Abstract
Purpose
The aim of this study was to evaluate the effect of BAY 57-1293, a helicase-primase inhibitor, on herpes simplex virus type 1 (HSV-1) reactivation in mice and its efficacy on established disease in rabbits.
Methods
BALB/c mice latent for McKrae-strain HSV-1 were reactivated via heat stress, treated with BAY 57-1293, and their corneas were swabbed for virus or the trigeminal ganglia (TG) obtained for quantification of viral DNA. New Zealand white rabbits were infected and treated topically or orally in comparison with trifluridine or valacyclovir.
Results
Oral BAY 57-1293 suppressed reactivation in HSV-1-infected mice and reduced the viral load in TG up to four orders of magnitude. In the rabbits, the therapeutic efficacies of topical BAY 57-1293 and trifluridine were similar. Once-daily oral BAY 57-1293 was significantly more effective than valacyclovir and as effective as twice a day topical trifluridine.
Conclusions
BAY 57-1293 may be more effective than valacyclovir, without the cytotoxicity or potential healing retardation seen with trifluridine. Oral BAY 57-1293 may be a substitute for eye drops as an effective treatment for herpetic keratitis and might be useful in treating stromal keratitis and iritis, as well as preventing recurrences of ocular herpes.
INTRODUCTION
Kleymann and others described the in vivo activity of a new class of potent antiviral compounds that inhibit particular steps in HSV-1 DNA viral replication, the helicase-primase inhibitors.1-3 These compounds differ significantly from the antiviral compounds in common use in terms of their mechanism of action.4,5 For example, acyclovir and its l-valyl ester valacyclovir, as well as penciclovir and its prodrug famciclovir, inhibit HSV-1 DNA polymerase and terminate synthesis or elongation of the sugar backbone of viral DNA, preventing its long-strand synthesis.4-7 They, as well as trifluridine, require phosphorylation by the infected cell; therefore, their antiviral activity cannot take place until the infection has progressed to the point where specific viral thymidine kinase is synthesized. By contrast, the helicase-primase inhibitors act by preventing the unwinding of the double-stranded DNA and the initiation of the new strand synthesis that is necessary for virus production, and thus do not require processing by the target cell to become active.1
Kleymann et al. found that one of these compounds, BAY 57-1293, was particularly potent1-3 and more effective than valacyclovir, and was not associated with significant systemic toxicity.1 They reported that BAY 57-1293 was effective when given orally in a variety of mouse,1,2 rat,2 and guinea pig herpes models,1,3 and that, when given approximately 6 h postinjection, it was also effective topically in mice.2 Because of the promise of this compound, we felt it was important to confirm and extend these observations and to study the efficacy of BAY 57-1293 in animal models of HSV-1 disease.
The rabbit model of herpetic keratitis is a good predictor of the effect of antiviral drugs on the treatment of human disease.8 BAY 57-1293 is difficult to solubilize (solubility ∼2.7 mg/L in neutral phosphate-buffered saline, pH 7.2-7.4); therefore, we compared various topical preparations of BAY 57-1293 with trifluridine in the rabbit model, beginning treatment 3 days postinfection (PI) when the disease was well established. Additionally, because BAY 57-1293 was found to be potent systemically, we tested the effect of orally administered drug on established epithelial herpes in the rabbit model to determine whether the oral medication alone would be effective in treating epithelial disease. We also tested orally administered BAY 57-1293 in the mouse model of hyperthermia-induced reactivation9 for its effect on the shedding of HSV-1 in the tears and the quantity of viral DNA in the TG.
METHODS
The care and handling of the animals conformed to the NIH Guidelines on the Care and Use of Animals in Research and the Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research. The studies were approved by the Louisiana State University (New Orleans, LA) Health Sciences Center Institutional Animal Care and Use Committee.
Mouse reactivation studies
Five (5)-week-old female BALB/c mice were anesthetized and infected on both corneas. The corneas were lightly scratched in a cross-hatch pattern and 4 μl of a McKrae strain suspension containing 105 plaque forming units (PFUs) were placed on each ocular surface.
