenza A virus. J Virol. 1990 Apr;64(4):1487–1497. doi: 10.1128/jvi.64.4.1487-1497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman O. T., Donis R. O., Kawaoka Y., Webster R. G. Evolution of influenza A virus PB2 genes: implications for evolution of the ribonucleoprotein complex and origin of human influenza A virus. J Virol. 1990 Oct;64(10):4893–4902. doi: 10.1128/jvi.64.10.4893-4902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graur D. Pattern of nucleotide substitution and the extent of purifying selection in retroviruses. J Mol Evol. 1984;21(3):221–231. doi: 10.1007/BF02102356. [DOI] [PubMed] [Google Scholar]
- Hayashida H., Toh H., Kikuno R., Miyata T. Evolution of influenza virus genes. Mol Biol Evol. 1985 Jul;2(4):289–303. doi: 10.1093/oxfordjournals.molbev.a040352. [DOI] [PubMed] [Google Scholar]
- Jones B. R. Epidemic haemorrhagic conjunctivitis in London 1971: a conjunctival picornavirus infection. Trans Ophthalmol Soc U K. 1972;92:625–627. [PubMed] [Google Scholar]
- Kanegae Y., Sugita S., Endo A., Ishida M., Senya S., Osako K., Nerome K., Oya A. Evolutionary pattern of the hemagglutinin gene of influenza B viruses isolated in Japan: cocirculating lineages in the same epidemic season. J Virol. 1990 Jun;64(6):2860–2865. doi: 10.1128/jvi.64.6.2860-2865.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto H. Antigenic analysis of acute hemorrhagic conjunctivitis viruses (enterovirus type 70). Microbiol Immunol. 1979;23(9):859–866. doi: 10.1111/j.1348-0421.1979.tb02819.x. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Hierholzer J. C., Obijeski J. F. Oligonucleotide fingerprint analysis of enterovirus 70 isolates from the 1980 to 1981 pandemic of acute hemorrhagic conjunctivitis: evidence for a close genetic relationship among Asian and American strains. Infect Immun. 1983 Aug;41(2):631–635. doi: 10.1128/iai.41.2.631-635.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono R. Apollo 11 disease or acute hemorrhagic conjunctivitis: a pandemic of a new enterovirus infection of the eyes. Am J Epidemiol. 1975 May;101(5):383–390. doi: 10.1093/oxfordjournals.aje.a112106. [DOI] [PubMed] [Google Scholar]
- Kono R., Miyamura K., Tajiri E., Sasagawa A., Phuapradit P. Virological and serological studies of neurological complications of acute hemorrhagic conjunctivitis in Thailand. J Infect Dis. 1977 May;135(5):706–713. doi: 10.1093/infdis/135.5.706. [DOI] [PubMed] [Google Scholar]
- Kono R., Miyamura K., Yamazaki S., Sasagawa A., Kurahashi H., Tajiri E., Takeda N., Robin Y., Renaudet J., Ishii K. Seroepidemiologic studies of acute hemorrhagic conjunctivitis virus (enterovirus type 70) in West Africa. II. Studies with human sera collected in West African countries other than Ghana. Am J Epidemiol. 1981 Aug;114(2):274–283. doi: 10.1093/oxfordjournals.aje.a113192. [DOI] [PubMed] [Google Scholar]
- Kono R., Sasagawa A., Ishii K., Sugiura S., Ochi M. Pandemic of new type of conjunctivitis. Lancet. 1972 Jun 3;1(7762):1191–1194. doi: 10.1016/s0140-6736(72)90921-x. [DOI] [PubMed] [Google Scholar]
- Kono R., Sasagawa A., Miyamura K., Tajiri E. Serologic characterization and sero-epidemiologic studies on acute hemorrhagic conjunctivitis (AHC) virus. Am J Epidemiol. 1975 May;101(5):444–457. doi: 10.1093/oxfordjournals.aje.a112112. [DOI] [PubMed] [Google Scholar]
- Li W. H., Tanimura M., Sharp P. M. Rates and dates of divergence between AIDS virus nucleotide sequences. Mol Biol Evol. 1988 Jul;5(4):313–330. doi: 10.1093/oxfordjournals.molbev.a040503. [DOI] [PubMed] [Google Scholar]
- Li W. H., Wu C. I., Luo C. C. A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol. 1985 Mar;2(2):150–174. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- Li W. H., Wu C. I., Luo C. C. Nonrandomness of point mutation as reflected in nucleotide substitutions in pseudogenes and its evolutionary implications. J Mol Evol. 1984;21(1):58–71. doi: 10.1007/BF02100628. [DOI] [PubMed] [Google Scholar]
