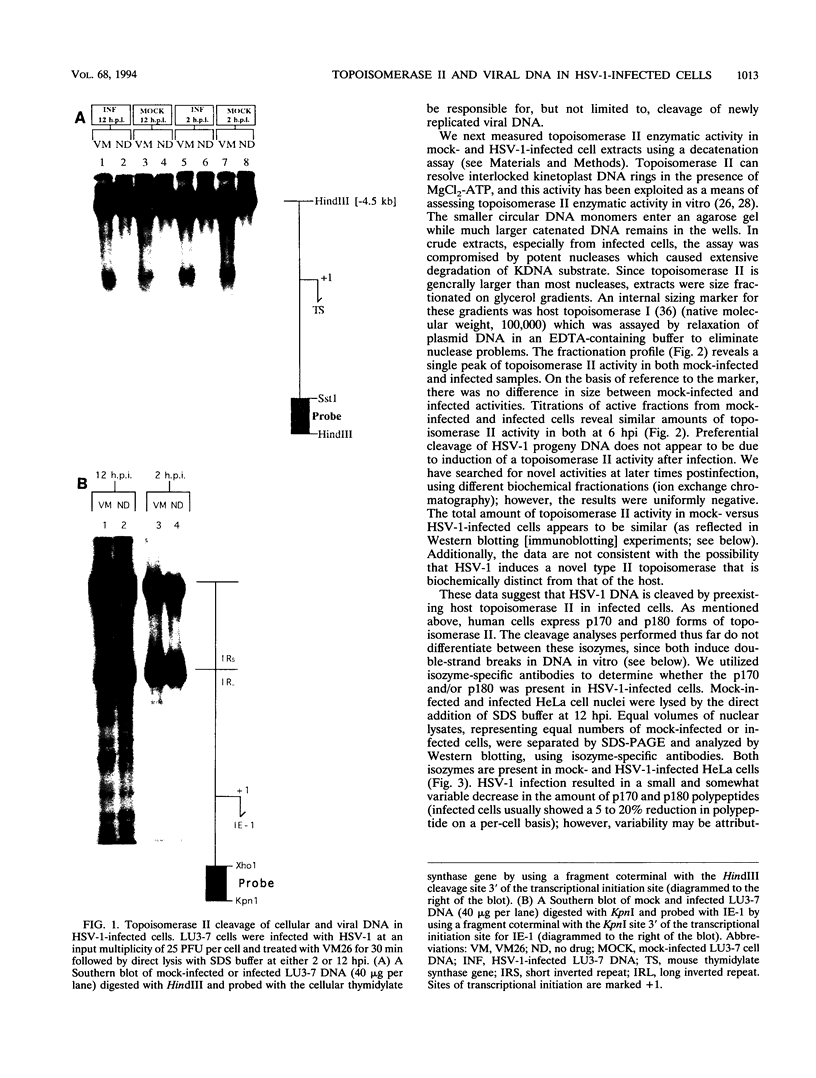
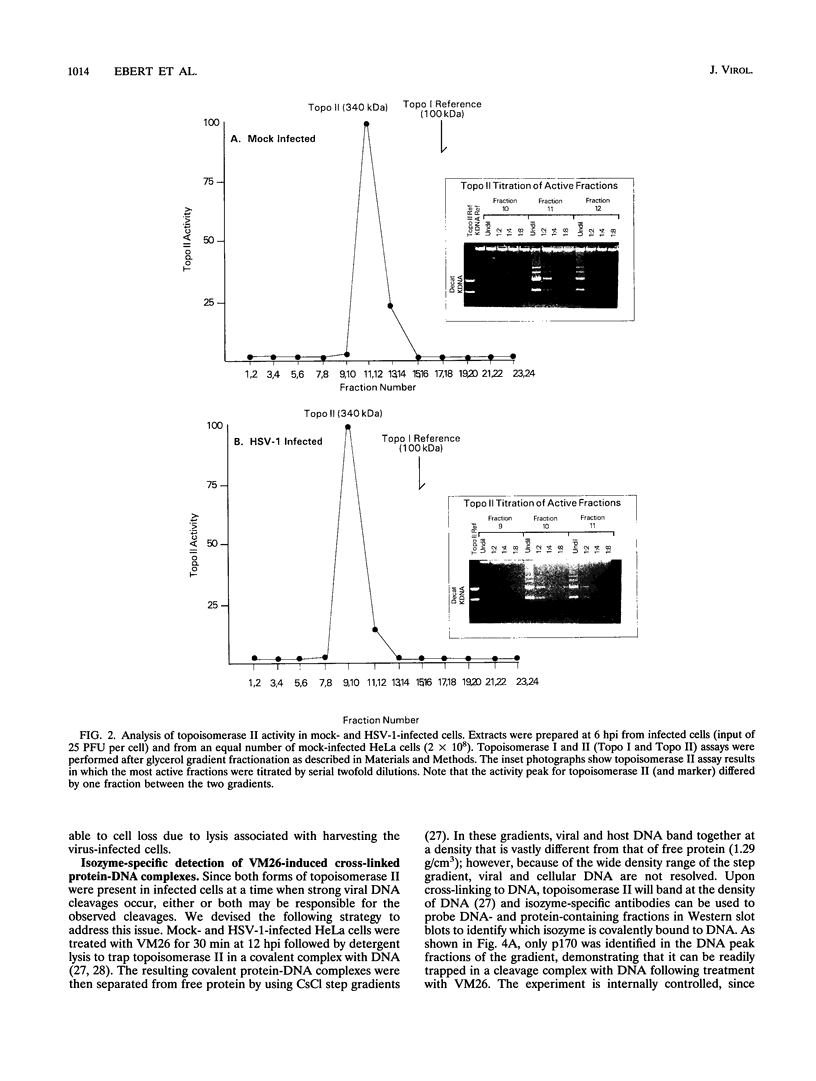
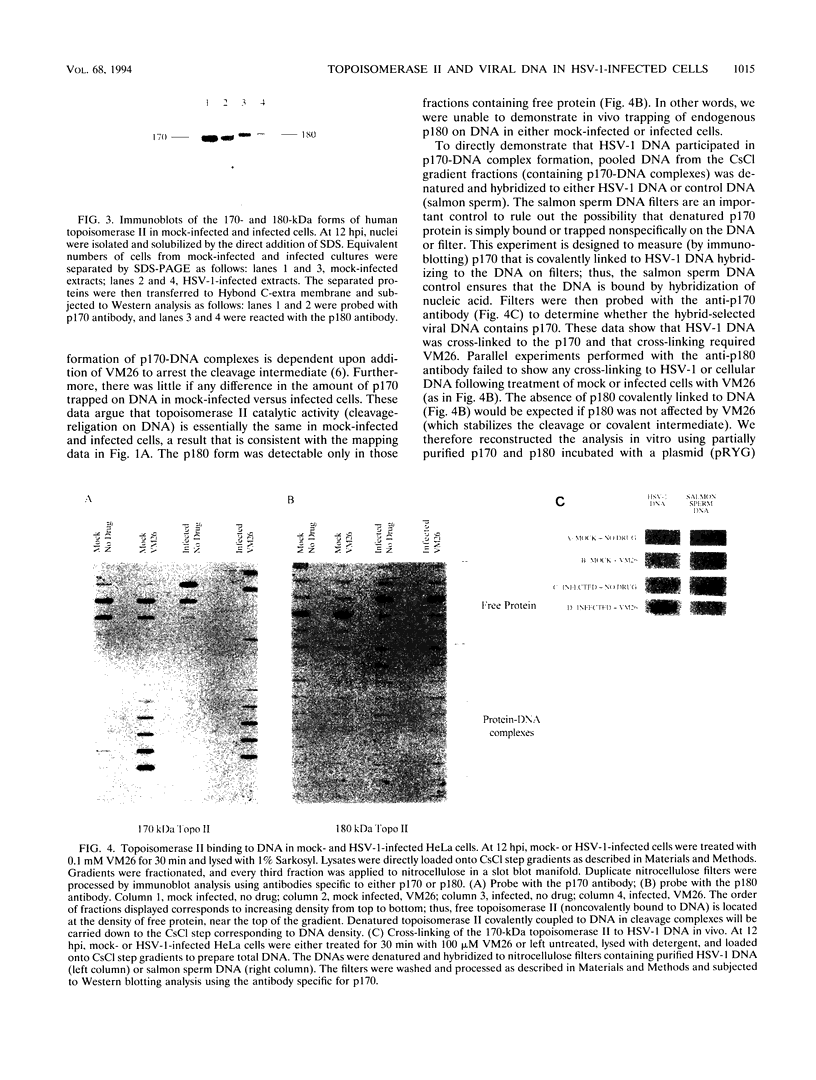
Abstract
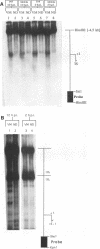
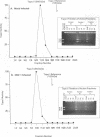
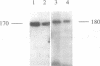
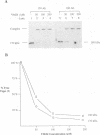
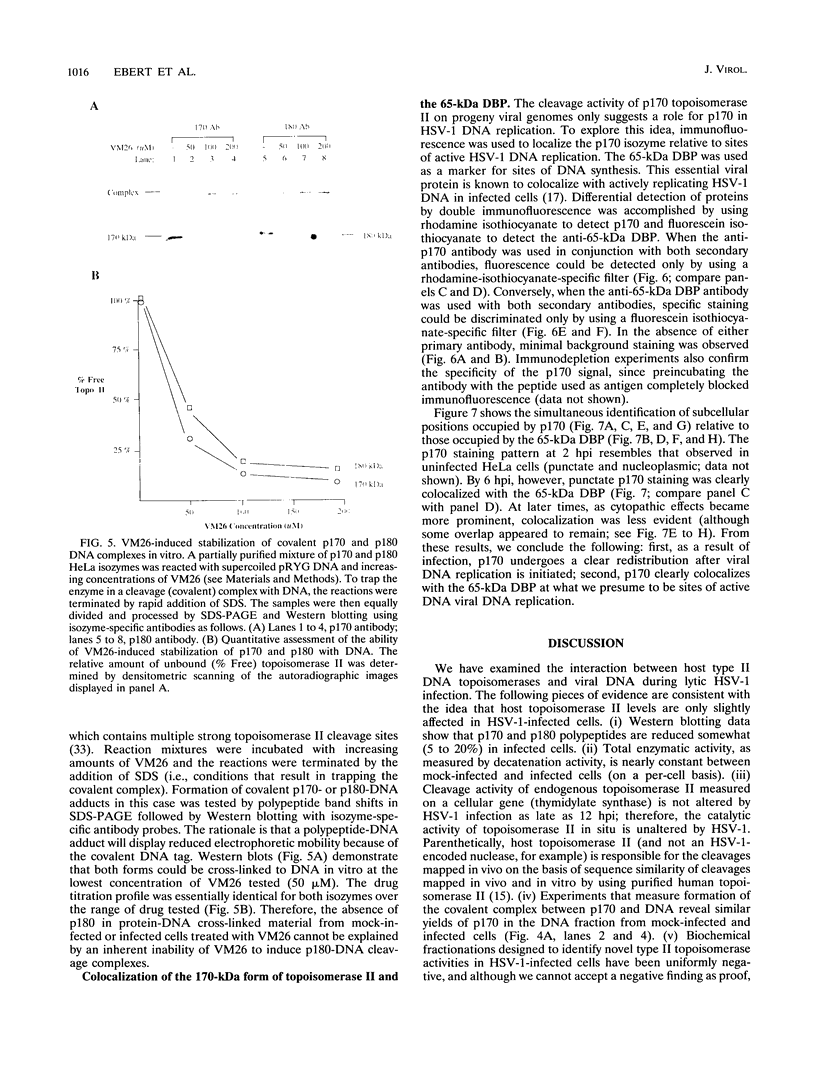
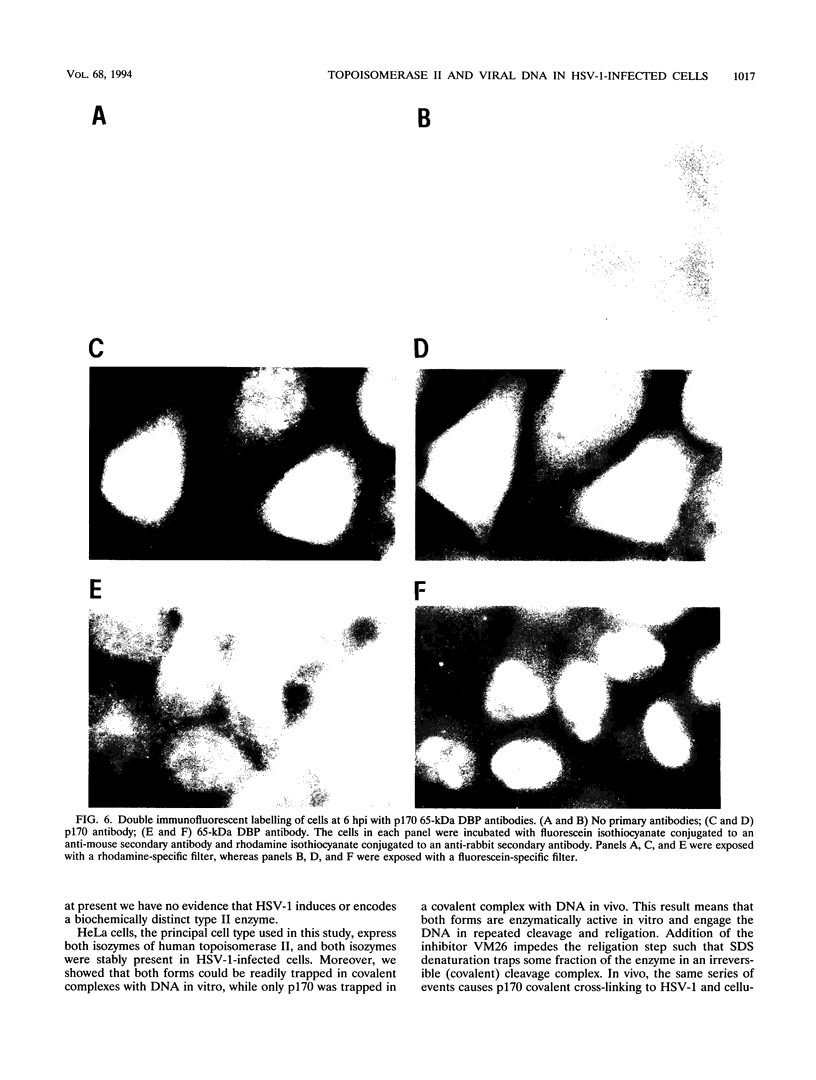
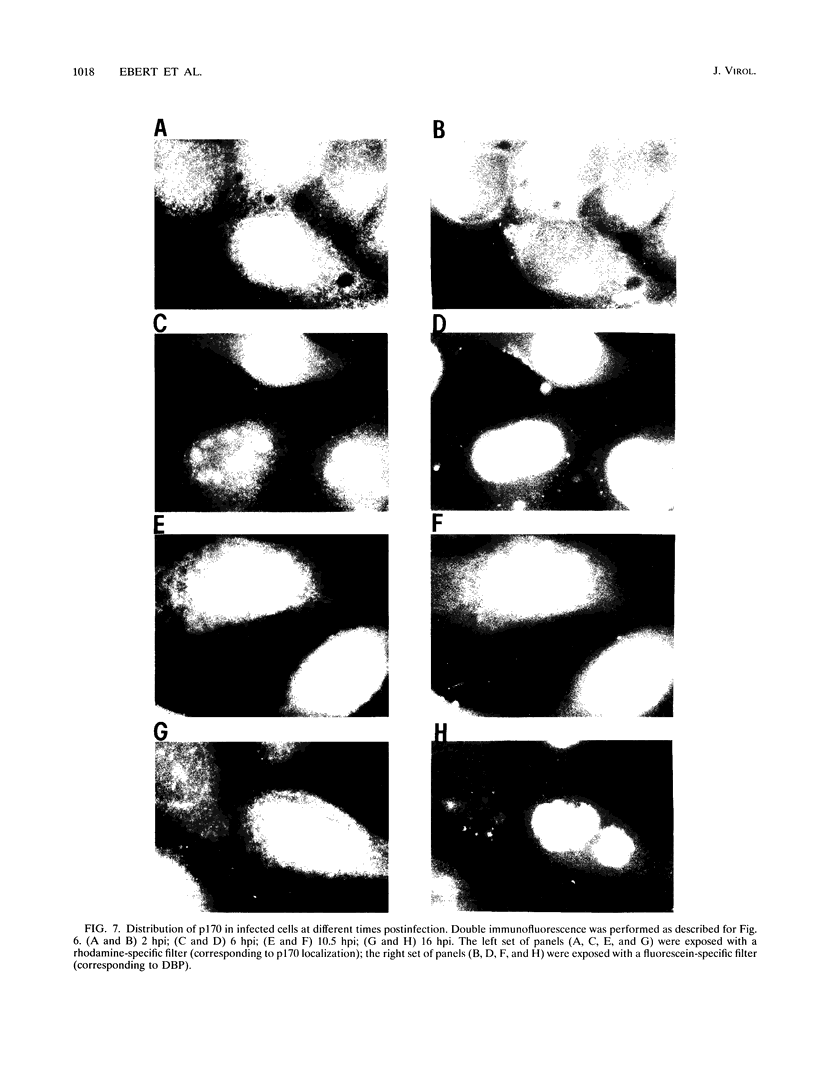
Endogenous host topoisomerase II acts upon herpes simplex virus type 1 (HSV-1) DNA in infected cells (S.N. Ebert, S.S. Shtrom, and M.T. Muller, J. Virol. 56:4059-4066, 1990), and cleavage is directed exclusively at progeny viral DNA while parental DNA is resistant. To evaluate the possibility that HSV-1 induces topoisomerase II activity which could account for the preferential cleavage of progeny viral DNA, we assessed topoisomerase II cleavage activity on cellular and viral DNA substrates before and after the initiation of viral DNA replication. We show that cleavage of a host gene in mock-infected cells was similar to that observed in HSV-1-infected cells, regardless of whether viral DNA replication had occurred. In addition, quantitative measurements revealed comparable amounts of topoisomerase II activity in infected and mock-infected cells; thus, HSV-1 neither induces nor encodes its own type II topoisomerase and cleavages in vivo are due to a preexisting host topoisomerase. Human cells contain two isozymes of