The success of the infections was confirmed by testing ocular swabs obtained 3 days post inoculation (PI) for the presence of virus in the tears. Both eyes of each mouse were swabbed with a single sterile swab. After collection, the swabs were processed for plaque assay. Viral cytopathic effect (CPE) and viral plaque assays were performed, using Vero cells as indicators. Vero cells were cultured in multiwell plates (96- or 24-well), depending on the experimental protocol. Volumes (0.1 or 0.5 mL) of culture medium from the tubes containing the swabs were incubated in each well for 1 h, the medium replaced with fresh tissue culture medium, and the plates incubated at 37°C in a CO2 incubator. The wells were examined for CPE daily, using low-power magnification. When quantification of infectious virus was required, the multiwell plates were coated with culture medium containing 0.5% methylcellulose. At the end of the incubation, the culture medium/methylcellulose was removed from the wells and the wells stained with crystal violet solution. The wells were rinsed, dried, and scanned and the plaques counted.
Thirty-five (35) days PI, the latent mice were heat-stressed by immersion up to their necks in 43°C water for 10 min9 to stimulate reactivation. The mice were dried and returned to their cages. After hyperthermic stress, the mice were randomized to control and drug treatment groups.
In a preliminary experiment to establish an effective dose, two groups of 30 mice latent for virus were heat-stressed and given two doses of either 10 or 50 mg/kg of BAY 57-1293 orally, one immediately after heat stress and one 12 h later. The drug was dissolved in cremophor (Cremophor EL; Sigma Chemical, St. Louis, MO) and diluted with balanced salt solution (BSS; Alcon Laboratories, Fort Worth, TX). A control group of 20 latent mice was given the drug vehicle only on the same schedule. All mice were tested for the presence of virus in the tears 24 h after heat stress, as described above.
In the second ocular swab experiment, 50 mice were given two oral doses of 50 mg/kg BAY 57-1293, one immediately after stress and one 12 h later; 30 mice were given vehicle alone on the same schedule. Twenty-four (24) h after heat stress, the eyes of the mice were swabbed. One sterile swab was used for both eyes of each mouse. The swabs were then cultured for virus, as described above.
To further analyze the effect of drug treatment on viral reactivation and shedding, the trigeminal ganglia (TG) from 50 latent mice were removed and analyzed for viral DNA by quantitative polymerase chain reaction (Q-PCR) analysis. Three groups of heat-stressed mice were used: 20 untreated; 10 oral vehicle-treated; and 20 oral drug-treated. Treatments were given as in the previous experiments: one immediately after heat stress and one 12 h later. The mice were sacrificed 24 h after heat stress, and the TG were collected. The Q-PCR method used was identical to that reported in detail in a previous study.10 In the current study, both TG of each mouse were pooled for analysis. The DNA data are reported as viral genome equivalents for ease of interpretation.
Pairs of ganglia were subjected to DNA extraction (Qiagen Extraction Kit; Qiagen, Inc., Chatsworth, CA) and the DNA quantified spectrophotometrically at 260 nm. For each sample, triplicate tubes containing 10× PCR buffer, dNTPs, Taq polymerase, and oligonucleotide primers complementary to the termini of a 476 base-pair sequence of the viral DNA polymerase gene were prepared. A competitor viral sequence, which amplifies a 530 base-pair fragment of the same viral gene, was added to triplicate tubes. Tubes received 108, 107, 106, 105, 104, 103, 102, 101, 100, or 10-1 molecules of competitor and 1 ng of ganglionic DNA. Following 35 thermal cycles, aliquots from each were resolved on 2% agarose gels, stained with ethidium bromide, and analyzed on a BioRad gel documentation system with ImageQuant® software (BioRad Laboratories, Hercules, CA). The equivalence point of staining intensity of the viral DNA and the competitor allowed determination of the quantity of viral DNA in each ganglion pair.
