Abstract
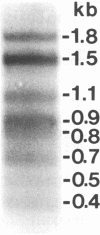
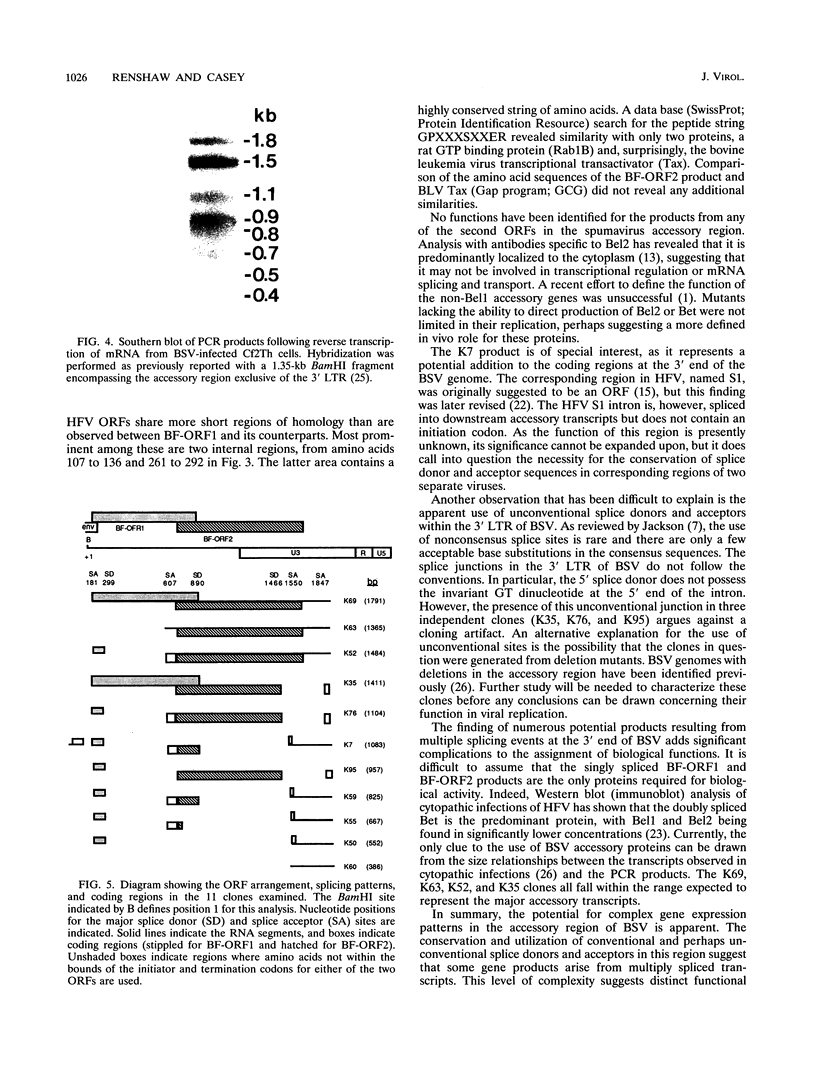
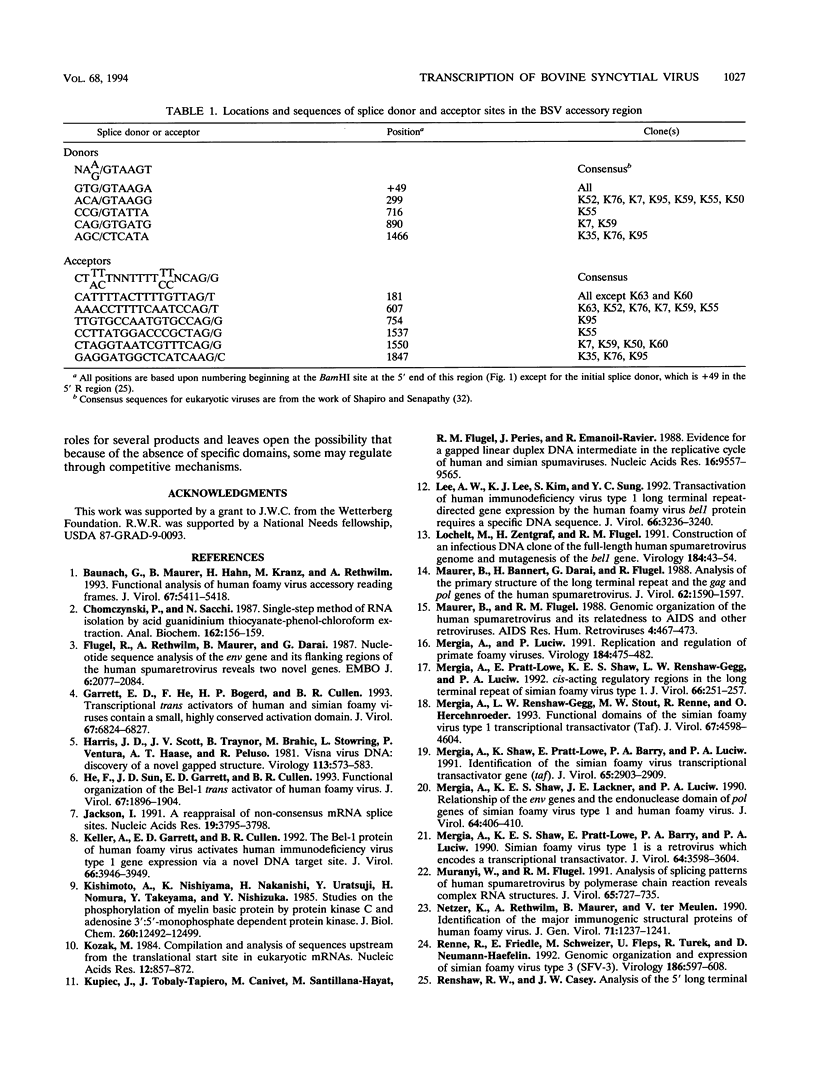
The bovine syncytial virus, a member of the retroviral subfamily Spumavirinae, causes a persistent, asymptomatic infection in cattle. Nucleotide sequence analysis of the viral genome revealed two overlapping reading frames in the 3' region, traditionally occupied by accessory-function genes in other complex retroviruses. In order to analyze the transcripts from the accessory-gene region, we designed oligonucleotide primers complementary to sequences within the 5' and 3' long terminal repeats (LTRs) for use with the PCR. Southern blot analysis of amplification products revealed eight major cDNA bands. Eleven distinct cDNA clones were subsequently isolated and characterized. The initial splice donor in each clone is located 49 bp downstream from the mRNA cap site in the 5' LTR. The primary splice acceptor site was located 17 bp upstream from the proximal 3' open reading frame known as BF-ORF1. A second major splice acceptor was localized to a region upstream of the second open reading frame, BF-ORF2. Clones were identified which spliced directly to each of these sites. Additional splice donor and acceptor sites within BF-ORF1 and BF-ORF2 and the 3' LTR were variously used to generate a complex array of multiply spliced transcripts. Each of these transcripts remained in frame and coded for a potential protein product.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baunach G., Maurer B., Hahn H., Kranz M., Rethwilm A. Functional analysis of human foamy virus accessory reading frames. J Virol. 1993 Sep;67(9):5411–5418. doi: 10.1128/jvi.67.9.5411-5418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Flügel R. M., Rethwilm A., Maurer B., Darai G. Nucleotide sequence analysis of the env gene and its flanking regions of the human spumaretrovirus reveals two novel genes. EMBO J. 1987 Jul;6(7):2077–2084. doi: 10.1002/j.1460-2075.1987.tb02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. D., He F., Bogerd H. P., Cullen B. R. Transcriptional trans activators of human and simian foamy viruses contain a small, highly conserved activation domain. J Virol. 1993 Nov;67(11):6824–6827. doi: 10.1128/jvi.67.11.6824-6827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Scott J. V., Traynor B., Brahic M., Stowring L., Ventura P., Haase A. T., Peluso R. Visna virus DNA: discovery of a novel gapped structure. Virology. 1981 Sep;113(2):573–583. doi: 10.1016/0042-6822(81)90185-9. [DOI] [PubMed] [Google Scholar]
