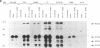Abstract
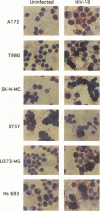
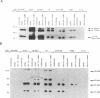
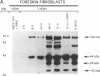
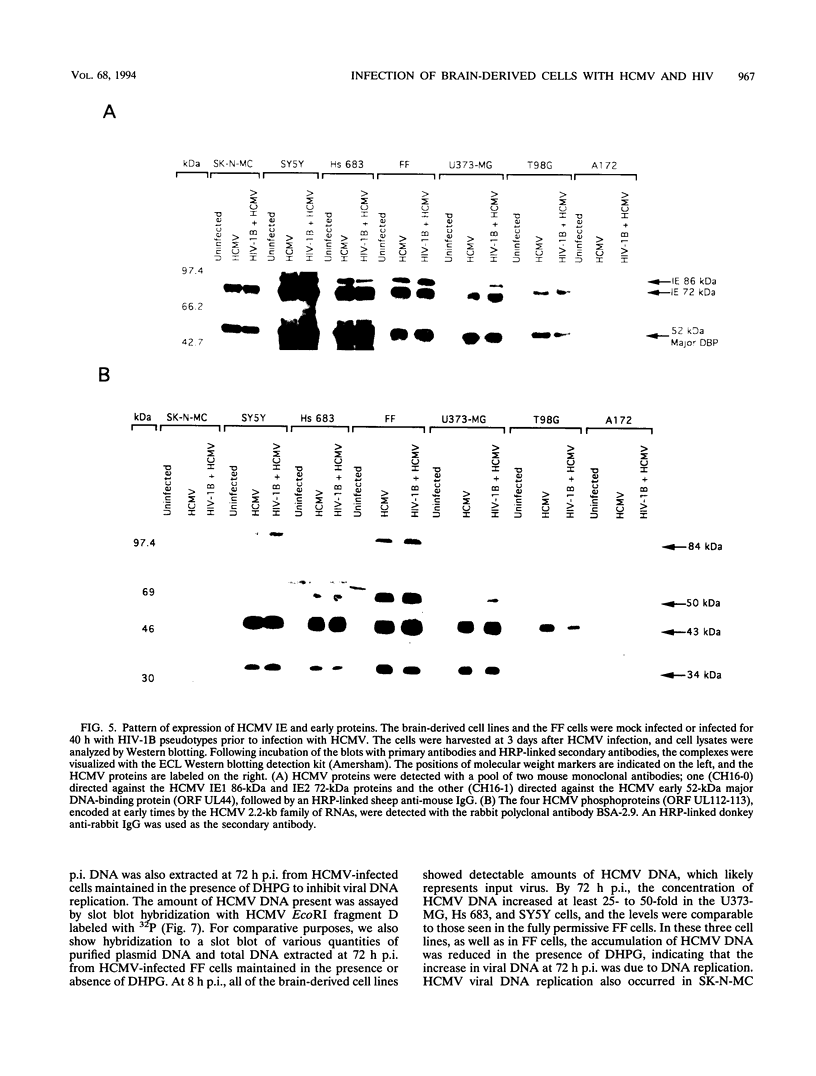
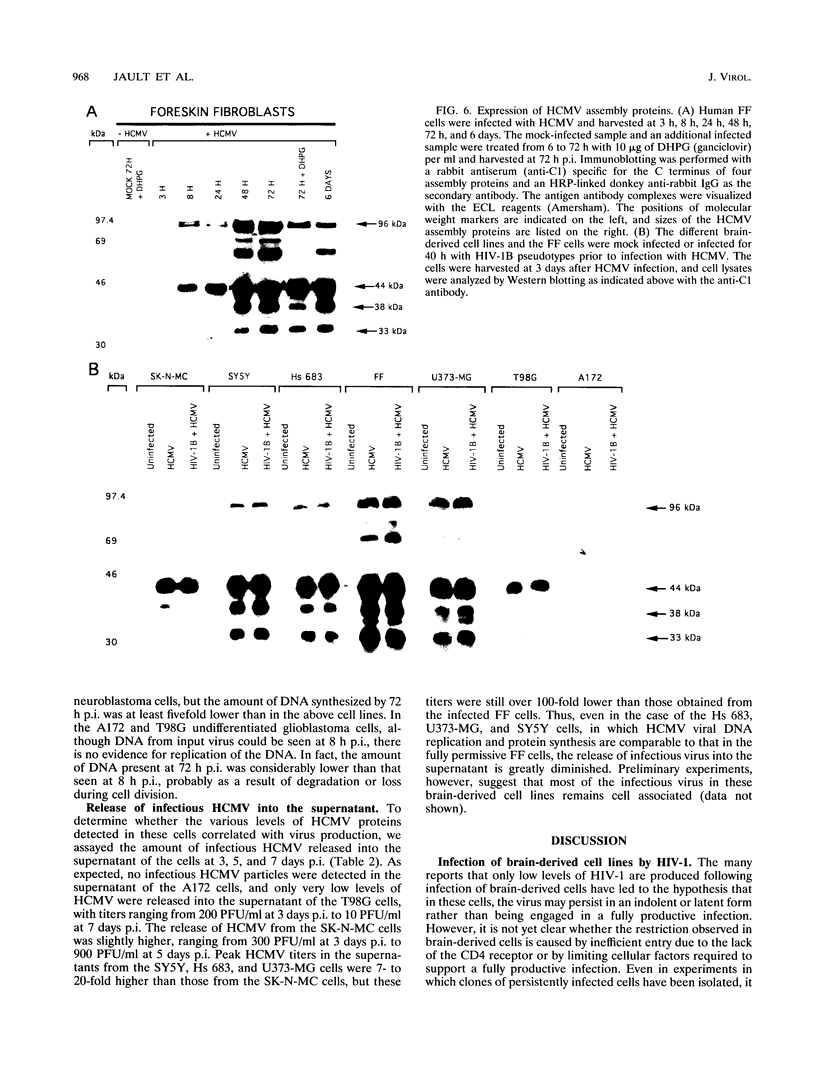
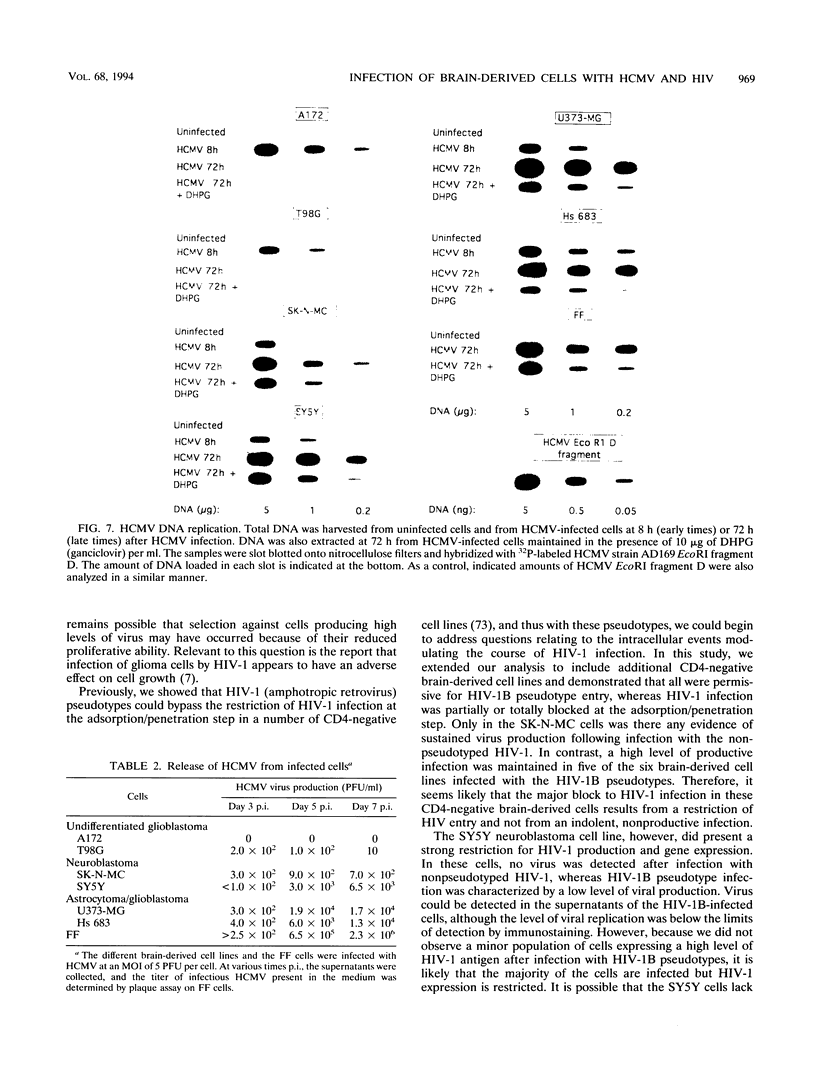
Human cytomegalovirus (HCMV) is commonly found in the brains of patients with AIDS and in some cases can be detected in the same cells as can human immunodeficiency virus type 1 (HIV-1). In this study, we analyzed the patterns of replication of HIV-1 and HCMV in singly infected cells and the effects of dual infection in human brain-derived cell lines of three different origins: neuroblastoma cell lines SK-N-MC and SY5Y; astrocytoma/glioblastoma cell lines U373-MG and Hs 683; and undifferentiated glioblastoma cell lines A172 and T98G. To bypass the restriction at the adsorption/penetration step in these CD4-negative cells, we used HIV-1 (amphotropic retrovirus) pseudotypes. These HIV-1 pseudotypes infected the majority of the cells in the cultures and expressed high levels of HIV-1 gene products in all except the SY5Y cells. The cell lines differed in the ability to support HCMV infection, but coinfection with HIV-1 had no effect on HCMV replication. The A172 cells were completely nonpermissive for HCMV gene expression, while HCMV replication in the singly infected T98G and SK-N-MC cell lines was restricted at the level of some early gene products. This resulted in complete and partial inhibition, respectively, of viral DNA synthesis. Dual infection of the A172, T98G, and SK-N-MC cells had no effect on HIV-1 replication. The other three cell lines, U373-MG, Hs 683, and SY5Y, were fully permissive for HCMV replication. In the U373-MG and Hs 683 cells, HCMV markedly inhibited the synthesis of HIV-1 gene products. In contrast, a transient stimulation of HIV-1 production followed by a repression was observed in the dually infected SY5Y cells. We conclude from these results that under conditions in which both HIV-1 and HCMV can undergo fully permissive infection, HCMV can repress HIV-1 gene expression. In cells in which HCMV replication is limited but HIV-1 replicates well, there is no effect on HIV-1 gene expression. However, activation of HIV-1, at least transiently, may occur in cells in which HIV-1 gene expression is limited. These studies suggest that a threshold level of some HIV-1 gene product(s) may obscure activation or promote repression of HIV replication by HCMV.
