Abstract
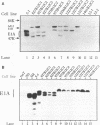
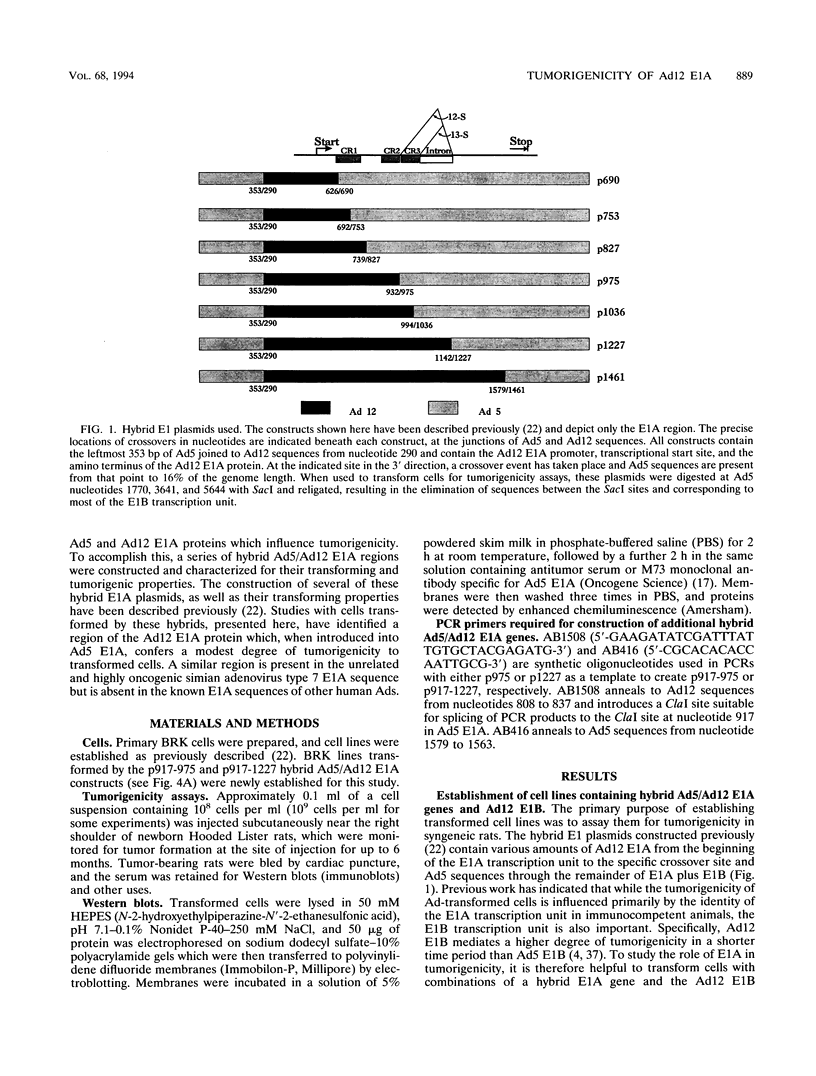
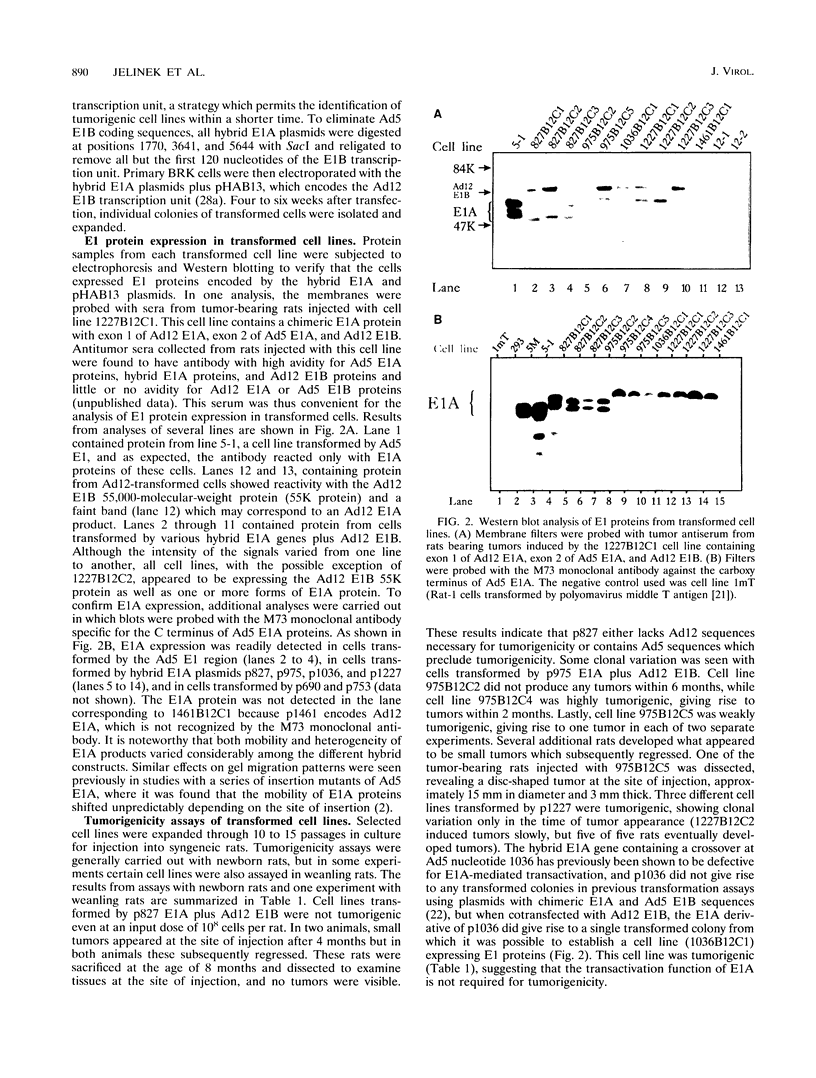
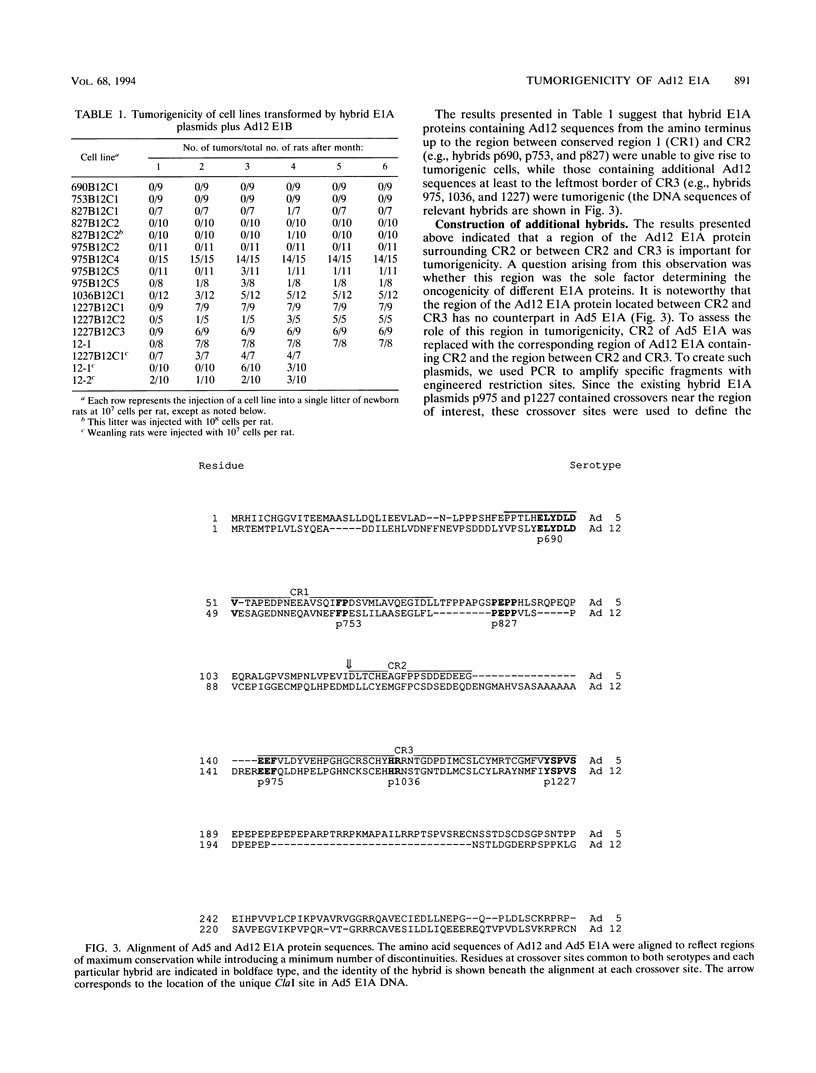
Chimeric adenovirus type 5 (Ad5)/Ad12 early region 1A (E1A) genes were used to transform primary baby rat kidney cells in cooperation with Ad12 E1B, and the resulting cell lines were assayed for tumorigenicity in syngeneic rats. It was found that lines were nontumorigenic when transformed by hybrid E1A genes consisting of the amino-terminal 80 amino acids from Ad12 including conserved region 1 (CR1), with the remaining portion from Ad5. In contrast, cell lines transformed by hybrids containing Ad12 E1A sequences from the amino terminus to the leftmost border of CR3 or beyond were tumorigenic. To extend these results, sequences spanning CR2 and CR3 of Ad5 E1A were replaced with the homologous regions of Ad12 E1A and additional transformed cell lines were established. These lines were weakly-to-moderately tumorigenic, suggesting that Ad12 E1A sequences between CR2 and CR3 may be involved in tumorigenicity but are not the sole factors influencing it. Interestingly, examination of an E1A sequence alignment indicated that the region between CR2 and CR3 of Ad12 E1A is also conserved in the corresponding sequence of simian adenovirus type 7, which, like Ad12, is highly oncogenic. This region is characterized by the presence of a stretch of several alanine residues and is similar to a motif present in a number of proteins with transcriptional repression activity. The possibility that this region may influence tumorigenicity by means of a transcriptional regulatory mechanism is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrill A. M., Blair G. E. Regulation of major histocompatibility class I gene expression at the level of transcription in highly oncogenic adenovirus transformed rat cells. Oncogene. 1988 Oct;3(4):483–487. [PubMed] [Google Scholar]
- Bautista D. S., Hitt M., McGrory J., Graham F. L. Isolation and characterization of insertion mutants in E1A of adenovirus type 5. Virology. 1991 Jun;182(2):578–596. doi: 10.1016/0042-6822(91)90599-7. [DOI] [PubMed] [Google Scholar]