- He F., Sun J. D., Garrett E. D., Cullen B. R. Functional organization of the Bel-1 trans activator of human foamy virus. J Virol. 1993 Apr;67(4):1896–1904. doi: 10.1128/jvi.67.4.1896-1904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J. A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res. 1991 Jul 25;19(14):3795–3798. doi: 10.1093/nar/19.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Garrett E. D., Cullen B. R. The Bel-1 protein of human foamy virus activates human immunodeficiency virus type 1 gene expression via a novel DNA target site. J Virol. 1992 Jun;66(6):3946–3949. doi: 10.1128/jvi.66.6.3946-3949.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec J. J., Tobaly-Tapiero J., Canivet M., Santillana-Hayat M., Flügel R. M., Périès J., Emanoil-Ravier R. Evidence for a gapped linear duplex DNA intermediate in the replicative cycle of human and simian spumaviruses. Nucleic Acids Res. 1988 Oct 25;16(20):9557–9565. doi: 10.1093/nar/16.20.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. H., Lee K. J., Kim S., Sung Y. C. Transactivation of human immunodeficiency virus type 1 long terminal repeat-directed gene expression by the human foamy virus bel1 protein requires a specific DNA sequence. J Virol. 1992 May;66(5):3236–3240. doi: 10.1128/jvi.66.5.3236-3240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löchelt M., Zentgraf H., Flügel R. M. Construction of an infectious DNA clone of the full-length human spumaretrovirus genome and mutagenesis of the bel 1 gene. Virology. 1991 Sep;184(1):43–54. doi: 10.1016/0042-6822(91)90820-2. [DOI] [PubMed] [Google Scholar]
- Maurer B., Bannert H., Darai G., Flügel R. M. Analysis of the primary structure of the long terminal repeat and the gag and pol genes of the human spumaretrovirus. J Virol. 1988 May;62(5):1590–1597. doi: 10.1128/jvi.62.5.1590-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer B., Flügel R. M. Genomic organization of the human spumaretrovirus and its relatedness to AIDS and other retroviruses. AIDS Res Hum Retroviruses. 1988 Dec;4(6):467–473. doi: 10.1089/aid.1988.4.467. [DOI] [PubMed] [Google Scholar]
- Mergia A., Luciw P. A. Replication and regulation of primate foamy viruses. Virology. 1991 Oct;184(2):475–482. doi: 10.1016/0042-6822(91)90417-a. [DOI] [PubMed] [Google Scholar]