Full text
PDF


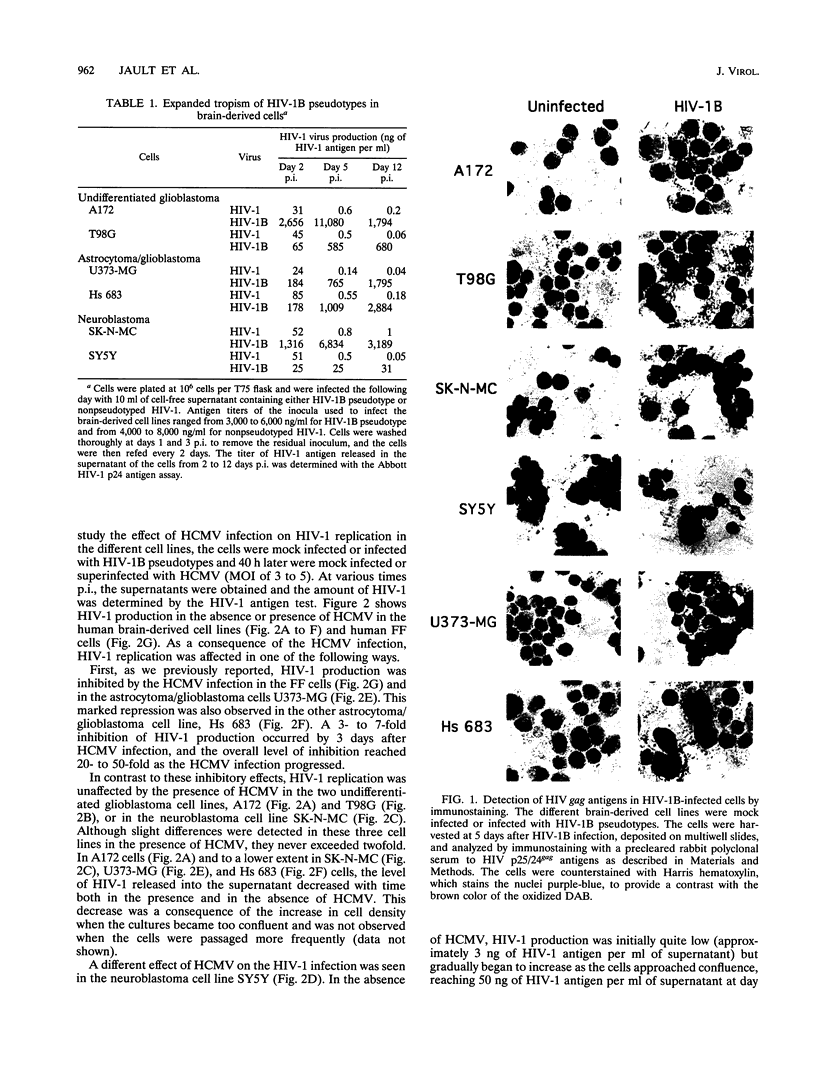
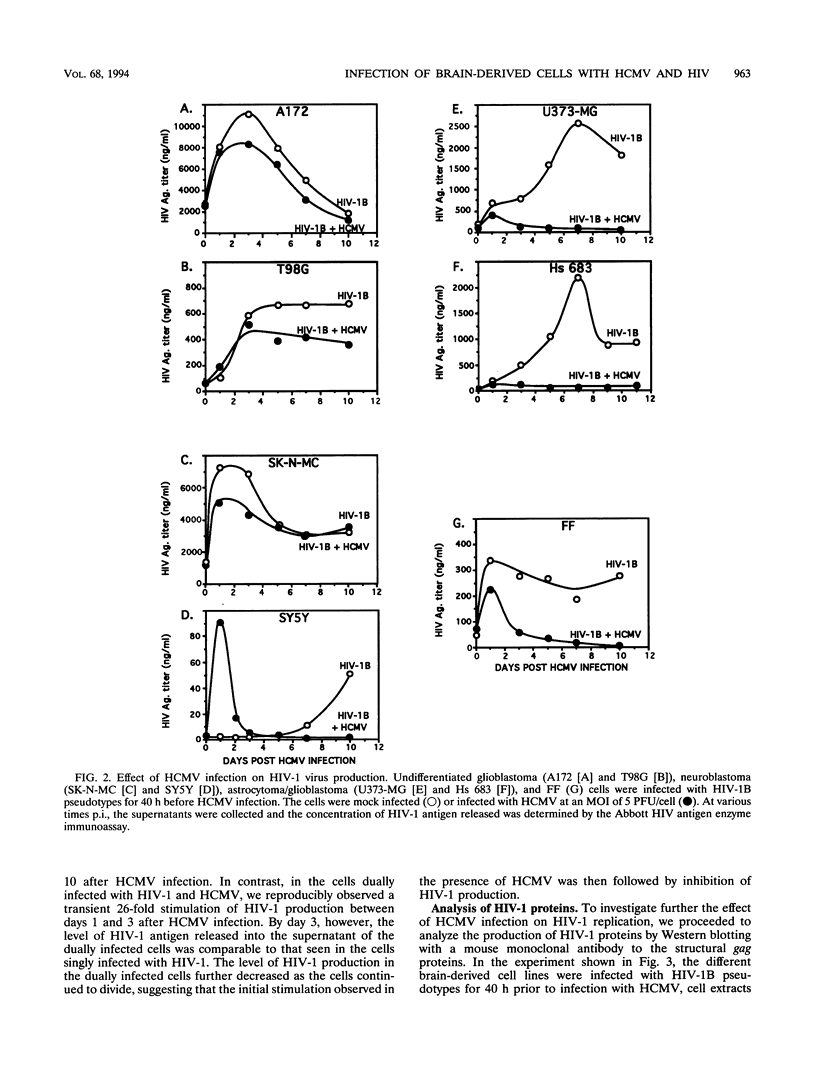
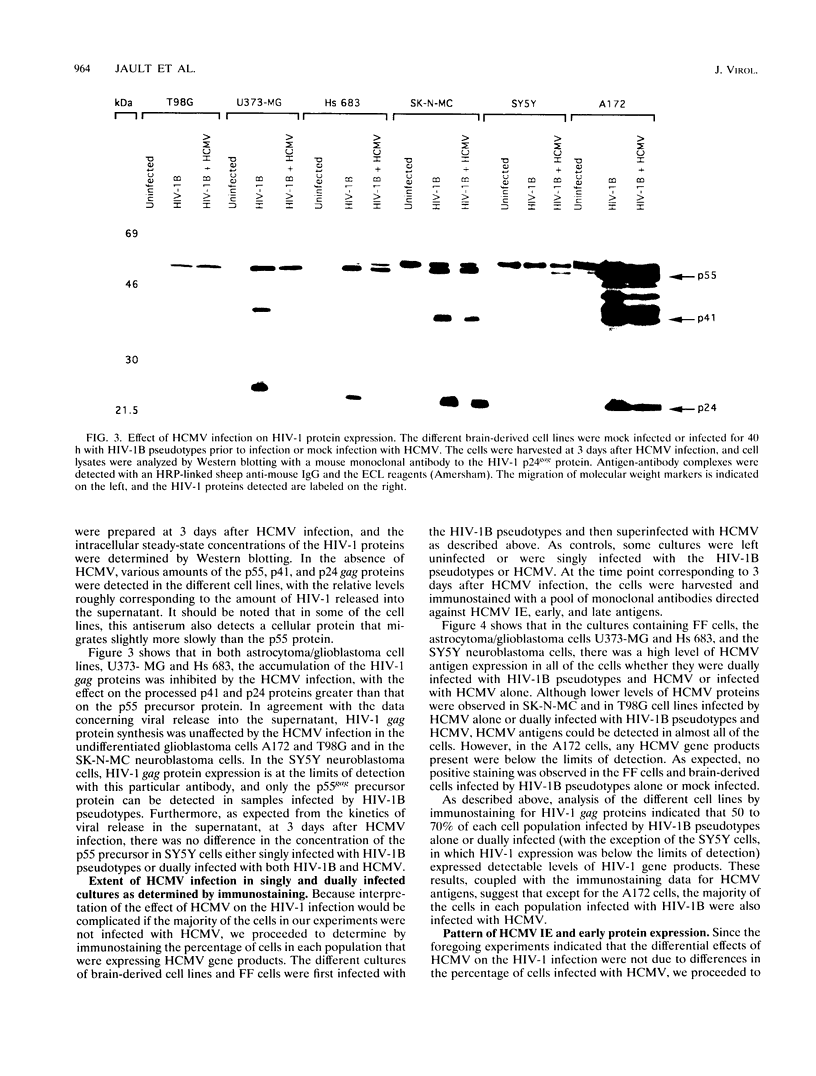
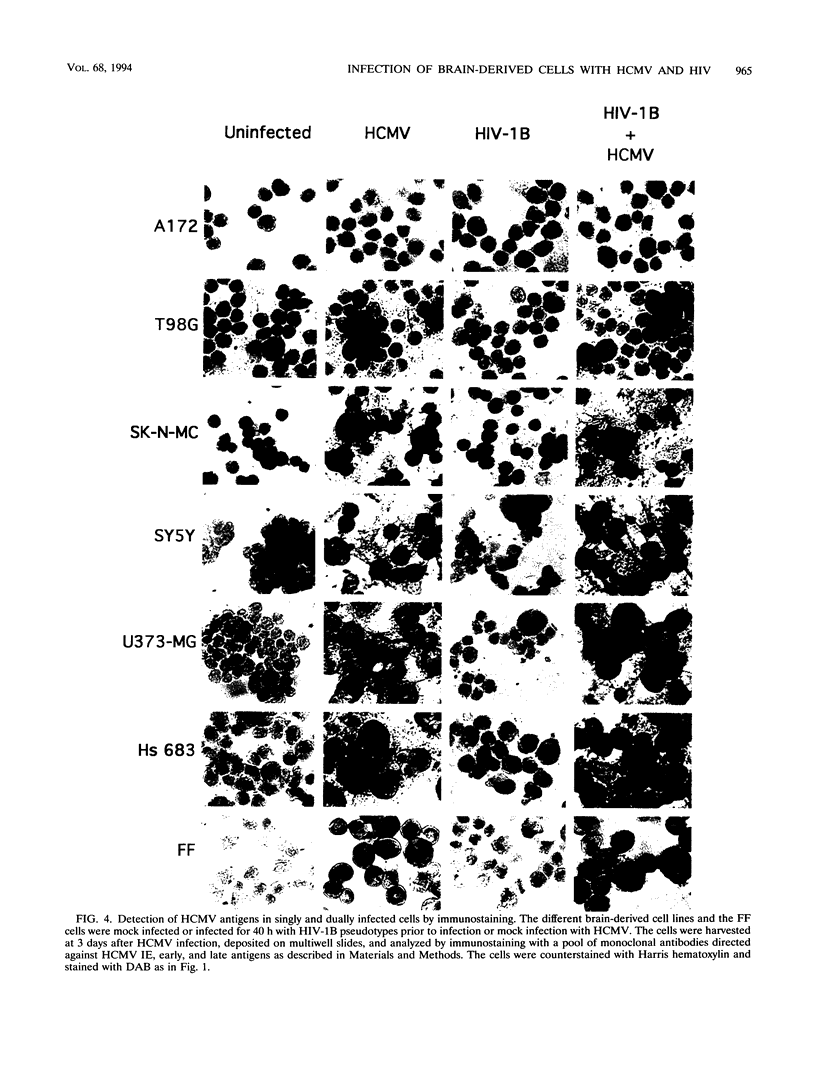





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht M. A., DeLuca N. A., Byrn R. A., Schaffer P. A., Hammer S. M. The herpes simplex virus immediate-early protein, ICP4, is required to potentiate replication of human immunodeficiency virus in CD4+ lymphocytes. J Virol. 1989 May;63(5):1861–1868. doi: 10.1128/jvi.63.5.1861-1868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Sethi A. Stimulation of the human immunodeficiency virus type 2 (HIV-2) gene expression by the cytomegalovirus and HIV-2 transactivator gene. AIDS Res Hum Retroviruses. 1990 May;6(5):649–658. doi: 10.1089/aid.1990.6.649. [DOI] [PubMed] [Google Scholar]
- Barry P. A., Pratt-Lowe E., Peterlin B. M., Luciw P. A. Cytomegalovirus activates transcription directed by the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2932–2940. doi: 10.1128/jvi.64.6.2932-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. A., Pratt-Lowe E., Unger R. E., Luciw P. A. Cellular factors regulate transactivation of human immunodeficiency virus type 1. J Virol. 1991 Mar;65(3):1392–1399. doi: 10.1128/jvi.65.3.1392-1399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegalke B. J., Geballe A. P. Sequence requirements for activation of the HIV-1 LTR by human cytomegalovirus. Virology. 1991 Jul;183(1):381–385. doi: 10.1016/0042-6822(91)90151-z. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R., Kleinschmidt A., Ludvigsen A., Mellert W., Neumann M., Herrmann R., Khim M. C., Burny A., Müller-Lantzsch N., Stavrou D. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992 Mar;6(3):273–285. [PubMed] [Google Scholar]
- Bélec L., Gray F., Mikol J., Scaravilli F., Mhiri C., Sobel A., Poirier J. Cytomegalovirus (CMV) encephalomyeloradiculitis and human immunodeficiency virus (HIV) encephalitis: presence of HIV and CMV co-infected multinucleated giant cells. Acta Neuropathol. 1990;81(1):99–104. doi: 10.1007/BF00662645. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K., Tapper M. A. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J Infect Dis. 1990 Oct;162(4):844–851. doi: 10.1093/infdis/162.4.844. [DOI] [PubMed] [Google Scholar]
- Casareale D., Fiala M., Chang C. M., Cone L. A., Mocarski E. S. Cytomegalovirus enhances lysis of HIV-infected T lymphoblasts. Int J Cancer. 1989 Jul 15;44(1):124–130. doi: 10.1002/ijc.2910440122. [DOI] [PubMed] [Google Scholar]
- Chapman C. J., Harris J. D., Collins M. K., Latchman D. S. A recombinant HIV provirus is synergistically activated by the HIV Tat protein and the HSV IE1 protein but not by the HSV IE3 protein. AIDS. 1991 Aug;5(8):945–950. doi: 10.1097/00002030-199108000-00004. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Robbins P. B., Fernie B., Ostrove J. M., Fauci A. S. Viral antigen stimulation of the production of human monokines capable of regulating HIV1 expression. J Immunol. 1989 Jul 15;143(2):470–475. [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C. P., Kulka M., Aurelian L. NF-kappa B-binding proteins induced by HSV-1 infection of U937 cells are not involved in activation of human immunodeficiency virus. Virology. 1993 Feb;192(2):491–500. doi: 10.1006/viro.1993.1065. [DOI] [PubMed] [Google Scholar]