Rabbit keratitis studies
To study the effect of BAY 57-1293 on herpetic keratitis, New Zealand white rabbits (1-2 kg body weight) were anesthetized, their corneas lightly scratched in a cross-hatch pattern, and 10 μL of McKrae strain HSV-1 suspension (2.6 × 106 PFU) placed on the corneas. Seventy-two (72) h later, the corneas were stained with fluorescein and the severity of the herpetic keratitis was graded on a basis of 0 (clear cornea) to 4 (complete corneal epithelial ulceration). The rabbits were randomized to treatment groups, with both eyes treated with the same compound. The keratitis was graded daily in a masked fashion.
In the first study, 1 drop of a 2% suspension of BAY 57-1293 in methylcellulose (Murocel®, methylcellulose 1%; Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL) was applied topically five times a day to each eye for 5 days, beginning 3 days PI (10 rabbits per group). Controls consisted of 1% trifluridine (Falcon Pharmaceuticals, Fort Worth, TX) and BSS applied on the same schedule. In the second study, the dosage schedule was reduced to twice a day (8 rabbits treated with BAY 57-1293, 6 rabbits with BSS, and 7 with trifluridine,).
Similar studies were performed with BAY 57-1293 dissolved in either cremophor diluted with BSS (8 rabbits per group: BAY 57-1293, trifluridine, or BSS twice a day; all treatments on PI days 3-7) or cyclodextrin diluted with BSS (Sigma Chemical, St. Louis, MO) (8 rabbits treated with BAY 57-1293 twice a day, 8 rabbits treated with the drug four times a day, 7 rabbits treated with trifluridine twice a day, and 7 rabbits treated with BSS twice a day; all treatments on PI days 3-7).
Two studies were performed using orally administered preparations of BAY 57-1293. In the first study, the drug was suspended in BSS and administered as a 500 mg/kg/day suspension one dose per day on PI days 3-6 (6 rabbits). As controls, BSS drops were administered twice a day (7 rabbits) and trifluridine drops were administered twice a day (7 rabbits). In the second study, an oral suspension of BAY 57-1293 was prepared in 50% Ora-Plus and 50% Ora-Sweet (Paddock Laboratories, Inc., Minneapolis, MN) and sonicated for a minimum of 2 h. The drug was compared with an oral suspension of valacyclovir (Valtrex®, GlaxoSmithKline, Research Triangle Park, NC) sonicated in the same vehicle. The animals received either 40 mg/kg/day valacyclovir, 40 mg/kg/day valacyclovir plus 40 mg/kg/day BAY 57-1293, or 40 mg/kg/day BAY 57-1293 (10 rabbits per group) in one dose per day on PI days 3-10.
Statistical analysis
The frequencies of reactivation on the mouse models were analyzed with exact chi-square analysis.11
The slit-lamp scores of the rabbit model were analyzed with a nested ANOVA.12 In this analysis, each rabbit received only one type of treatment and was followed over time for the duration of the experiment so that a within-subject design (nested ANOVA) was applied, with rabbit response variability over time nested within the responses to one treatment. The alpha level was controlled for the number of multiple comparisons performed using the simulation method.13
RESULTS
Mouse reactivation studies
Based on the preliminary experiment demonstrating a dose-dependent effect of BAY 57-1293 treatment (Table 1), 50-mg/kg doses were used in the mouse studies. In an additional heat-stressed mouse study, orally administered BAY 57-1293 reduced the presence of herpes virus in the tears statistically significantly (P = 0.0061; exact chi-square test) more effectively than vehicle alone (Table 1).
Table 1.
Effect of Orally Administered BAY 57-1293 on Viral Reactivation in Heat-Stressed Micea
| Treatment | Number of mice | No. of positive corneal swabs/total no. of swabs (%) |
|---|---|---|
| Preliminary studyb | ||
| 10 mg/kg BAY 57-1293 | 30 | 14/30 (46) |
| 50 mg/kg BAY 57-1293 | 30 | 6/30 (20) |
| Vehicle | 20 | 14/20 (70) |
| Second studyc | ||
| 50 mg/kg BAY 57-1293 | 50 | 16/50 (32) |
| Vehicle | 30 | 19/30 (63) |
BALB/c strain female mice, 5 weeks of age, were infected with McKrae strain HSV-1. Thirty-five (35) days later, the mice were heat stressed (43°C for 10 min) and treated immediately, and 12 h later with BAY 57-1293 or vehicle orally. Eyes were swabbed (both eyes of each mouse with one swab) 24 h after hear stress, and the swabs were cultured for infectious virus.