topoisomerase II (p170 and p180), encoded by separate genes. Through the use of isozyme-specific antibodies, we demonstrate that only p170 was found to be cross-linked to HSV-1 DNA even though both forms were present at nearly constant levels in HSV-1-infected cells. Immunofluorescence revealed that by 6 h postinfection, p170 becomes redistributed and localized to sites of active viral DNA synthesis. The data suggest that p170 gains preferential access to replicated viral DNA molecules, which explains why topoisomerase II activity is concentrated on progeny DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Luke M., Laemmli U. K. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 1991 Jan 11;64(1):137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Kawasaki I., Ikeda H., Liu L. F. Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2076–2080. doi: 10.1073/pnas.85.7.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. D., Huang E. S. Human cytomegalovirus induces expression of cellular topoisomerase II. J Virol. 1990 Jan;64(1):9–15. doi: 10.1128/jvi.64.1.9-15.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Chung I. K., Muller M. T. Aggregates of oligo(dG) bind and inhibit topoisomerase II activity and induce formation of large networks. J Biol Chem. 1991 May 25;266(15):9508–9514. [PubMed] [Google Scholar]
- Chung T. D., Drake F. H., Tan K. B., Per S. R., Crooke S. T., Mirabelli C. K. Characterization and immunological identification of cDNA clones encoding two human DNA topoisomerase II isozymes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9431–9435. doi: 10.1073/pnas.86.23.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Deng T. L., Li D. W., Jenh C. H., Johnson L. F. Structure of the gene for mouse thymidylate synthase. Locations of introns and multiple transcriptional start sites. J Biol Chem. 1986 Dec 5;261(34):16000–16005. [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan N., Cooke C., Rothfield N. The kinetochore is part of the metaphase chromosome scaffold. J Cell Biol. 1984 Jan;98(1):352–357. doi: 10.1083/jcb.98.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert S. N., Shtrom S. S., Muller M. T. Topoisomerase II cleavage of herpes simplex virus type 1 DNA in vivo is replication dependent. J Virol. 1990 Sep;64(9):4059–4066. doi: 10.1128/jvi.64.9.4059-4066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. D., Rixon F. J., Parris D. S. Kinetics of expression of the gene encoding the 65-kilodalton DNA-binding protein of herpes simplex virus type 1. J Virol. 1989 Jan;63(1):137–147. doi: 10.1128/jvi.63.1.137-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. D., Schaffer P. A., Dorsky D. I., Crumpacker C. S., Parris D. S. Localization of the herpes simplex virus type 1 65-kilodalton DNA-binding protein and DNA polymerase in the presence and absence of viral DNA synthesis. J Virol. 