- Mergia A., Pratt-Lowe E., Shaw K. E., Renshaw-Gegg L. W., Luciw P. A. cis-acting regulatory regions in the long terminal repeat of simian foamy virus type 1. J Virol. 1992 Jan;66(1):251–257. doi: 10.1128/jvi.66.1.251-257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergia A., Renshaw-Gegg L. W., Stout M. W., Renne R., Herchenröeder O. Functional domains of the simian foamy virus type 1 transcriptional transactivator (Taf). J Virol. 1993 Aug;67(8):4598–4604. doi: 10.1128/jvi.67.8.4598-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergia A., Shaw K. E., Lackner J. E., Luciw P. A. Relationship of the env genes and the endonuclease domain of the pol genes of simian foamy virus type 1 and human foamy virus. J Virol. 1990 Jan;64(1):406–410. doi: 10.1128/jvi.64.1.406-410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergia A., Shaw K. E., Pratt-Lowe E., Barry P. A., Luciw P. A. Identification of the simian foamy virus transcriptional transactivator gene (taf). J Virol. 1991 Jun;65(6):2903–2909. doi: 10.1128/jvi.65.6.2903-2909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergia A., Shaw K. E., Pratt-Lowe E., Barry P. A., Luciw P. A. Simian foamy virus type 1 is a retrovirus which encodes a transcriptional transactivator. J Virol. 1990 Aug;64(8):3598–3604. doi: 10.1128/jvi.64.8.3598-3604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranyi W., Flügel R. M. Analysis of splicing patterns of human spumaretrovirus by polymerase chain reaction reveals complex RNA structures. J Virol. 1991 Feb;65(2):727–735. doi: 10.1128/jvi.65.2.727-735.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer K. O., Rethwilm A., Maurer B., ter Meulen V. Identification of the major immunogenic structural proteins of human foamy virus. J Gen Virol. 1990 May;71(Pt 5):1237–1241. doi: 10.1099/0022-1317-71-5-1237. [DOI] [PubMed] [Google Scholar]
- Renne R., Friedl E., Schweizer M., Fleps U., Turek R., Neumann-Haefelin D. Genomic organization and expression of simian foamy virus type 3 (SFV-3). Virology. 1992 Feb;186(2):597–608. doi: 10.1016/0042-6822(92)90026-l. [DOI] [PubMed] [Google Scholar]
- Renshaw R. W., Gonda M. A., Casey J. W. Structure and transcriptional status of bovine syncytial virus in cytopathic infections. Gene. 1991 Sep 15;105(2):179–184. doi: 10.1016/0378-1119(91)90149-6. [DOI] [PubMed] [Google Scholar]
- Rethwilm A., Darai G., Rösen A., Maurer B., Flügel R. M. Molecular cloning of the genome of human spumaretrovirus. Gene. 1987;59(1):19–28. doi: 10.1016/0378-1119(87)90262-9. [DOI] [PubMed] [Google Scholar]