- Bellgrau D., Walker T. A., Cook J. L. Recognition of adenovirus E1A gene products on immortalized cell surfaces by cytotoxic T lymphocytes. J Virol. 1988 May;62(5):1513–1519. doi: 10.1128/jvi.62.5.1513-1519.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Differential NK cell and macrophage killing of hamster cells infected with nononcogenic or oncogenic adenovirus. Science. 1984 May 11;224(4649):612–615. doi: 10.1126/science.6710160. [DOI] [PubMed] [Google Scholar]
- Cook J. L., May D. L., Lewis A. M., Jr, Walker T. A. Adenovirus E1A gene induction of susceptibility to lysis by natural killer cells and activated macrophages in infected rodent cells. J Virol. 1987 Nov;61(11):3510–3520. doi: 10.1128/jvi.61.11.3510-3520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., Walker T. A., Lewis A. M., Jr, Ruley H. E., Graham F. L., Pilder S. H. Expression of the adenovirus E1A oncogene during cell transformation is sufficient to induce susceptibility to lysis by host inflammatory cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6965–6969. doi: 10.1073/pnas.83.18.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Pfizenmaier K., Ricciardi R. P. Modulation of major histocompatibility complex (MHC) class I genes in adenovirus 12 transformed cells: interferon-gamma increases class I expression by a mechanism that circumvents E1A induced-repression and tumor necrosis factor enhances the effect of interferon-gamma. Oncogene. 1989 Jan;4(1):39–44. [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C., Bayley S. T., Branton P. E. Binding of the Rb1 protein to E1A products is required for adenovirus transformation. Oncogene. 1989 Mar;4(3):383–388. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. J., Ricciardi R. P. Adenovirus type 12 E1A gene represses accumulation of MHC class I mRNAs at the level of transcription. Virology. 1988 Jul;165(1):303–305. doi: 10.1016/0042-6822(88)90689-7. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Tumour production in immunosuppressed rats with cells transformed in vitro by adenovirus type 2. J Gen Virol. 1972 Jul;16(1):99–102. doi: 10.1099/0022-1317-16-1-99. [DOI] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Haddada H., Lewis A. M., Jr, Sogn J. A., Coligan J. E., Cook J. L., Walker T. A., Levine A. S. Tumorigenicity of hamster and mouse cells transformed by adenovirus types 2 and 5 is not influenced by the level of class I major histocompatibility antigens expressed on the cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9684–9688. doi: 10.1073/pnas.83.24.9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K. P., Overhauser J., Babiss L. E., Ginsberg H. S., Jones N. C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz D. R., Chinnadurai G. Evidence that a second tumor antigen coded by adenovirus early gene region E1a is required for efficient cell transformation. Proc Natl Acad Sci U S A. 1985 Jan;82(1):163–167. doi: 10.1073/pnas.82.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz D. R., Chinnadurai G. Immortalization of rat embryo fibroblasts by an adenovirus 2 mutant expressing a single functional E1a protein. J Virol. 1985 May;54(2):358–363. doi: 10.1128/jvi.54.2.358-363.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek M. A., Hassell J. A. Reversion of middle T antigen-transformed Rat-2 cells by Krev-1: implications for the role of p21c-ras in polyomavirus-mediated transformation. Oncogene. 1992 Sep;7(9):1687–1698. [PubMed] [Google Scholar]
- Jelinek T., Graham F. L. Recombinant human adenoviruses containing hybrid adenovirus type 5 (Ad5)/Ad12 E1A genes: characterization of hybrid E1A proteins and analysis of transforming activity and host range. J Virol. 1992 Jul;66(7):4117–4125. doi: 10.1128/jvi.66.7.4117-4125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen A. G., Bos J. L., van der Eb A. J. The first exon of region E1a genes of adenoviruses 5 and 12 encodes a separate functional protein domain. EMBO J. 1984 Dec 1;3(12):2923–2927. doi: 10.1002/j.1460-2075.1984.tb02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Kenyon D. J., Dougherty J., Raska K., Jr Tumorigenicity of adenovirus-transformed cells and their sensitivity to tumor necrosis factor alpha and NK/LAK cell cytolysis. Virology. 1991 Feb;180(2):818–821. doi: 10.1016/0042-6822(91)90099-w. [DOI] [PubMed] [Google Scholar]
- Lamberti C., Williams J. Differential requirement for adenovirus type 12 E1A gene products in oncogenic transformation. J Virol. 1990 Oct;64(10):4997–5007. doi: 10.1128/jvi.64.10.4997-5007.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht J. D., Grossel M. J., Figge J., Hansen U. M. Drosophila Krüppel protein is a transcriptional repressor. Nature. 1990 Jul 5;346(6279):76–79. doi: 10.1038/346076a0. [DOI] [PubMed] [Google Scholar]