Preliminary study: vehicle versus 10 mg/kg, P = 0.0899, vehicle versus 50 mg/kg, P = 0.0005, exact chi-square test.
Second study: Vehicle versus 50 mg/kg, P = 0.0061 (exact chi-square test).
As in a previous study,10 the Q-PCR method for viral DNA quantification yielded viral DNA values, based on the amplification of the viral DNA polymerase gene. The results showed that the untreated and vehicle-treated mice had similar and overlapping viral DNA levels in their TG following heat-stress-induced reactivation, whereas the TG of mice treated orally with BAY 57-1293 had up to four orders of magnitude lower levels of viral DNA in their TG (Table 2), compared with the other groups. This indicates that BAY 57-1293 statistically significantly (P = 0.0001) prevented the reactivation of HSV-1 in the mouse TG.
Table 2.
Viral DNA in the Trigeminal Ganglion After Heat-Stress-Induced Reactivation in Micea
| Treatment | No. of ganglion pairs analyzed | Mean (± SD) gene copy Numberb |
|---|---|---|
| Untreated | 20 | 1.4 × 106 ± 0.9 × 101 |
| Oral vehicle | 10 | 1.4 × 106 ± 0.8 × 101 |
| Oral BAY 57-1293 | 20 | 2.4 × 102 ± 0.5 × 101 |
Latent mice were heat-stressed and not treated, vehicle-treated, or drug-treated by oral administration. Twenty-four (24) h later, the mice were euthanized and their ganglia analyzed for viral DNA.
The mean number of viral polymerase gene copies in the ganglia of the mice treated orally with BAY 57-1293 was significantly smaller than the numbers of copies in the ganglia from the other groups of mice (P < 0.0001).
Rabbit keratitis studies
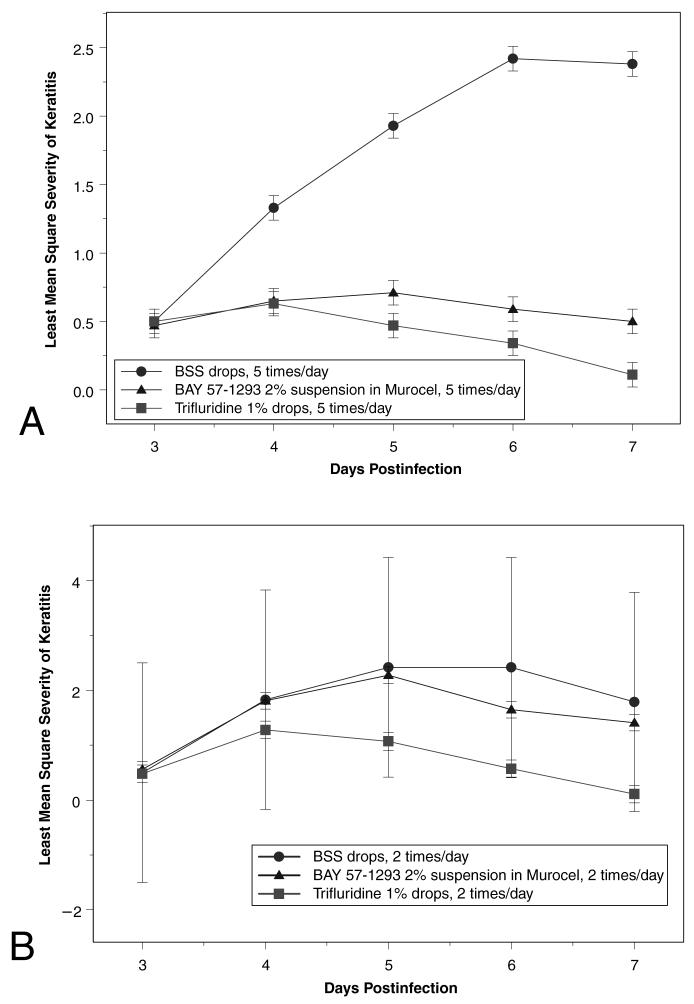
