Abstract
Hypericum perforatum (Hp) is commonly known for its antiviral, antidepressant, and cytotoxic properties, but traditionally Hp was also used to treat inflammation. In this study, the anti-inflammatory activity and cytotoxicity of different Hp extractions and accessions and constituents present within Hp extracts were characterized. In contrast to the antiviral activity of Hp, the anti-inflammatory activity observed with all Hp extracts was light-independent. When pure constituents were tested, the flavonoids, amentoflavone, hyperforin, and light-activated pseudohypericin, displayed anti-inflammatory activity, albeit at concentrations generally higher than the amount present in the Hp extracts. Constituents that were present in the Hp extracts at concentrations that inhibited the production of prostaglandin E2 (PGE2) were pseudohypericin and hyperforin, suggesting that they are the primary anti-inflammatory constituents along with the flavonoids, and perhaps the interactions of these constituents and other unidentified compounds are important for the anti-inflammatory activity of the Hp extracts.
Keywords: Hypericum perforatum, St. John’s wort, hyperforin, hypericin, pseudohypericin, quercetin, flavonoids, anti, inflammatory, cytotoxicity, PGE2, RAW264.7
INTRODUCTION
Hypericum perforatum (Hp) is an herbaceous perennial plant native to Europe and Asia (1). Traditionally, Hp extracts were used both externally, for the treatment of inflammation, wounds, and skin diseases, and internally, for the treatment of anxiety, headache, bedwetting, neuralgia, inflammation, and mild-to-moderate depression (2). The use of Hp supplements has been prevalent for many years, but increased use of these supplements and the identification of bioactive constituents present within Hp have intensified interest in the mechanisms by which Hp extracts exert specific bioactivities, such as inhibiting inflammation.
Hp extracts possess anti-inflammatory properties in a variety of in vivo systems. The cyclooxygenase (COX) enzymes metabolize arachidonic acid to eicosanoids. The products of COX-2 metabolism of arachidonic acid are the 2-series prostanoids, of which prostaglandin E2 (PGE2) is important in mediating pain, inflammation, and swelling (3). Raso et al. found that 100 mg/kg by gavage of Hp root dry powder extract twice daily significantly inhibited COX-2 protein levels and significantly reduced carrageenan-induced paw edema in mice (4). Herold et al. reported that a hydroalcoholic Hp extract significantly inhibited 5-lipoxygenase but did not affect COX-2 protein in cell-free systems (5). Mice fed 50–300 mg/kg Hp extract by gavage showed a dose-related and significant inhibition of carrageenan-induced paw edema (6). A 50% ethanol Hp extract administered at both 100 and 200 mg/kg reduced inflammation and analgesia in carrageenan-induced paw edema and cotton pellet-induced granuloma (7), and an Hp extract suppressed inflammatory and leukocyte infiltration in carrageenan-and prostaglandin E1-induced Wistar rats (8).
The minimal dose of Hp to provide a therapeutic effect is unknown; however, Hp treatments for humans generally range from 500 to 650 mg/day and vary depending upon study design (9). Others report that the dosage for a fluid or powder extract would be the amount of extract equivalent to 0.5–3.0 mg of hypericin and pseudohypericin daily (2). Relatively few studies have determined the levels of many constituents present in plasma after administration of Hp extracts. Schulz et al. administered 612 mg of dry Hp extract to 18 healthy male volunteers as a single oral dose for 14 days. The maximal plasma concentrations for hypericin, pseudohypericin, and quercetin were 3.14, 8.5, and 47.7 ng/mL, respectively (10).
The bioactive constituents of Hp extracts are complex and include many different classes of chemicals (11). Two of these classes of constituents are present in only select plant species: naphthodianthrones such as hypericin and pseudohypericin and phloroglucinols like hyperforin (11). Other classes of constituents present within Hp are also present in many plant species; these include the flavonoids and biflavonoids, tannins, procyanidins, and caffeic acid derivatives, among others. The flavonoids present in Hp are quercetin, the aglycone form, and its glycosylated derivatives, quercitrin, isoquercitrin, hyperoside, and rutin, whereas a biflavonoid present in Hp is I3′,II8-biapigenin, also known as amentoflavone.
