Abstract
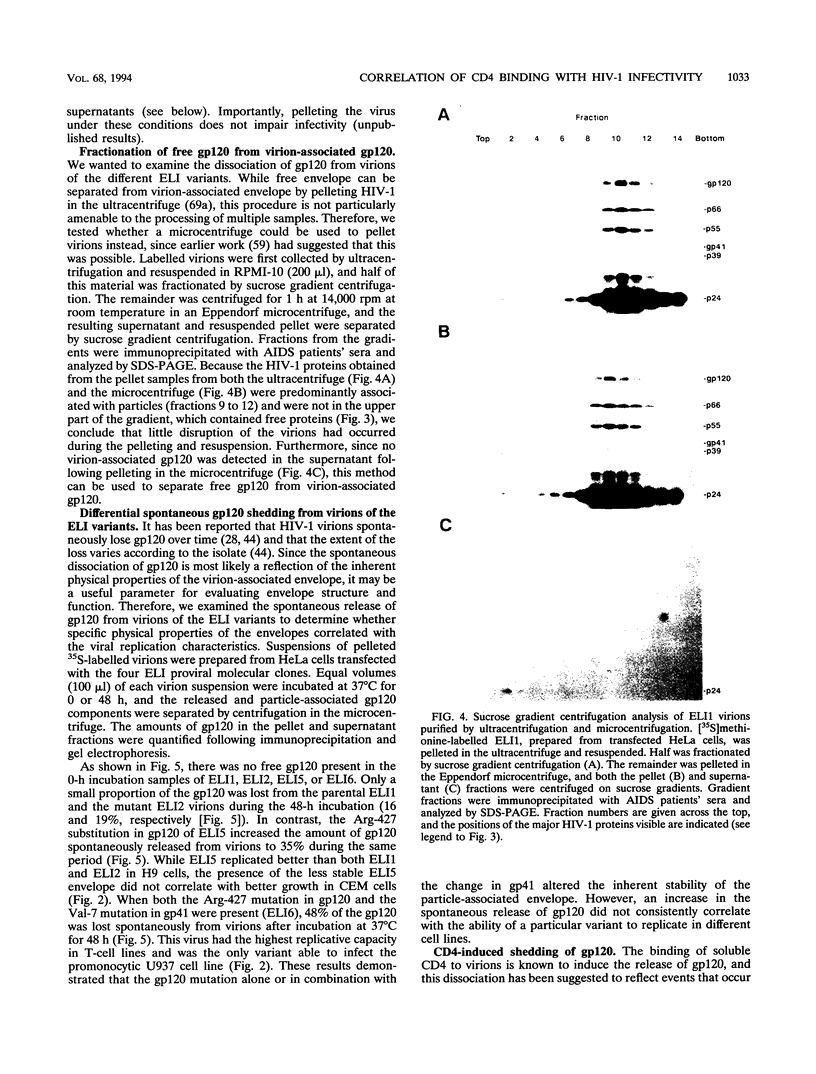
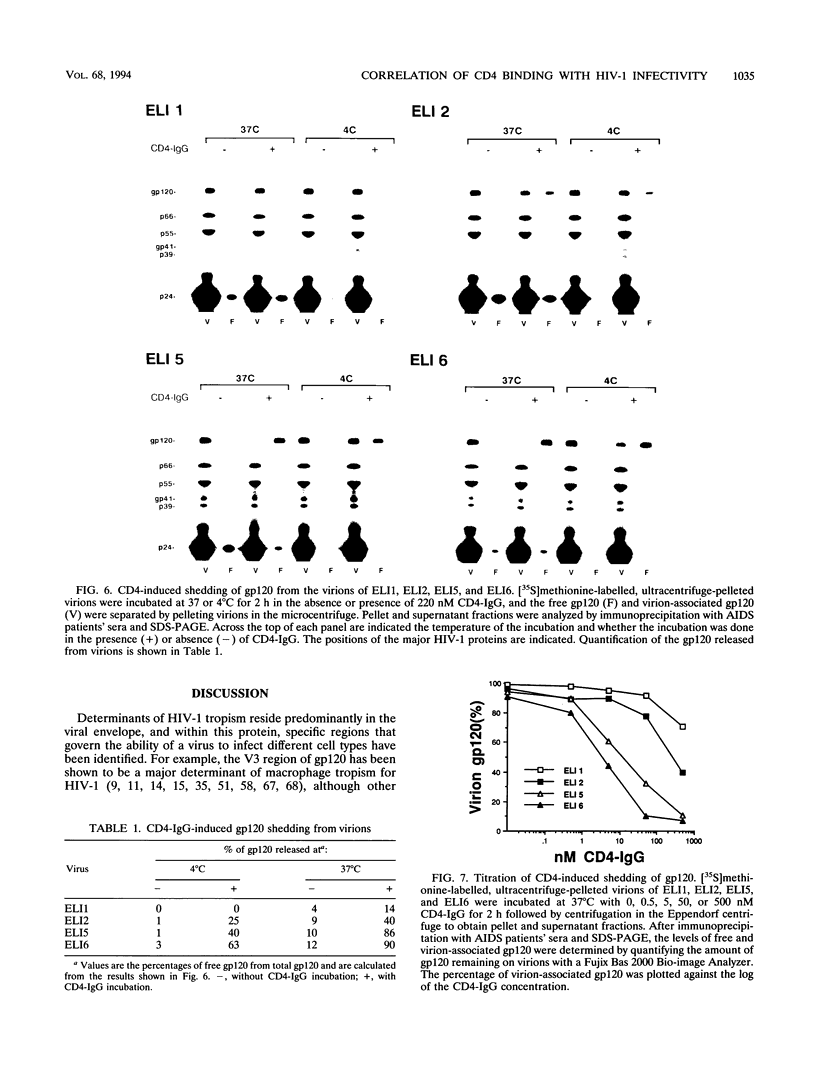
Mutations in the human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins gp120 and gp41, previously shown to confer an enhanced replicative capacity and broadened host range to the ELI1 strain of HIV-1, were analyzed for their biochemical effects on envelope structure and function. The tendency of purified virions to release their extracellular gp120 component, either spontaneously or after interacting with soluble CD4 (CD4-induced shedding) was assessed. A single amino acid substitution in part of the CD4 binding site of gp120 (Gly-427 to Arg) enhanced both spontaneous and CD4-induced shedding of gp120 from virions, while a single change in the fusogenic region of gp41 (Met-7 to Val) affected only CD4-induced shedding. Although each codon change alone conferred increased growth ability, virus with both mutations exhibited the most rapid replication kinetics. In addition, when both of these mutations were present, virions had the highest tendency to shed gp120, both spontaneously and after exposure to soluble CD4. Analysis of CD4 binding to virion-associated gp120 showed that the changes in both gp120 and gp41 contributed to increased binding. These results demonstrated that the increased replicative capacity of the ELI variants in human CD4+ cell lines was associated with altered physical and functional properties of the virion envelope glycoproteins.