In the first rabbit study, topical treatment with a 2% suspension of BAY 57-1293 in methylcellulose five times a day demonstrated therapeutic efficacy similar to that of 1% trifluridine, although the helicase-primase inhibitor was difficult to solubilize and the final preparation was not entirely satisfactory (Fig. 1A). The severity of keratitis in the Bay 57-1293-treated eyes and in the trifluridine-treated eyes was significantly less, compared with eyes treated with BSS on PI days 4, 5, 6, and 7 (P = 0.0002, 0.0001, 0.0001, and 0.0001, respectively). On PI days 4, 5, and 6, there was no significant difference between the trifluridine- and BAY 57-1293-treated eyes (P = 1.000, 0.8954, and 0.8509, respectively). When the treatment schedule was reduced to twice a day (Fig. 1B), the keratitis in the BAY 57-1293-treated eyes was similar in severity to that in the BSS-treated eyes on PI days 4 (P = 0.9269) and 5 (P = 0.5513) and less severe on PI days 6 (P = 0.0010) and 7 (P = 0.0910). Throughout this study, the eyes treated with trifluridine showed significantly less severe keratitis than either the BAY 57-1293- or the BSS-treated eyes (P = 0.0001 at all examination times).
FIG. 1.
Severity of keratitis in rabbits infected with McKrae strain HSV-1 in both eyes and treated topically with BAY 57-1293 2% suspension in methylcellulose, 1% trifluridine, or balanced salt solution on postinjection days 3-7. Severity was graded on a masked basis on a scale of 1-4. Treatment was administered (A) 5 times or (B) twice a day.
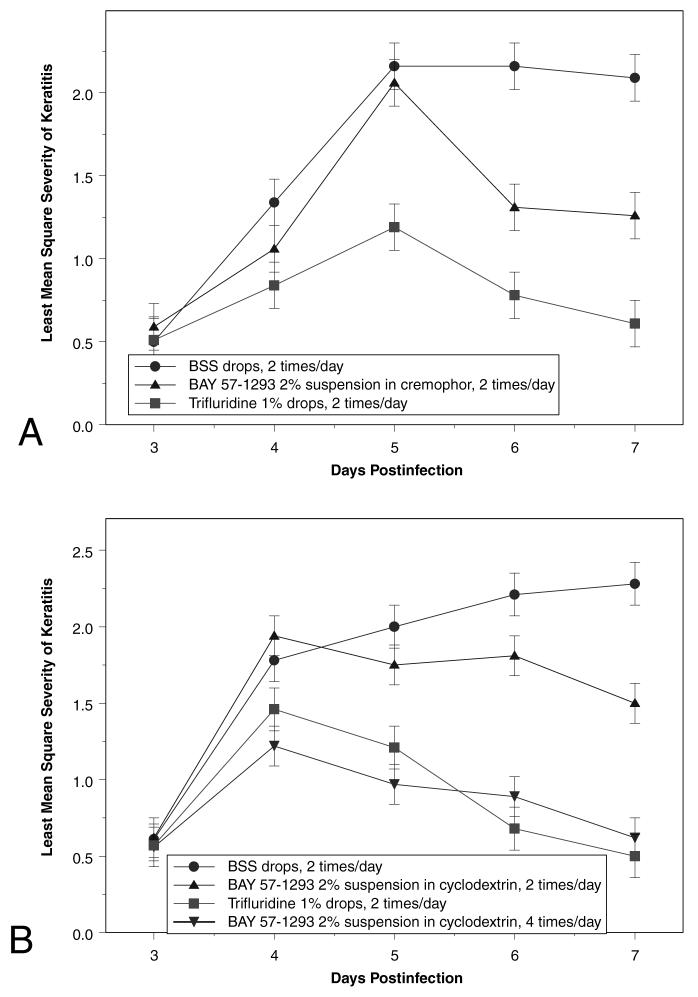
In additional topical application studies, BAY 57-1293 dissolved in either cremophor (Fig. 2A) or cyclodextrin (Fig. 2B), administered twice a day, was not as effective as twice-a-day trifluridine treatment. Efficacy was increased, however, with four-times-a-day treatment using the cyclodextrin formulation (Fig. 2B); the severity of keratitis was similar to that seen with twice-a-day treatment with trifluridine (P = 0.9998, 0.9998, 1.0000, and 1.0000 on PI days 4, 5, 6, and 7, respectively), indicating that an optimal ocular formulation of BAY 57-1293 may further increase efficacy.