Although individual constituents have been shown to provide bioactivity alone, the interaction among constituents may account for diverse bioactivities of the supplements. Work by Schmitt et al. supports the role of unknown compounds in the bioactivity of Hp extracts in which chlorogenic acid and porphyrin, which were present in Hp extracts, attenuated hypericin’s light-dependent toxicity in HaCaT keratinocytes extracts (12, 13).
Because comprehensive research on the anti-inflammatory activity of Hp is lacking, to begin to identify anti-inflammatory constituents we hypothesized that Hp extracts made using several extraction procedures and accessions would yield distinct chemical profiles which could be related to the anti-inflammatory activity of the Hp extracts. The goal of this study was to identify Hp extracts from different extraction procedures and Hp accessions that maximize anti-inflammatory activity in RAW264.7 macrophage cells. To assess which constituents may be responsible for the anti-inflammatory activity seen in the extracts, constituents known to be present within Hp were also tested. Because Hp extracts are known to possess cytotoxic properties, the cytotoxicity of these Hp extracts and constituents was assessed. The dependence of the anti-inflammatory activity on light activation of Hp extracts, a treatment condition important for several Hp bioactivities, was also evaluated.
MATERIALS AND METHODS
Plant Material and Extractions
All plant material was obtained from either Frontier Natural Products Co-op (FNPC) (Norway, IA) or the North Central Regional Plant Introduction Station (NCRPIS) (Ames, IA) of the U.S. Department of Agriculture and processed as described in Schmitt et al. (12). Six accessions of Hp were provided by the NCRPIS: Plant Introductions (PI) 325351 and 371528 and the commercial varieties, Common, ‘Medizinal’ (Elixir) (Ames 27452), ‘Helos’ (Ames 27453), and ‘Topas’ (Ames 27455). Accessions PI 325351 and 371528 were collected in the former Soviet Union; Common was grown from seeds supplied by Johnny’s Selected Seeds (Winslow, ME), and the other varieties were grown from seeds supplied by Richter’s Herb Specialists (Goodwood, ON, Canada). ‘Topas’, bred to increase overall commercial production, was developed in Germany, and Elixir, bred to contain a higher amount of napthodianthrones, and ‘Helos’, bred for tolerance to anthracnose disease, were developed in Denmark (14, 15).
Six grams of dried plant material was extracted by either Soxhlet extraction for 6 h or room temperature shaking for 24 h, evaporated to dryness, and dissolved in 15 mL of dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) as described previously (12). Extracts were stored at −30 °C in the dark and used as stock solutions for treatments. Because preliminary testing determined that the highest amount of DMSO that could be added onto the cells was 0.1% of the media and because the Hp extracts were dissolved in DMSO, each stock extract was added at a final DMSO concentration of 0.1% of the media that was added onto the RAW264.7 cells for an initial test of anti-inflammatory activity. Thus, the extracts were initially compared at different (μg/mL) concentrations on the basis of adding 0.1% DMSO to allow comparison of the relative anti-inflammatory activity of the constituents extracted from 6 g of dried plant material. Hp extracts screened in this way will be referred to as “highest concentration tested” in this paper. The stock extract was further diluted in DMSO to allow for comparisons of the extracts at the same (μg/mL) concentration.
Endotoxin levels of the plant extracts were assayed using the Limulus Amebocyte Lysate Test (BioWhittaker, Inc., Walkersville, MD) because the presence of high levels of endotoxin in the extracts would stimulate the macrophages to release inflammatory mediators including PGE2 Endotoxin levels ranged from 0.001 to 0.2 endotoxin units per milliliter (EU/mL). Because the extracts are further diluted in media, the range of endotoxin levels present in the RAW264.7 macrophage cell media was 0.000001–0.0002 EU/mL. Pure endotoxin up to 5 EU/mL did not significantly increase the RAW264.7 cells’ production of PGE2 in the assay (data not shown).
Chemicals
Hypericin was purchased from Molecular Probes (Eugene, OR) and pseudohypericin from Calbiochem-Novabiochem (La Jolla, CA). Chlorogenic acid, quercetin, hyperoside, hyperforin, and rutin were purchased from Fisher Scientific (Hanover Park, IL), and quercitrin, isoquercitrin, and amentoflavone were purchased from ChromaDex (Santa Ana, CA).
