Abstract
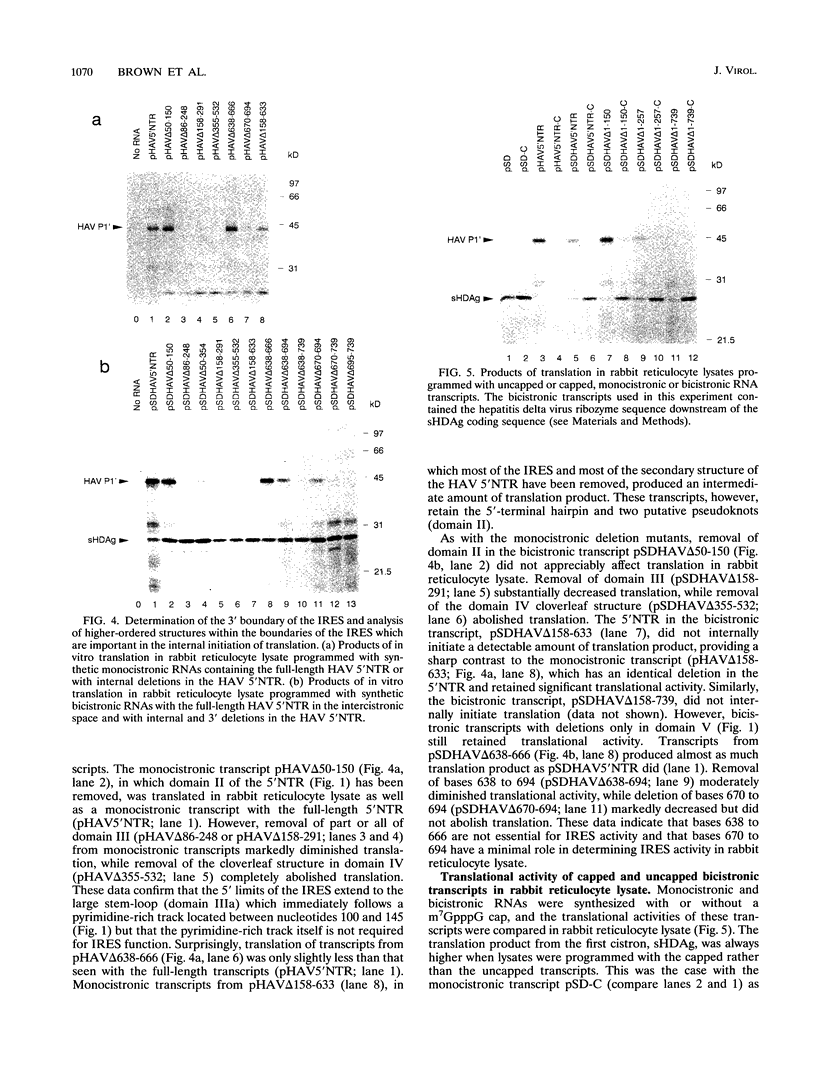
The lengthy 5' nontranslated region (5'NTR) of hepatitis A virus (HAV) forms a highly ordered secondary structure, which has been suggested to play an important role in controlling viral translation by allowing for translation initiation by internal ribosome entry. To test this hypothesis, synthetic bicistronic RNAs, with all or part of the HAV 5'NTR in the intercistronic space, were translated in rabbit reticulocyte lysates. In the presence of an upstream cistron designed to block ribosomal scanning, the HAV 5'NTR was capable of directing the internal initiation of translation, confirming the presence of an internal ribosome entry site (IRES). Analysis of various deletion mutants demonstrated that the 5' border of the IRES is located between nucleotides 151 and 257, while the 3' border extends to the 3' end of the 5'NTR, between nucleotide 695 and the first initiation codon at 735. Except for a segment between bases 638 and 694, deletion of stem-loop structures between bases 151 and the 3' end of the 5'NTR inhibited or abolished translation. The addition of a 5' cap structure (m7GpppN) to monocistronic or bicistronic transcripts decreased the translation of a reporter gene downstream of the HAV 5'NTR but enhanced translation of the upstream cistron in bicistronic transcripts. This finding indicates that a 5' cap structure is inhibitory to HAV IRES-directed translation initiation and that the cap structure and the HAV IRES probably compete for the same limiting translation factors. The efficiency with which monocistronic constructs containing the HAV 5'NTR directed translation in reticulocyte lysates was compared with results for monocistronic constructs containing the IRES of the more rapidly growing encephalomyocarditis virus (EMCV). These results indicated that the HAV 5'NTR was more than 25-fold less active than the EMCV IRES in producing translation product. HAV 5'NTR-directed translation was inhibited by the presence of a one-fifth molar quantity of RNA containing the EMCV IRES, while a fivefold molar excess of the HAV 5'NTR did not inhibit EMCV IRES-directed translation. The relatively weak activity of the HAV IRES may thus be due to a reduced affinity for cellular translation factors which are present in limiting quantities in rabbit reticulocyte lysate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony D. D., Merrick W. C. Eukaryotic initiation factor (eIF)-4F. Implications for a role in internal initiation of translation. J Biol Chem. 1991 Jun 5;266(16):10218–10226. [PubMed] [Google Scholar]
- Borman A., Jackson R. J. Initiation of translation of human rhinovirus RNA: mapping the internal ribosome entry site. Virology. 1992 Jun;188(2):685–696. doi: 10.1016/0042-6822(92)90523-r. [DOI] [PubMed] [Google Scholar]