1990 Dec;64(12):5738–5749. doi: 10.1128/jvi.64.12.5738-5749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck M. M., Hittelman W. N., Earnshaw W. C. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1086–1090. doi: 10.1073/pnas.85.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Howard M. T., Lee M. P., Hsieh T. S., Griffith J. D. Drosophila topoisomerase II-DNA interactions are affected by DNA structure. J Mol Biol. 1991 Jan 5;217(1):53–62. doi: 10.1016/0022-2836(91)90610-i. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N. DNA topoisomerases as potential targets of antiviral action. Pharmacol Ther. 1989;43(3):377–395. doi: 10.1016/0163-7258(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C., Miller K. G., Englund P. T. Decatenation of kinetoplast DNA by topoisomerases. J Biol Chem. 1980 Jun 10;255(11):4976–4979. [PubMed] [Google Scholar]
- Muller M. T., Helal K., Soisson S., Spitzner J. R. A rapid and quantitative microtiter assay for eukaryotic topoisomerase II. Nucleic Acids Res. 1989 Nov 25;17(22):9499–9499. doi: 10.1093/nar/17.22.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Mehta V. B. DNase I hypersensitivity is independent of endogenous topoisomerase II activity during chicken erythrocyte differentiation. Mol Cell Biol. 1988 Sep;8(9):3661–3669. doi: 10.1128/mcb.8.9.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Spitzner J. R., DiDonato J. A., Mehta V. B., Tsutsui K., Tsutsui K. Single-strand DNA cleavages by eukaryotic topoisomerase II. Biochemistry. 