- Feng C. P., Kulka M., Aurelian L. The GGTCA palindrome and cognate cellular factors in trans-regulation of human immunodeficiency virus long terminal repeat by herpes simplex virus. J Gen Virol. 1993 Apr;74(Pt 4):715–723. doi: 10.1099/0022-1317-74-4-715. [DOI] [PubMed] [Google Scholar]
- Finkle C., Tapper M. A., Knox K. K., Carrigan D. R. Coinfection of cells with the human immunodeficiency virus and cytomegalovirus in lung tissues of patients with AIDS. J Acquir Immune Defic Syndr. 1991;4(7):735–737. [PubMed] [Google Scholar]
- Geleziunas R., Schipper H. M., Wainberg M. A. Pathogenesis and therapy of HIV-1 infection of the central nervous system. AIDS. 1992 Dec;6(12):1411–1426. doi: 10.1097/00002030-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. Q., Chandran B., Josephs S. F., Wood C. Identification and characterization of a human herpesvirus 6 gene segment that trans activates the human immunodeficiency virus type 1 promoter. J Virol. 1992 Mar;66(3):1564–1570. doi: 10.1128/jvi.66.3.1564-1570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Young J., Giulietti E., DeMattei C., Garcia J., Gaynor R., Stenberg R. M., Nelson J. A. A discrete cis element in the human immunodeficiency virus long terminal repeat mediates synergistic trans activation by cytomegalovirus immediate-early proteins. J Virol. 1991 Dec;65(12):6735–6742. doi: 10.1128/jvi.65.12.6735-6742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Murphy T. L., Roby C. Cytomegalovirus-infected cells contain a DNA-binding protein. Virology. 1981 May;111(1):251–262. doi: 10.1016/0042-6822(81)90669-3. [DOI] [PubMed] [Google Scholar]
- Golden M. P., Kim S., Hammer S. M., Ladd E. A., Schaffer P. A., DeLuca N., Albrecht M. A. Activation of human immunodeficiency virus by herpes simplex virus. J Infect Dis. 1992 Sep;166(3):494–499. doi: 10.1093/infdis/166.3.494. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Simurda M. C. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through induction of NF-kappa B activity. J Virol. 1992 Nov;66(11):6496–6501. doi: 10.1128/jvi.66.11.6496-6501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. E., Yang J. Y., Zhang R. D., Bealer M. Altered HIV expression and EBV-induced transformation in coinfected PBLs and PBL subpopulations. Virology. 1991 May;182(1):186–198. doi: 10.1016/0042-6822(91)90662-u. [DOI] [PubMed] [Google Scholar]
- Hirka G., Prakash K., Kawashima H., Plotkin S. A., Andrews P. W., Gönczöl E. Differentiation of human embryonal carcinoma cells induces human immunodeficiency virus permissiveness which is stimulated by human cytomegalovirus coinfection. J Virol. 1991 May;65(5):2732–2735. doi: 10.1128/jvi.65.5.2732-2735.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W. Z., Harouse J. M., Rando R. F., Gönczöl E., Srinivasan A., Plotkin S. A. Reciprocal enhancement of gene expression and viral replication between human cytomegalovirus and human immunodeficiency virus type 1. J Gen Virol. 1990 Jan;71(Pt 1):97–103. doi: 10.1099/0022-1317-71-1-97. [DOI] [PubMed] [Google Scholar]