Cell Culture
RAW264.7 macrophages were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in high-glucose Dulbecco’s Modified Eagle’s medium (4500 mg/L D-glucose) (Invitrogen, Carlsbad, CA) and supplemented with 100 IU/mL penicillin/streptomycin (Invitrogen) and 10% fetal bovine serum (FBS) (Invitrogen). Cells were maintained in a 5% CO2 incubator with 70% humidity at 37 °C until 70% confluent in 75 cm2 flasks.
Cell Treatments
Cells were plated at a density of 1.0 × 105 cells/well in 24-well cell culture plates and allowed to attach overnight. Cells were incubated with or without 1 μg/mL lipopolysaccharide (LPS) (Escherichia coli 02B:B6) (Sigma) and solvent alone, DMSO, or plant extract or constituent simultaneously for 8 h. DMSO concentration did not exceed 0.1% of the media, an amount determined by preliminary testing. Four controls were included in each treatment: media alone, media and DMSO, media and LPS, and media and LPS and DMSO. In addition, 10 μM quercetin and 6 μM baicalien were used as positive controls to ensure the assay was working properly.
After initial testing of FNPC plant material in ambient light, the anti-inflammatory and cytotoxicity screening was always performed in both light-activated and dark conditions, due to the well-known light-activated properties of the naphthodianthrone compounds present within the Hp extracts. Experimental conditions for light activation were as previously described (12). Cell supernatants were collected on ice and stored in a −70 °C freezer for use in the PGE2 assay as described below.
PGE2 Assay
The supernatant samples were assayed with a Prostaglandin E2 EIA kit (GE Biosciences, Piscataway, NJ) according to the manufacturer’s instructions. Supernatants were diluted 1:15 in water to ensure the concentrations of PGE2 present within the samples were within the linear range of the standard curve for the assay.
Cytotoxicity Assay
CellTiter96 Aqueous One Solution cell proliferation assay (Promega Corp., Madison, WI) was used as previously described in Schmitt et al. with an 8 h treatment incubation to parallel the anti-inflammatory studies (12). Following the 8 h incubation, treatment solutions were removed and fresh media and CellTiter96 dye were added for 3 h and 15 min (12). The metabolized dye solutions were transferred to 96-well plates for absorbance measurement at 490 nm. The number of viable cells for each treatment was compared to the media + DMSO solvent control. Light-activated and dark treatments of 20 μM hypericin were used as positive controls to ensure the assay was working properly.
LC-MS-UV Analysis
Samples in DMSO were diluted 1:2 with methanol prior to injection into an Agilent Technologies 100 ion trap liquid chromatography–electron spray ionization–mass spectrometer, with a coupled UV absorption detector (LC-MS-UV). Standards were injected in triplicate for each concentration, and extracts were injected in duplicate. A Synergi Max-RP 150 × 4.6 mm column (Phenomenex, Torrance, CA) was used for analytical separation. For the mobile phase an acetonitrile/methanol (ACN/MeOH) 9:1 v/v (A) and 10 mM ammonium acetate (B) gradient was used. The gradient consisted of 85% A/15% B in 10 min to 80% A/20% B, then to 100% B in 25 min and held for 5 min at 40 °C. The flow rate was 0.75 mL/min (16). External calibration curves for standards were constructed from coupled UV absorption data at 254 nm.
Concentrated stock Hp extracts were analyzed by LC-MS-UV, and calculations were made to estimate the concentration of constituents present in the amount of Hp extract used as a treatment at the highest concentration tested. The data are presented in this way for comparison between the amount of constituents needed to observe an effect and the amount of constituent that would be present in the Hp extracts when tested at the highest concentration.
Statistical Analysis
The anti-inflammatory data were logarithmically transformed to eliminate unequal variances and skewed distribution. An F-protected two-way ANOVA was used followed by a Tukey–Kramer test for multiple comparisons for all PGE2 samples (17). For the anti-inflammatory data in the tables, the data are shown as mean percent reduction in LPS-induced PGE2 levels ± the 95% confidence interval as compared to the media + LPS + DMSO control. For the anti-inflammatory data presented in the figures, original PGE2 levels ± standard error are shown and statistical significance was determined by an F-protected two-way ANOVA followed by a Tukey–Kramer test for multiple comparisons as compared to the media + LPS + DMSO control. For cytotoxicity, data are presented as mean percent reduction in cell viability ± standard error, and the p value was adjusted using the Dunnett–Hsu method for multiple comparisons against the media + DMSO control (18). To determine light versus dark differences, the data were compared using the Tukey–Kramer test for multiple comparisons. For LC-MS-UV detection of compounds in NCRPIS Hp extracts, data are presented as mean ± standard error, and differences in concentrations of constituents for each extract were determined using a one-way ANOVA for each constituent followed by a Tukey–Kramer test. p values of <0.05 were considered to be statistically significant.