- Lin K. H., Takeda N., Miyamura K., Yamazaki S., Chen C. W. The nucleotide sequence of 3C proteinase region of the coxsackievirus A24 variant: comparison of the isolates in Taiwan in 1985-1988. Virus Genes. 1991 Apr;5(2):121–131. doi: 10.1007/BF00571927. [DOI] [PubMed] [Google Scholar]
- Martínez M. A., Dopazo J., Hernández J., Mateu M. G., Sobrino F., Domingo E., Knowles N. J. Evolution of the capsid protein genes of foot-and-mouth disease virus: antigenic variation without accumulation of amino acid substitutions over six decades. J Virol. 1992 Jun;66(6):3557–3565. doi: 10.1128/jvi.66.6.3557-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Tagaya I., von Magnus H. Enteroviruses 69, 70, and 71. Intervirology. 1974;4(6):369–370. doi: 10.1159/000149872. [DOI] [PubMed] [Google Scholar]
- Mirkovic R. R., Kono R., Yin-Murphy M., Sohier R., Schmidt N. J., Melnick J. L. Enterovirus type 70: the etiologic agent of pandemic acute haemorrhagic conjunctivitis. Bull World Health Organ. 1973;49(4):341–346. [PMC free article] [PubMed] [Google Scholar]
- Miyamura K., Takeda N., Tanimura M., Ogino T., Yamazaki S., Chen C. W., Lin K. H., Lin S. Y., Ghafoor A., Yin-Murphy M. Evolutionary study on the Coxsackievirus A 24 variant causing acute hemorrhagic conjunctivitis by oligonucleotide mapping analysis of RNA genome. Arch Virol. 1990;114(1-2):37–51. doi: 10.1007/BF01311010. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Tanimura M., Takeda N., Kono R., Yamazaki S. Evolution of enterovirus 70 in nature: all isolates were recently derived from a common ancestor. Arch Virol. 1986;89(1-4):1–14. doi: 10.1007/BF01309875. [DOI] [PubMed] [Google Scholar]
- Nejmi S., Gaudin O. G., Chomel J. J., Baaj A., Sohier R., Bosshard S. Isolation of a virus responsible for an outbreak of acute haemorrhagic conjunctivitis in Morocco. J Hyg (Lond) 1974 Apr;72(2):181–183. doi: 10.1017/s002217240002338x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. D., Jenkins O., Hughes P. J., Brown A., Knowles N. J., Booth D., Minor P. D., Almond J. W. The complete nucleotide sequence of enterovirus type 70: relationships with other members of the picornaviridae. J Gen Virol. 1990 Oct;71(Pt 10):2291–2299. doi: 10.1099/0022-1317-71-10-2291. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sakurai N., Nishioka H., Yoshikawa H., Shiomi T., Okada S., Niwa T. [Studies on acute hemorrhagic conjunctivitis (AHC) virus. I. Isolation of AHC virus from conjunctival scrapings, throat swabs and feces of patients (author's transl)]. Uirusu. 1975;25(4):237–241. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Okamoto T., Moriyama E. N., Takeuchi Y., Gojobori T., Hoshino H. Patterns of nucleotide substitutions and implications for the immunological diversity of human immunodeficiency virus. FEBS Lett. 1989 Jul 3;250(2):591–595. doi: 10.1016/0014-5793(89)80802-6. [DOI] [PubMed] [Google Scholar]
- Shu L. L., Bean W. J., Webster R. G. Analysis of the evolution and variation of the human influenza A virus nucleoprotein gene from 1933 to 1990. J Virol. 1993 May;67(5):2723–2729. doi: 10.1128/jvi.67.5.2723-2729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Rico-Hesse R., Scott T. W. Genetic diversity and slow rates of evolution in New World alphaviruses. Curr Top Microbiol Immunol. 1992;176:99–117. doi: 10.1007/978-3-642-77011-1_7. [DOI] [PubMed] [Google Scholar]
- Whitcher J. P., Schmidt N. J., Mabrouk R., Messadi M., Daghfous T., Hoshiwara I., Dawson C. R. Acute hemorrhagic conjunctivitis in Tunisia. Report of viral isolations. Arch Ophthalmol. 1976 Jan;94(1):51–55. doi: 10.1001/archopht.1976.03910030017006. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Krystal M., Fitch W. M., Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988 Mar;163(1):112–122. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]
- Yin-Murphy M. The picornaviruses of epidemic conjunctivitis. Southeast Asian J Trop Med Public Health. 1973 Mar;4(1):11–14. [PubMed] [Google Scholar]