- Ho W. Z., Song L., Douglas S. D. Human cytomegalovirus infection and trans-activation of HIV-1 LTR in human brain-derived cells. J Acquir Immune Defic Syndr. 1991;4(11):1098–1106. [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Balachandran N. Transactivation of human immunodeficiency virus promoter by human herpesvirus 6. J Virol. 1989 Feb;63(2):970–973. doi: 10.1128/jvi.63.2.970-973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Josephs S. F., Balachandran N. Transactivation of the human immunodeficiency virus promoter by human herpesvirus 6 (HHV-6) strains GS and Z-29 in primary human T lymphocytes and identification of transactivating HHV-6(GS) gene fragments. J Virol. 1991 Jun;65(6):2895–2902. doi: 10.1128/jvi.65.6.2895-2902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari B., Radeke R., Gehrz R. Processing of human cytomegalovirus envelope glycoproteins in and egress of cytomegalovirus from human astrocytoma cells. J Gen Virol. 1992 Feb;73(Pt 2):253–260. doi: 10.1099/0022-1317-73-2-253. [DOI] [PubMed] [Google Scholar]
- Keller A., Garrett E. D., Cullen B. R. The Bel-1 protein of human foamy virus activates human immunodeficiency virus type 1 gene expression via a novel DNA target site. J Virol. 1992 Jun;66(6):3946–3949. doi: 10.1128/jvi.66.6.3946-3949.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Markovitz D., Fenrick R., Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S., Garcia J., Pearson L., Soultanakis E., Dasgupta A., Gaynor R. Multiple transcriptional regulatory domains in the human immunodeficiency virus type 1 long terminal repeat are involved in basal and E1A/E1B-induced promoter activity. J Virol. 1989 Nov;63(11):4616–4625. doi: 10.1128/jvi.63.11.4616-4625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval V., Clark C., Vaishnav M., Spector S. A., Spector D. H. Human cytomegalovirus inhibits human immunodeficiency virus replication in cells productively infected by both viruses. J Virol. 1991 Dec;65(12):6969–6978. doi: 10.1128/jvi.65.12.6969-6978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. H., Lee K. J., Kim S., Sung Y. C. Transactivation of human immunodeficiency virus type 1 long terminal repeat-directed gene expression by the human foamy virus bel1 protein requires a specific DNA sequence. J Virol. 1992 May;66(5):3236–3240. doi: 10.1128/jvi.66.5.3236-3240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levrero M., Balsano C., Natoli G., Avantaggiati M. L., Elfassi E. Hepatitis B virus X protein transactivates the long terminal repeats of human immunodeficiency virus types 1 and 2. J Virol. 1990 Jun;64(6):3082–3086. doi: 10.1128/jvi.64.6.3082-3086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Landay A., Lennette E. T. Human herpesvirus 6 inhibits human immunodeficiency virus type 1 replication in cell culture. J Clin Microbiol. 1990 Oct;28(10):2362–2364. doi: 10.1128/jcm.28.10.2362-2364.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. M., Bredesen D. E., Rosenblum M. L. Opportunistic central nervous system pathology in patients with AIDS. Ann Neurol. 1988;23 (Suppl):S7–12. doi: 10.1002/ana.410230706. [DOI] [PubMed] [Google Scholar]