RESULTS
Anti-inflammatory Activity of Extracts of FNPC Hp Plant Material Prepared by Different Extraction Procedures
To determine the relative bioactivity of material within the Hp extracts made by different extraction procedures, an initial screen was conducted using the FNPC Hp extracts. None of the extracts made from FNPC plant material significantly reduced PGE2 levels when added to the media without LPS (data not shown). Soxhlet extracts generally reduced LPS-induced PGE2 levels greater than room temperature extracts (room temperature extracts not shown), and all of the Soxhlet extracts significantly reduced PGE2 levels at the highest concentration tested (Table 1). Although the HPLC analysis suggested that the Soxhlet and room temperature extracts had similar chemical profiles, the Soxhlet method extracted more plant material than the room temperature shaking method (12). Only the room temperature 70% ethanol (–chloroform) extract tested at 65 μg/mL significantly reduced LPS-induced PGE2 levels by 66%. The other room temperature extracts tested [70% ethanol, chloroform, hexane, and 70% ethanol (–hexane)] reduced LPS-induced PGE2 levels from 0 to 46% at concentrations ranging from 6 to 74 μg/mL. Although the Soxhlet hexane extract caused the greatest reduction in LPS-induced PGE2 levels (81%) at the lowest concentration (17 μg/mL) tested in the screening among the Soxhlet FNPC extracts, Soxhlet chloroform and Soxhlet ethanol extracts were used for the remaining studies for two reasons. First, ethanol extracts are used primarily in the supplement industry, and, second, chloroform extracts do not contain the light-activated hypericin and pseudohypericin compounds, which allows for determination of the anti-inflammatory nature of other compounds in the extract under light-activated and dark treatment conditions.
Table 1.
Anti-inflammatory Activity of Extracts of FNPC Hp Plant Material Prepared by Different Soxhlet Extraction Procedures of Dried Hp and Tested at Concentrations That Represent the Relative Amount Extracted from 6 g of Plant Material
| Soxhlet extraction | [μg/mL] testeda | % reduction in LPS-induced PGE2b (95% CI) |
|---|---|---|
| 70% ethanol | 116 | 78 (64–87)** |
| chloroform | 43 | 79 (64–87)** |
| hexane | 17 | 81 (61–91)** |
| 70% ethanol (–hexane) | 65 | 73 (55–84)** |
| 70% ethanol(–chloroform) | 69 | 82 (62–91)** |
Concentration tested in μ g/mL represents the final concentration of the extract in the media. Anti-inflammatory activity
[mean percent reduction in LPS-induced PGE2 levels as compared to media + LPS + DMSO control (95% confidence intervals)] was screened using the PGE2 assay (n = 4 for each) in ambient light. Addition of LPS to the culture media + DMSO control increased the level of PGE2 24-fold over media + DMSO control alone (0.08 ± 0.03 ng/mL for media + DMSO, 1.9 ± 0.3 ng/mL for media + DMSO + LPS). Extracts in the culture media without LPS did not affect the concentration of PGE2 as compared to the media + DMSO control.
, p value < 0.0001 as compared to control.
The cytotoxicity of the FNPC Hp extracts was reported in Schmitt et al. (12). All of the ethanol and chloroform extracts and the Soxhlet hexane extract possessed significant cytotoxicity against NIH3T3 mouse fibroblasts, SW480 human colon cancer cells, and HaCaT human keratinocytes. The concentrations of the extracts tested in Schmitt et al. (12) were higher than those used in the initial anti-inflammatory screen due to the greater sensitivity of RAW264.7 macrophage cells to DMSO.