- Brown E. A., Day S. P., Jansen R. W., Lemon S. M. The 5' nontranslated region of hepatitis A virus RNA: secondary structure and elements required for translation in vitro. J Virol. 1991 Nov;65(11):5828–5838. doi: 10.1128/jvi.65.11.5828-5838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. H., Brown E. A., Lemon S. M. Cell type-specific proteins which interact with the 5' nontranslated region of hepatitis A virus RNA. J Virol. 1993 Nov;67(11):6716–6725. doi: 10.1128/jvi.67.11.6716-6725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. V., Kaufman R. J. The sequence context of the initiation codon in the encephalomyocarditis virus leader modulates efficiency of internal translation initiation. J Virol. 1992 Apr;66(4):1924–1932. doi: 10.1128/jvi.66.4.1924-1932.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S. P., Murphy P., Brown E. A., Lemon S. M. Mutations within the 5' nontranslated region of hepatitis A virus RNA which enhance replication in BS-C-1 cells. J Virol. 1992 Nov;66(11):6533–6540. doi: 10.1128/jvi.66.11.6533-6540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke G. M., Hoffman M. A., Palmenberg A. C. Sequence and structural elements that contribute to efficient encephalomyocarditis virus RNA translation. J Virol. 1992 Mar;66(3):1602–1609. doi: 10.1128/jvi.66.3.1602-1609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S., Maier K., Wiedmann K. H., Sacher M., Thaler H., Vallbracht A. Clonal analysis of infiltrating T lymphocytes in liver tissue in viral hepatitis A. Immunology. 1990 Jan;69(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Glass M. J., Jia X. Y., Summers D. F. Identification of the hepatitis A virus internal ribosome entry site: in vivo and in vitro analysis of bicistronic RNAs containing the HAV 5' noncoding region. Virology. 1993 Apr;193(2):842–852. doi: 10.1006/viro.1993.1193. [DOI] [PubMed] [Google Scholar]
- Glass M. J., Summers D. F. A cis-acting element within the hepatitis A virus 5'-non-coding region required for in vitro translation. Virus Res. 1992 Oct;26(1):15–31. doi: 10.1016/0168-1702(92)90143-w. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Howell M. T., Kaminski A. The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem Sci. 1990 Dec;15(12):477–483. doi: 10.1016/0968-0004(90)90302-r. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Complete nucleotide sequence of a cell culture-adapted variant of hepatitis A virus: comparison with wild-type virus with restricted capacity for in vitro replication. Virology. 1988 Apr;163(2):299–307. doi: 10.1016/0042-6822(88)90270-x. [DOI] [PubMed] [Google Scholar]
- Kaminski A., Howell M. T., Jackson R. J. Initiation of encephalomyocarditis virus RNA translation: the authentic initiation site is not selected by a scanning mechanism. EMBO J. 1990 Nov;9(11):3753–3759. doi: 10.1002/j.1460-2075.1990.tb07588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Luz N., Beck E. Functional analysis of the internal translation initiation site of foot-and-mouth disease virus. J Virol. 