- Lusso P., De Maria A., Malnati M., Lori F., DeRocco S. E., Baseler M., Gallo R. C. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991 Feb 7;349(6309):533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Mallon R., Borkowski J., Albin R., Pepitoni S., Schwartz J., Kieff E. The Epstein-Barr virus BZLF1 gene product activates the human immunodeficiency virus type 1 5' long terminal repeat. J Virol. 1990 Dec;64(12):6282–6285. doi: 10.1128/jvi.64.12.6282-6285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis D. M., Rabson A. B., Straus S. E., Ostrove J. M. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology. 1992 Feb;186(2):788–791. doi: 10.1016/0042-6822(92)90048-t. [DOI] [PubMed] [Google Scholar]
- Markovitz D. M., Kenney S., Kamine J., Smith M. S., Davis M., Huang E. S., Rosen C., Pagano J. S. Disparate effects of two herpesvirus [corrected] immediate-early gene trans-activators on the HIV-1 LTR. Virology. 1989 Dec;173(2):750–754. doi: 10.1016/0042-6822(89)90591-6. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Griffiths P. D., Weiss R. A. HIV susceptibility conferred to human fibroblasts by cytomegalovirus-induced Fc receptor. Nature. 1990 Feb 15;343(6259):659–661. doi: 10.1038/343659a0. [DOI] [PubMed] [Google Scholar]
- Morgello S., Cho E. S., Nielsen S., Devinsky O., Petito C. K. Cytomegalovirus encephalitis in patients with acquired immunodeficiency syndrome: an autopsy study of 30 cases and a review of the literature. Hum Pathol. 1987 Mar;18(3):289–297. doi: 10.1016/s0046-8177(87)80012-6. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Reynolds-Kohler C., Oldstone M. B., Wiley C. A. HIV and HCMV coinfect brain cells in patients with AIDS. Virology. 1988 Jul;165(1):286–290. doi: 10.1016/0042-6822(88)90685-x. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Leonard J., Weck K. E., Rabson A. B., Gendelman H. E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paya C. V., Virelizier J. L., Michelson S. Modulation of T-cell activation through protein kinase C- or A-dependent signalling pathways synergistically increases human immunodeficiency virus long terminal repeat induction by cytomegalovirus immediate-early proteins. J Virol. 1991 Oct;65(10):5477–5484. doi: 10.1128/jvi.65.10.5477-5484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Gallo D., Cremer N. Monoclonal antibodies to human cytomegalovirus: three surface membrane proteins with unique immunological and electrophoretic properties specify cross-reactive determinants. Infect Immun. 1982 Jun;36(3):924–932. doi: 10.1128/iai.36.3.924-932.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Gekker G., Chao C. C., Hu S. X., Edelman C., Balfour H. H., Jr, Verhoef J. Human cytomegalovirus-stimulated peripheral blood mononuclear cells induce HIV-1 replication via a tumor necrosis factor-alpha-mediated mechanism. J Clin Invest. 1992 Feb;89(2):574–580. doi: 10.1172/JCI115623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland S. D., Costello P., Dekaban G. A., Rice G. P. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J Infect Dis. 1990 Dec;162(6):1252–1262. doi: 10.1093/infdis/162.6.1252. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L. Cytomegalovirus preferentially infects a monocyte derived macrophage/microglial cell in human brain cultures: neuropathology differs between strains. J Neuropathol Exp Neurol. 1991 Jul;50(4):432–440. doi: 10.1097/00005072-199107000-00004. [DOI] [PubMed] [Google Scholar]