- Mak I., Mak S. Transformation of rat cells by cyt mutants of adenovirus type 12 and mutants of adenovirus type 5. J Virol. 1983 Mar;45(3):1107–1117. doi: 10.1128/jvi.45.3.1107-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer I., Jochemsen A. G., de Wit C. M., Bos J. L., Morello D., van der Eb A. J. Adenovirus type 12 E1A down regulates expression of a transgene under control of a major histocompatibility complex class I promoter: evidence for transcriptional control. J Virol. 1989 Sep;63(9):4039–4042. doi: 10.1128/jvi.63.9.4039-4042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow G. H., Föhring B., Dougherty J., Gallimore P. H., Raska K., Jr Tumorigenicity of adenovirus-transformed rat cells and expression of class I major histocompatibility antigen. Virology. 1984 Apr 30;134(2):460–465. doi: 10.1016/0042-6822(84)90313-1. [DOI] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Moran E., Grodzicker T., Roberts R. J., Mathews M. B., Zerler B. Lytic and transforming functions of individual products of the adenovirus E1A gene. J Virol. 1986 Mar;57(3):765–775. doi: 10.1128/jvi.57.3.765-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska K., Jr, Gallimore P. H. An inverse relation of the oncogenic potential of adenovirus-transformed cells and their sensitivity to killing by syngeneic natural killer cells. Virology. 1982 Nov;123(1):8–18. doi: 10.1016/0042-6822(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Raska K., Jr, Morrongiello M. P., Föhring B. Adenovirus type-12 tumor antigen. III. Tumorigenicity and immune response to syngeneic rat cells transformed with virions and isolated transforming fragment of adenovirus 12 DNA. Int J Cancer. 1980 Jul 15;26(1):79–86. doi: 10.1002/ijc.2910260113. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Quade K., Gallimore P., Cedar H., Grosveld F. Increased MHC H-2K gene transcription in cultured mouse embryo cells after adenovirus infection. Nature. 1985 Jun 13;315(6020):579–581. doi: 10.1038/315579a0. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Föhring B., Shenk T. E., Raska K., Jr Tumorigenicity of adenovirus-transformed cells: region E1A of adenovirus 12 confers resistance to natural killer cells. Virology. 1985 Dec;147(2):413–421. doi: 10.1016/0042-6822(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Raska K., Jr, Shenk T. Adenovirus type 5 and adenovirus type 12 recombinant viruses containing heterologous E1 genes are viable, transform rat cells, but are not tumorigenic in rats. Virology. 