1990 Oct;64(10):4625–4631. doi: 10.1128/jvi.64.10.4625-4631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Chen J. H., Sonenberg N., Maizel J. V., Jr Conserved tertiary structural elements in the 5' nontranslated region of cardiovirus, aphthovirus and hepatitis A virus RNAs. Nucleic Acids Res. 1993 May 25;21(10):2445–2451. doi: 10.1093/nar/21.10.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Whetter L., Chang K. H., Brown E. A. Why do human hepatitis viruses replicate so poorly in cell cultures? FEMS Microbiol Lett. 1992 Dec 15;100(1-3):455–459. doi: 10.1111/j.1574-6968.1992.tb14076.x. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Nicholson R., Sonenberg N. In vitro mutational analysis of cis-acting RNA translational elements within the poliovirus type 2 5' untranslated region. J Virol. 1991 Nov;65(11):5895–5901. doi: 10.1128/jvi.65.11.5895-5901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R., Pelletier J., Le S. Y., Sonenberg N. Structural and functional analysis of the ribosome landing pad of poliovirus type 2: in vivo translation studies. J Virol. 1991 Nov;65(11):5886–5894. doi: 10.1128/jvi.65.11.5886-5894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Translational efficiency of poliovirus mRNA: mapping inhibitory cis-acting elements within the 5' noncoding region. J Virol. 1988 Jul;62(7):2219–2227. doi: 10.1128/jvi.62.7.2219-2227.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Chernov B. K., Dmitrieva T. M., Agol V. I. Conservation of the secondary structure elements of the 5'-untranslated region of cardio- and aphthovirus RNAs. Nucleic Acids Res. 1989 Jul 25;17(14):5701–5711. doi: 10.1093/nar/17.14.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Romanova L. I., Sinyakov A. N., Maslova S. V., Agol V. I. Conserved structural domains in the 5'-untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology. 1989 Feb;168(2):201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Gmyl A. P., Maslova S. V., Svitkin Y. V., Sinyakov A. N., Agol V. I. Prokaryotic-like cis elements in the cap-independent internal initiation of translation on picornavirus RNA. Cell. 1992 Jan 10;68(1):119–131. doi: 10.1016/0092-8674(92)90211-t. [DOI] [PubMed] [Google Scholar]
- Scheper G. C., Thomas A. A., Voorma H. O. The 5' untranslated region of encephalomyocarditis virus contains a sequence for very efficient binding of eukaryotic initiation factor eIF-2/2B. Biochim Biophys Acta. 1991 Jun 13;1089(2):220–226. doi: 10.1016/0167-4781(91)90011-a. [DOI] [PubMed] [Google Scholar]
- Tesar M., Harmon S. A., Summers D. F., Ehrenfeld E. Hepatitis A virus polyprotein synthesis initiates from two alternative AUG codons. Virology. 1992 Feb;186(2):609–618. doi: 10.1016/0042-6822(92)90027-m. [DOI] [PubMed] [Google Scholar]
- Trono D., Andino R., Baltimore D. An RNA sequence of hundreds of nucleotides at the 5' end of poliovirus RNA is involved in allowing viral protein synthesis. J Virol. 1988 Jul;62(7):2291–2299. doi: 10.1128/jvi.62.7.2291-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]