- Rando R. F., Pellett P. E., Luciw P. A., Bohan C. A., Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987 Mar;1(1):13–18. [PubMed] [Google Scholar]
- Rando R. F., Srinivasan A., Feingold J., Gonczol E., Plotkin S. Characterization of multiple molecular interactions between human cytomegalovirus (HCMV) and human immunodeficiency virus type 1 (HIV-1). Virology. 1990 May;176(1):87–97. doi: 10.1016/0042-6822(90)90233-h. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Trans-activation of the human immunodeficiency virus long terminal repeat sequences, expressed in an adenovirus vector, by the adenovirus E1A 13S protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4200–4204. doi: 10.1073/pnas.85.12.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Quinto I., Ruocco M. R., Mallardo M., Ambrosino C., Squitieri B., Tassone P., Venuta S. Epstein-Barr virus nuclear antigen 2 transactivates the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1993 May;67(5):2853–2861. doi: 10.1128/jvi.67.5.2853-2861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk P., Woods A. S., Gibson W. The 45-kilodalton protein of cytomegalovirus (Colburn) B-capsids is an amino-terminal extension form of the assembly protein. J Virol. 1991 Mar;65(3):1525–1529. doi: 10.1128/jvi.65.3.1525-1529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Gaynor R., Srinivasan A., Mapoles J., Farr R. W. trans-activation of viral enhancers including long terminal repeat of the human immunodeficiency virus by the hepatitis B virus X protein. Virology. 1989 Apr;169(2):479–484. doi: 10.1016/0042-6822(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Skolnik P. R., Kosloff B. R., Hirsch M. S. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J Infect Dis. 1988 Mar;157(3):508–514. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- Skolnik P. R., Pomerantz R. J., de la Monte S. M., Lee S. F., Hsiung G. D., Foos R. Y., Cowan G. M., Kosloff B. R., Hirsch M. S., Pepose J. S. Dual infection of retina with human immunodeficiency virus type 1 and cytomegalovirus. Am J Ophthalmol. 1989 Apr 15;107(4):361–372. doi: 10.1016/0002-9394(89)90659-4. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Wade E., Wright D. A., Koval V., Clark C., Jaquish D., Spector S. A. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990 May;64(5):2298–2308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. C., Price R. W. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–693. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Staprans S. I., Rabert D. K., Spector D. H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988 Sep;62(9):3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Puma J. P., Power M. D., Powers M. A., George-Nascimento C., Stephans J. C., Levy J. A., Sanchez-Pescador R., Luciw P. A., Barr P. J. Differential antibody responses of individuals infected with AIDS-associated retroviruses surveyed using the viral core antigen p25gag expressed in bacteria. Virology. 1986 Apr 15;150(1):283–290. doi: 10.1016/0042-6822(86)90289-8. [DOI] [PubMed] [Google Scholar]