Full text
PDF


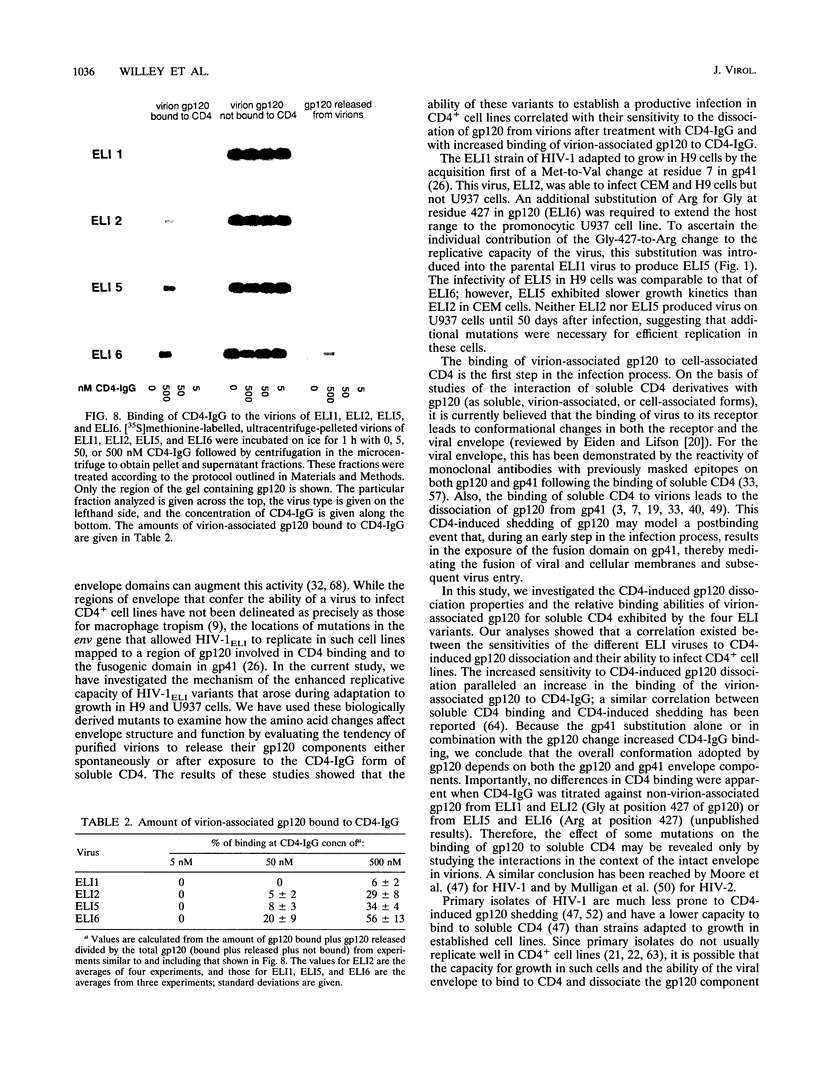



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Lifson J. D., Eiden L. E. Stimulation of glycoprotein gp120 dissociation from the envelope glycoprotein complex of human immunodeficiency virus type 1 by soluble CD4 and CD4 peptide derivatives: implications for the role of the complementarity-determining region 3-like region in membrane fusion. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8082–8086. doi: 10.1073/pnas.88.18.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Sisler J. R., Earl P. L. Human immunodeficiency virus type 1 envelope glycoprotein molecules containing membrane fusion-impairing mutations in the V3 region efficiently undergo soluble CD4-stimulated gp120 release. J Virol. 1992 Oct;66(10):6208–6212. doi: 10.1128/jvi.66.10.6208-6212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron L., Sullivan N., Sodroski J. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J Virol. 1992 Apr;66(4):2389–2397. doi: 10.1128/jvi.66.4.2389-2397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P. HIV antibodies and vaccine design. AIDS. 1989;3 (Suppl 1):S111–S118. doi: 10.1097/00002030-198901001-00016. [DOI] [PubMed] [Google Scholar]
- Bugelski P. J., Ellens H., Hart T. K., Kirsh R. L. Soluble CD4 and dextran sulfate mediate release of gp120 from HIV-1: implications for clinical trials. J Acquir Immune Defic Syndr. 1991;4(9):923–924. [PubMed] [Google Scholar]
- Byrn R. A., Mordenti J., Lucas C., Smith D., Marsters S. A., Johnson J. S., Cossum P., Chamow S. M., Wurm F. M., Gregory T. Biological properties of a CD4 immunoadhesin. Nature. 1990 Apr 12;344(6267):667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Churcher M. J., Boyd M., O'Brien W., Zhao J. Q., Zack J., Chen I. S. The region of the envelope gene of human immunodeficiency virus type 1 responsible for determination of cell tropism. J Virol. 1992 Jan;66(1):305–309. doi: 10.1128/jvi.66.1.305-309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chamow S. M., Mordenti J., Marsters S. A., Gregory T., Mitsuya H., Byrn R. A., Lucas C., Wurm F. M., Groopman J. E. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989 Feb 9;337(6207):525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Levy J. A. Altered host range of HIV-1 after passage through various human cell types. Virology. 1991 Mar;181(1):288–294. doi: 10.1016/0042-6822(91)90494-v. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Shioda T., Levy J. A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991 Dec;65(12):6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Nishio J., Perryman S., Cann A., O'Brien W., Chen I. S., Wehrly K. Identification of human immunodeficiency virus envelope gene sequences influencing viral entry into CD4-positive HeLa cells, T-leukemia cells, and macrophages. J Virol. 1991 Nov;65(11):5782–5789. doi: 10.1128/jvi.65.11.5782-5789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Zajac R. A., Boswell R. N., Gebel H. M., Takahashi H., Berzofsky J. A., Shearer G. M. Detection of cytotoxic T lymphocytes specific for synthetic peptides of gp160 in HIV-seropositive individuals. J Immunol. 1991 Apr 1;146(7):2214–2219. [PubMed] [Google Scholar]