FIG. 2.
Severity of keratitis in rabbits infected with McKrae strain HSV-1 in both eyes and treated topically with BAY 57-1293 2% suspension of cremophor or cyclodextrin, 1% trifluridine, or balanced salt solution (BSS) on postinjection days 3-7. Severity was graded on a masked basis on a scale of 1-4. (A) BAY 57-1293 was solubilized in cremophor and diluted in BSS and administered twice a day. (B) BAY 57-1293 was solubilized in cyclodextrin and diluted in BSS and administered twice or four times a day.
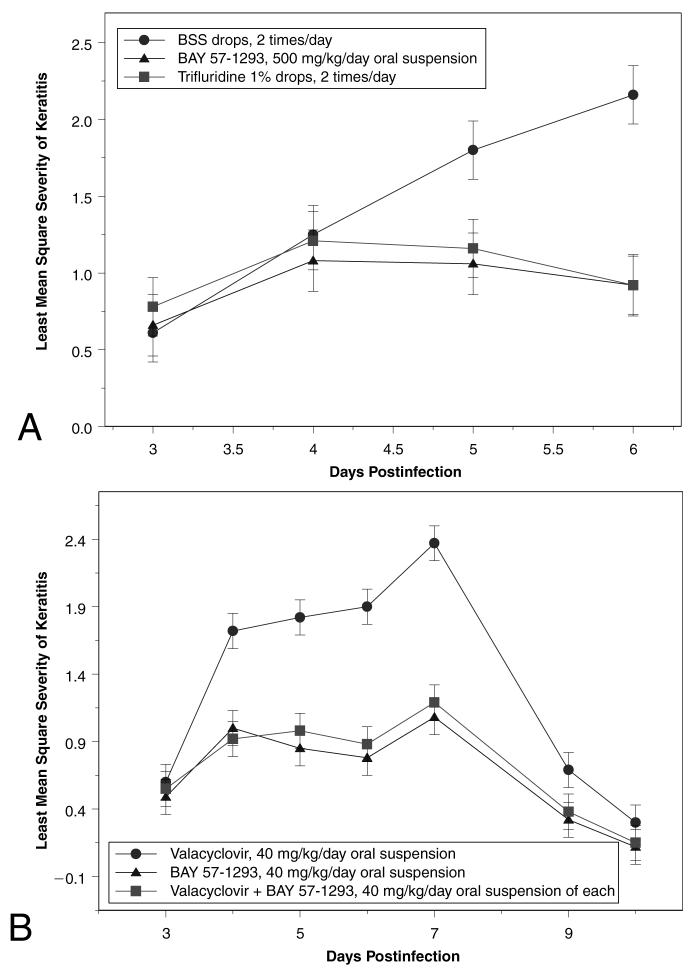
In the first oral dosage study, once-daily oral treatment with 500 mg/kg BAY 57-1293 was as effective as twice-a-day topical treatment with trifluridine (Fig. 3A), with no statistically significant differences seen in the severity of keratitis on PI days 4, 5, or 6 (P = 1.000 all times). Treatment with BAY 57-1293 was more effective than treatment with BSS on PI days 5 (P = 0.0029) and 6 (P < 0.0001).
FIG. 3.