- Stellrecht K. A., Sperber K., Pogo B. G. Activation of the human immunodeficiency virus type 1 long terminal repeat by vaccinia virus. J Virol. 1992 Apr;66(4):2051–2056. doi: 10.1128/jvi.66.4.2051-2056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M., Gornitsky M., Wainberg M. A. Active replication of human immunodeficiency virus type 1 by peripheral blood mononuclear cells following coincubation with herpes viruses. J Med Virol. 1989 Oct;29(2):109–114. doi: 10.1002/jmv.1890290207. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Chu K., Robinson W. S. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5168–5172. doi: 10.1073/pnas.86.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Robinson W. S. Hepatitis B virus X gene can transactivate heterologous viral sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2046–2050. doi: 10.1073/pnas.86.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Rosen C. A., Haseltine W. A., Robinson W. S. Identification of a region within the human immunodeficiency virus type 1 long terminal repeat that is essential for transactivation by the hepatitis B virus gene X. J Virol. 1989 Jun;63(6):2857–2860. doi: 10.1128/jvi.63.6.2857-2860.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlach J., Pitha P. M. Herpes simplex virus type 1-mediated induction of human immunodeficiency virus type 1 provirus correlates with binding of nuclear proteins to the NF-kappa B enhancer and leader sequence. J Virol. 1992 Jun;66(6):3616–3623. doi: 10.1128/jvi.66.6.3616-3623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S., Hagemeier C., Sissons J. G., Sinclair J. H. A 10-base-pair element of the human immunodeficiency virus type 1 long terminal repeat (LTR) is an absolute requirement for transactivation by the human cytomegalovirus 72-kilodalton IE1 protein but can be compensated for by other LTR regions in transactivation by the 80-kilodalton IE2 protein. J Virol. 1992 Mar;66(3):1543–1550. doi: 10.1128/jvi.66.3.1543-1550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch A. R., McNally L. M., Gibson W. Cytomegalovirus assembly protein nested gene family: four 3'-coterminal transcripts encode four in-frame, overlapping proteins. J Virol. 1991 Aug;65(8):4091–4100. doi: 10.1128/jvi.65.8.4091-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigdahl B., Kunsch C. Role of HIV in human nervous system dysfunction. AIDS Res Hum Retroviruses. 1989 Aug;5(4):369–374. doi: 10.1089/aid.1989.5.369. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Denaro F. J., Nelson J. A., Lampert P. W., Oldstone M. B. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J Neuropathol Exp Neurol. 1986 Mar;45(2):127–139. doi: 10.1097/00005072-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Wright D. A., Spector D. H. Posttranscriptional regulation of a class of human cytomegalovirus phosphoproteins encoded by an early transcription unit. J Virol. 1989 Jul;63(7):3117–3127. doi: 10.1128/jvi.63.7.3117-3127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. A., Staprans S. I., Spector D. H. Four phosphoproteins with common amino termini are encoded by human cytomegalovirus AD169. J Virol. 1988 Jan;62(1):331–340. doi: 10.1128/jvi.62.1.331-340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]