- Cordonnier A., Montagnier L., Emerman M. Single amino-acid changes in HIV envelope affect viral tropism and receptor binding. Nature. 1989 Aug 17;340(6234):571–574. doi: 10.1038/340571a0. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Lifson J. D. HIV interactions with CD4: a continuum of conformations and consequences. Immunol Today. 1992 Jun;13(6):201–206. doi: 10.1016/0167-5699(92)90154-Y. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R., Stålhanske P., von Gegerfelt A., Lind B., Aman P., Rassart E., Fenyö E. M. Biological characterization of infectious molecular clones derived from a human immunodeficiency virus type-1 isolate with rapid/high replicative capacity. Virology. 1991 Mar;181(1):55–61. doi: 10.1016/0042-6822(91)90469-r. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Fujita K., Silver J., Peden K. Changes in both gp120 and gp41 can account for increased growth potential and expanded host range of human immunodeficiency virus type 1. J Virol. 1992 Jul;66(7):4445–4451. doi: 10.1128/jvi.66.7.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F., Waxham M. N., Ross A. M., Hoxie J. A. Sequence similarities between human immunodeficiency virus gp41 and paramyxovirus fusion proteins. AIDS Res Hum Retroviruses. 1987 Fall;3(3):245–252. doi: 10.1089/aid.1987.3.245. [DOI] [PubMed] [Google Scholar]
- Goudsmit J. Immunodominant B-cell epitopes of the HIV-1 envelope recognized by infected and immunized hosts. AIDS. 1988;2 (Suppl 1):S41–S45. doi: 10.1097/00002030-198800001-00006. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groenink M., Andeweg A. C., Fouchier R. A., Broersen S., van der Jagt R. C., Schuitemaker H., de Goede R. E., Bosch M. L., Huisman H. G., Tersmette M. Phenotype-associated env gene variation among eight related human immunodeficiency virus type 1 clones: evidence for in vivo recombination and determinants of cytotropism outside the V3 domain. J Virol. 1992 Oct;66(10):6175–6180. doi: 10.1128/jvi.66.10.6175-6180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Furman C., Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991 Apr;65(4):2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Liu Z. Q., Wood C., Levy J. A., Cheng-Mayer C. The viral envelope gene is involved in macrophage tropism of a human immunodeficiency virus type 1 strain isolated from brain tissue. J Virol. 1990 Dec;64(12):6148–6153. doi: 10.1128/jvi.64.12.6148-6153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney D. J., Hayashi S., Nicklas M., Redfield R. R., Broder S., Wong-Staal F., Mitsuya H. Differences in the interaction of HIV-1 and HIV-2 with CD4. J Acquir Immune Defic Syndr. 1990;3(7):649–657. [PubMed] [Google Scholar]
- Martin M., Weiss R. A. AIDS 1989. Virology: overview. AIDS. 1989;3 (Suppl 1):S3–S4. [PubMed] [Google Scholar]
- McClure M. O., Marsh M., Weiss R. A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- McKeating J. A., McKnight A., Moore J. P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991 Feb;65(2):852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Burkly L. C., Connor R. I., Cao Y., Tizard R., Ho D. D., Fisher R. A. Adaptation of two primary human immunodeficiency virus type 1 isolates to growth in transformed T cell lines correlates with alterations in the responses of their envelope glycoproteins to soluble CD4. AIDS Res Hum Retroviruses. 1993 Jun;9(6):529–539. doi: 10.1089/aid.1993.9.529. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Klasse P. J. Thermodynamic and kinetic analysis of sCD4 binding to HIV-1 virions and of gp120 dissociation. AIDS Res Hum Retroviruses. 1992 Apr;8(4):443–450. doi: 10.1089/aid.1992.8.443. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Huang Y. X., Ashkenazi A., Ho D. D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992 Jan;66(1):235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Norton W. A., Sattentau Q. J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991 Mar;65(3):1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Mulligan M. J., Ritter G. D., Jr, Chaikin M. A., Yamshchikov G. V., Kumar P., Hahn B. H., Sweet R. W., Compans R. W. Human immunodeficiency virus type 2 envelope glycoprotein: differential CD4 interactions of soluble gp120 versus the assembled envelope complex. Virology. 1992 Mar;187(1):233–241. doi: 10.1016/0042-6822(92)90311-c. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Orloff S. L., Kennedy M. S., Belperron A. A., Maddon P. J., McDougal J. S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993 Mar;67(3):1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. J., Tanner C. C., Mulligan M. J., Srinivas R. V., Compans R. W. Oligopeptide inhibitors of HIV-induced syncytium formation. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1289–1296. doi: 10.1089/aid.1990.6.1289. [DOI] [PubMed] [Google Scholar]
- Peden K., Emerman M., Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991 Dec;185(2):661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- Pollard S. R., Meier W., Chow P., Rosa J. J., Wiley D. C. CD4-binding regions of human immunodeficiency virus envelope glycoprotein gp120 defined by proteolytic digestion. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11320–11324. doi: 10.1073/pnas.88.24.11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Sinangil F., Loyter A., Volsky D. J. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 1988 Oct 24;239(1):88–92. doi: 10.1016/0014-5793(88)80551-9. [DOI] [PubMed] [Google Scholar]
- Slepushkin V. A., Kornilaeva G. V., Andreev S. M., Sidorova M. V., Petrukhina A. O., Matsevich G. R., Raduk S. V., Grigoriev V. B., Makarova T. V., Lukashov V. V. Inhibition of human immunodeficiency virus type 1 (HIV-1) penetration into target cells by synthetic peptides mimicking the N-terminus of the HIV-1 transmembrane glycoprotein. Virology. 1993 May;194(1):294–301. doi: 10.1006/viro.1993.1260. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Engleman E. G. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J Biol Chem. 1990 Feb 15;265(5):2640–2649. [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Tersmette M., de Goede R. E., Al B. J., Winkel I. N., Gruters R. A., Cuypers H. T., Huisman H. G., Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988 Jun;62(6):2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Furman C., Helseth E., Repke H., Sodroski J. Lack of correlation between soluble CD4-induced shedding of the human immunodeficiency virus type 1 exterior envelope glycoprotein and subsequent membrane fusion events. J Virol. 1992 Sep;66(9):5516–5524. doi: 10.1128/jvi.66.9.5516-5524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Plata F. Cytotoxic T lymphocytes against HIV. AIDS. 1990 Mar;4(3):177–184. doi: 10.1097/00002030-199003000-00001. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Klimkait T., Frucht D. M., Bonifacino J. S., Martin M. A. Mutations within the human immunodeficiency virus type 1 gp160 envelope glycoprotein alter its intracellular transport and processing. Virology. 1991 Sep;184(1):319–329. doi: 10.1016/0042-6822(91)90848-6. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Martin M. A. Association of human immunodeficiency virus type 1 envelope glycoprotein with particles depends on interactions between the third variable and conserved regions of gp120. J Virol. 1993 Jun;67(6):3639–3643. doi: 10.1128/jvi.67.6.3639-3643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]