Severity of keratitis in rabbits infected with McKrae strain HSV-1 in both eyes and treated with oral suspensions of BAY 57-1293. Severity was graded on a masked basis on a scale of 1-4. (A) BAY 57-1293 suspended in balanced salt solution (BSS) administered orally at 500 mg/kg/day on postinjection (PI) days 3-6. Controls: topical 1% trifluridine and BSS twice a day. (B) Oral treatment with BAY 57-1293 (40 mg/kg/day), valacyclovir (40 mg/kg/day), or BAY 57-1293 and valacyclovir (40 mg/kg/day each) on postinjection days 3-10. The oral suspensions of the drug were prepared in Ora-Plus/Ora-Sweet and sonicated.
In the second oral dosage study, there was no difference in the severity of keratitis in the animals receiving 40 mg/kg/day BAY 57-1293 alone or 40 mg/kg/day BAY 57-1293 plus 40 mg/kg/day valacyclovir (P = 1.0000 at all times; Fig. 3B). Keratitis in the animals treated with 40 mg/kg/day BAY-1293 was significantly less severe, compared with the animals treated with valacyclovir alone (P = 0.0116, < 0.0001, < 0.0001, and < 0.0001, on PI days 4, 5, 6, and 7, respectively). Treatment with oral valacyclovir resulted in a keratitis that was similar to that seen in the previous studies in animals treated with BSS.
DISCUSSION
Previous studies in humans indicate that the present treatments for herpes simplex keratitis, while efficient for epithelial disease, are relatively ineffective for stromal disease and iritis, and that a drug such as acyclovir is also relatively ineffective.14 It is not certain that a drug with increased potency and efficacy would facilitate the treatment of these syndromes, but we believe that continued viral replication takes place even in stromal keratitis, and that a more potent antiviral may offer significant benefits. Although oral acyclovir alone with or without debridement of any active lesions may provide sufficient treatment in epithelial disease, this is not established. An effective oral drug that would eliminate the need for eye drops may be of benefit to patients who have a problem complying with topical medication regimens, and our studies suggest that BAY 57-1293 may be such a drug. In addition, this study shows that oral BAY 57-1293 inhibits viral production in the TG of mice, which suggests that it may be effective in preventing recurrences of herpes. This may be especially important, since acyclovir in the HEDS trial prevented only 40% of recurrences.15
The susceptibility of various human, bovine and pseudorabies herpesviruses to helicase primase inhibitors has been reported.1 We determined the 50% plaque reduction concentration (IC50 on Vero cells = 1.5 × 10-8 M) of BAY 57-1293 for the McKrae virus used in this study to be 15 nM, which matches IC50 values of 10-20 nM reported for other HSV-1 strains by other groups.1
The suppression of viral reactivation in mice by oral BAY 57-1293 in these studies (Table 1) was more effective than acyclovir administered in drinking water for 3 days in a previous study.17 In the present study, only 20%-30% of mice showed reactivation after oral treatment with BAY 57-1293, compared with 40% reactivation after acyclovir treatment in the previous study.
In this study, rabbits were infected with the McKrae strain of HSV-1, and 72 h later were randomized and treated with coded 2% BAY 57-1293, trifluridine 1%, or BSS. Even though the BAY 57-1293 was difficult to solubilize and the preparation was not entirely satisfactory, the therapeutic effectiveness of the methylcellulose formulation, given five times a day, was approximately the same as that of trifluridine. Two percent (2%) suspensions of BAY 57-1293 in methylcellulose, cremophor, or cyclodextrin given twice a day were less effective than the methylcellulose formulation given five times a day. However, when the cyclodextrin formulation was given four times a day, its activity was the same as that of trifluridine. The fact that increasing the frequency of treatment greatly improved efficacy suggests that if a better formulation and a higher concentration could be obtained, efficacy would greatly increase. The comparable results of five-times-a-day treatment with BAY 57-1293 with trifluridine, given five times a day, suggests that it may be possible to treat with maximal efficacy but without the cytotoxicity or the potential effects on healing retardation seen with trifluridine.