1988 Sep;166(1):281–284. doi: 10.1016/0042-6822(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Urbanelli D., Raskova J., Shenk T. E., Raska K., Jr Adenovirus tumor-specific transplantation antigen is a function of the E1A early region. J Exp Med. 1986 Mar 1;163(3):563–572. doi: 10.1084/jem.163.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Shemesh J., Rotem-Yehudar R., Ehrlich R. Transcriptional and posttranscriptional regulation of class I major histocompatibility complex genes following transformation with human adenoviruses. J Virol. 1991 Oct;65(10):5544–5548. doi: 10.1128/jvi.65.10.5544-5548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soddu S., Lewis A. M., Jr Driving adenovirus type 12-transformed BALB/c mouse cells to express high levels of class I major histocompatibility complex proteins enhances, rather than abrogates, their tumorigenicity. J Virol. 1992 May;66(5):2875–2884. doi: 10.1128/jvi.66.5.2875-2884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling G. C., Williams J. Constructing chimeric type 12/type 5 adenovirus E1A genes and using them to identify an oncogenic determinant of adenovirus type 12. J Virol. 1994 Feb;68(2):877–887. doi: 10.1128/jvi.68.2.877-887.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada R., Eager K. B., Barbanti-Brodano G., Caputo A., Ricciardi R. P. Adenovirus type 12 early region 1A proteins repress class I HLA expression in transformed human cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5257–5261. doi: 10.1073/pnas.83.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. A., Wilson B. A., Lewis A. M., Jr, Cook J. L. E1A oncogene induction of cytolytic susceptibility eliminates sarcoma cell tumorigenicity. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6491–6495. doi: 10.1073/pnas.88.15.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. G., Rikitake Y., Carter M. C., Yaciuk P., Abraham S. E., Zerler B., Moran E. Identification of specific adenovirus E1A N-terminal residues critical to the binding of cellular proteins and to the control of cell growth. J Virol. 1993 Jan;67(1):476–488. doi: 10.1128/jvi.67.1.476-488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]