1988 Nov 1;27(22):8369–8379. doi: 10.1021/bi00422a012. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Biochemical basis for the interactions of type I and type II topoisomerases with DNA. Pharmacol Ther. 1989;41(1-2):223–241. doi: 10.1016/0163-7258(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Schaack J., Schedl P., Shenk T. Topoisomerase I and II cleavage of adenovirus DNA in vivo: both topoisomerase activities appear to be required for adenovirus DNA replication. J Virol. 1990 Jan;64(1):78–85. doi: 10.1128/jvi.64.1.78-85.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzner J. R., Chung I. K., Muller M. T. Eukaryotic topoisomerase II preferentially cleaves alternating purine-pyrimidine repeats. Nucleic Acids Res. 1990 Jan 11;18(1):1–11. doi: 10.1093/nar/18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzner J. R., Muller M. T. A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids Res. 1988 Jun 24;16(12):5533–5556. doi: 10.1093/nar/16.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzner J. R., Muller M. T. Application of a degenerate consensus sequence to quantify recognition sites by vertebrate DNA topoisomerase II. J Mol Recognit. 1989 Sep;2(2):63–74. doi: 10.1002/jmr.300020204. [DOI] [PubMed] [Google Scholar]
- Trask D. K., Muller M. T. Biochemical characterization of topoisomerase I purified from avian erythrocytes. Nucleic Acids Res. 1983 May 11;11(9):2779–2800. doi: 10.1093/nar/11.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai-Pflugfelder M., Liu L. F., Liu A. A., Tewey K. M., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Wang J. C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Uemura T., Tanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986 May;5(5):1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Caron P. R., Kim R. A. The role of DNA topoisomerases in recombination and genome stability: a double-edged sword? Cell. 1990 Aug 10;62(3):403–406. doi: 10.1016/0092-8674(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases: why so many? J Biol Chem. 1991 Apr 15;266(11):6659–6662. [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Woessner R. D., Mattern M. R., Mirabelli C. K., Johnson R. K., Drake F. H. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991 Apr;2(4):209–214. [PubMed] [Google Scholar]
- Wood E. R., Earnshaw W. C. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J Cell Biol. 1990 Dec;111(6 Pt 2):2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Nelson E. M., Liu L. F. In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin. Cell. 1985 May;41(1):127–132. doi: 10.1016/0092-8674(85)90067-4. [DOI] [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechiedrich E. L., Osheroff N. Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J. 1990 Dec;9(13):4555–4562. doi: 10.1002/j.1460-2075.1990.tb07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]