CONCLUSIONS
The very potent oral activity of BAY 57-1293 suggests that drops may not be necessary and that stromal disease and iritis might be treatable. The effective suppression of herpes reactivation and suppression of viral replication in the trigeminal ganglion suggest that this drug may prevent recurrences and shedding more effectively than acyclovir, valacyclovir, penciclovir, and famciclovir. Further extension of the potential for successful antiviral therapy with this drug depends on the results of careful studies in humans.
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. Public Health Service grants R01EY002672 and P30EY002377 from the National Eye Institute, National Institutes of Health (Bethesda, MD) and an unrestricted challenge grant from Research to Prevent Blindness (New York, NY).
REFERENCES
- 1.Kleymann G, Fischer R, Betz UAK, et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat. Med. 2002;8:392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 2.Betz UAK, Fischer R, Kleymann G, et al. Potent in vivo antiviral activity of the herpes simplex virus primase-helicase inhibitor BAY 57-1293. Antimicrob. Agents Chemother. 2002;46:1766–1772. doi: 10.1128/AAC.46.6.1766-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumeister J, Fischer R, Eckenberg P, et al. Superior efficacy of helicase-primase inhibitor BAY 57-1293 for herpes infection and latency in the guinea pig model of human genital herpes disease. Antiviral Chem. Chemother. 2007;18:35–48. doi: 10.1177/095632020701800104. [DOI] [PubMed] [Google Scholar]
- 4.Kleymann G. New antiviral drugs that target herpesvirus helicase primase enzymes. HERPES. 2003;10:46–52. [PubMed] [Google Scholar]
- 5.Kleymann G. Antiviral treatment. In: Studahl M, Cinque P, Bergström T, editors. Herpes Simplex Viruses. Taylor & Francis Group; New York: 2006. pp. 153–176. [Google Scholar]
- 6.Kleymann G. Agents and strategies in development for improved management of herpes simplex virus infection and disease. Expert Opin. Investig. Drugs. 2005;14:135–161. doi: 10.1517/13543784.14.2.135. [DOI] [PubMed] [Google Scholar]
- 7.Kleymann G. Helicase primase: Targeting the Achilles heel of herpes simplex viruses. Antiviral Chem. Chemother. 2004;15:135–140. doi: 10.1177/095632020401500303. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman HE, Martola EL, Dohlman CH. The use of 5-iodo-2′-deoxyuridine (IDU) in the treatment of herpes simplex keratitis. Arch. Ophthalmol. 1962;68:235–239. doi: 10.1001/archopht.1962.00960030239015. [DOI] [PubMed] [Google Scholar]
- 9.Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebhardt BM, Wright GE, Xu H, et al. 9-(4-hydroxybutyl)-N2-phenylguanine, HBPG, a thymidine kinase inhibitor, suppresses herpes virus reactivation in mice. Antiviral Res. 1996;30:87–94. doi: 10.1016/0166-3542(95)00900-0. [DOI] [PubMed] [Google Scholar]
- 11.Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. 2nd ed. Chapman & Hall/CRC; Boca Raton, FL: 2000. [Google Scholar]
- 12.Milliken GA, Johnson DE. Analysis of Messy Data. Volume I: Designed Experiments. Van Nostrand Reinhold; New York: 1984. p. 473. [Google Scholar]
- 13.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. [PubMed] [Google Scholar]
- 14.Kaufman HE. Treatment of viral diseases of the cornea and external eye. Prog. Retin. Eye Res. 2000;19:69–85. doi: 10.1016/s1350-9462(99)00004-x. [DOI] [PubMed] [Google Scholar]
- 15.The Herpetic Eye Disease Study Group Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N. Engl. J. Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 16.Kleymann G. Discovery and SAR of herpesvirus helicase primase inhibitors. Curr. Med. Chem. 2004;3:69–83. [Google Scholar]
- 17.Gebhardt BM, Kaufman HE, Hill JM. Effect of acyclovir on thermal stress-induced herpesvirus reactivation. Curr. Eye Res. 2004;29:137–144. doi: 10.1080/02713680490504560. [DOI] [PubMed] [Google Scholar]