- Rethwilm A., Erlwein O., Baunach G., Maurer B., ter Meulen V. The transcriptional transactivator of human foamy virus maps to the bel 1 genomic region. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):941–945. doi: 10.1073/pnas.88.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethwilm A., Mori K., Maurer B., ter Meulen V. Transacting transcriptional activation of human spumaretrovirus LTR in infected cells. Virology. 1990 Apr;175(2):568–571. doi: 10.1016/0042-6822(90)90442-t. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Renne R., Neumann-Haefelin D. Structural analysis of proviral DNA in simian foamy virus (LK-3)-infected cells. Arch Virol. 1989;109(1-2):103–114. doi: 10.1007/BF01310521. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. K., Cywinski A., Taylor J. M. Initiation of plus-strand DNA synthesis during reverse transcription of an avian retrovirus genome. J Virol. 1984 Jan;49(1):200–204. doi: 10.1128/jvi.49.1.200-204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaly-Tapiero J., Kupiec J. J., Santillana-Hayat M., Canivet M., Peries J., Emanoil-Ravier R. Further characterization of the gapped DNA intermediates of human spumavirus: evidence for a dual initiation of plus-strand DNA synthesis. J Gen Virol. 1991 Mar;72(Pt 3):605–608. doi: 10.1099/0022-1317-72-3-605. [DOI] [PubMed] [Google Scholar]
- Tobaly-Tapiero J., Santillana-Hayat M., Giron M. L., Guillemin M. C., Rozain F., Périès J., Emanoil-Ravier R. Molecular differences between two immunologically related spumaretroviruses: the human prototype HSRV and the chimpanzee isolate SFV6. AIDS Res Hum Retroviruses. 1990 Jul;6(7):951–957. doi: 10.1089/aid.1990.6.951. [DOI] [PubMed] [Google Scholar]
- Venkatesh L. K., Chinnadurai G. The carboxy-terminal transcription enhancement region of the human spumaretrovirus transactivator contains discrete determinants of the activator function. J Virol. 1993 Jul;67(7):3868–3876. doi: 10.1128/jvi.67.7.3868-3876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh L. K., Theodorakis P. A., Chinnadurai G. Distinct cis-acting regions in U3 regulate trans-activation of the human spumaretrovirus long terminal repeat by the viral bel1 gene product. Nucleic Acids Res. 1991 Jul 11;19(13):3661–3666. doi: 10.1093/nar/19.13.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh L. K., Yang C., Theodorakis P. A., Chinnadurai G. Functional dissection of the human spumaretrovirus transactivator identifies distinct classes of dominant-negative mutants. J Virol. 1993 Jan;67(1):161–169. doi: 10.1128/jvi.67.1.161-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]