Quantification of Constituents Present within NCRPIS Hp Plant Material Extracts
The NCRPIS Soxhlet ethanol and chloroform extracts were characterized by LC-MS-UV for detection of 10 known constituents present within the extracts. Chlorogenic acid was one of the most abundant constituents detected in the accessions (Table 2). Higher levels of chlorogenic acid were observed for Soxhlet ethanol Common, ‘Helos’, and Elixir than PI 325351, PI 371528, and ‘Topas’. Rutin was the most abundant flavonoid detected in all accessions. Higher levels of rutin were observed for Soxhlet ethanol Common, PI 325351, ‘Helos’, PI 371528, ‘Topas’, and Elixir than ‘Topas’ and PI 371528. Levels of hyperforin, isoquercitrin, hyperoside, quercitrin, amentoflavone, hypericin, and pseudohypericin in the Soxhlet ethanol extracts differed among the accessions. Very few constituents were detected in the Soxhlet chloroform extracts, but hyperforin was the most abundant. Quercitrin was detected in the Soxhlet chloroform Common and ‘Helos’ extracts, amentoflavone was detected in the Soxhlet chloroform Common, ‘Helos’, and ‘Topas’ extracts, and hypericin was detected in the Soxhlet chloroform Elixir extract.
Table 2.
Constituents Identified and Quantified within NCRPIS Hp Extractsa
| compound concn (μM)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| accession | extract | chlorogenic acid | rutin | hyperoside | isoquercitrin | quercitrin | quercetin | amentoflavone | pseudo hypericin | Hyperiicin | hyperforin |
| Common | ethanol | 25 ± 0.1 b | 15 ± 0.02 b | 5 ± 0.02 b | 1 ± 0.01 b | 0.2 ± 0.02 b | 1 ± 0.02 c | 0.5 ± 0.006 a | 1 ± 0.05 c | 0.2 ± 0.05 a | 22 ± 0.05 |
| chloroform | – | – | – | – | 0.08 ± 0.02 a | – | detected | – | – | 8 ± 0.05 c | |
| PI 325351 | ethanol | 13 ± 0.5 a | 13 ± 0.1 b | 3 ± 0.03 a | 0.5 ± 0.006 a | 0.2 ± 0.02 b | 0.5 ± 0.02 b | 0.2 ± 0.06 a | 0.5 ± 0.003 b | 0.1 ± 2.5 a | 11 ± 0.02 c |
| chloroform | – | – | – | – | – | – | – | – | – | 5 ± 0.04 b | |
| PI 371528 | ethanol | 11 ± 0.4 a | 8 ± 0.06 a | 4 ± 0.02 ab | 0.9 ± 0.01 b | 0.1 ± 0.02 ab | 0.2 ± 0.02 a | 0.2 ± 0.06 a | 0.3 ± 0.006 a | 0.06 ± 3.9 a | 17 ± 0.03 d |
| chloroform | – | – | – | – | – | – | – | – | – | 7 ± 0.01 bc | |
| ‘Helos’ | ethanol | 28 ± 0.2 b | 13 ± 0.5 b | 7 ± 0.03 c | 2 ± 0.0006 c | 0.3 ± 0.02 c | 0.8 ± 0.02 c | 2 ± 0.06 b | 1 ± 0.01 c | 0.4 ± 0.0005 b | 70 ± 0.02 e |
| chloroform | – | – | – | – | 0.07 ± 0.02 a | – | detected | – | – | 6 ± 0.04 b | |
| Elixir | ethanol | 25 ± 0.2 b | 11 ± 0.03 b | 7 ± 0.05 c | 0.8 ± 0.01 b | 0.2 ± 0.02 b | 0.5 ± 0.02 b | 0.7 ± 0.06a | 0.7 ± 0.04 bc | 0.6 ± 0.0004 c | 11 ± 0.05 c |
| chloroform | – | – | – | – | – | – | – | – | detected | – | |
| ‘Topas’ | ethanol | 10 ± 0.1 a | 9 ± 0.02 a | 3 ± 0.06 a | 0.4 ± 0.02 a | 0.1 ± 0.02 ab | 0.2 ± 0.02 a | detected | 0.2 ± 0.05 a | 0.04 ± 0.005 a | 8 ± 0.02 b, c |
| chloroform | – | – | – | – | – | – | – | – | – | 1 ± 0.04 a | |
Compounds identified and quantified by LC-MS-UV analysis. Hp extracts were analyzed in the concentrated stock extract, and calculations were made to estimate the concentration of constituents present in the amount of Hp extract used for treatment denoted as highest concentration tested in this paper. Ten metabolites from 12 Soxhlet Hp extracts were quantified. The data are represented as mean concentration ± standard error. “Detected” indicates detection by the MS; however, the amount was too low for quantification with the UV absorption data. “–” represents compounds not detected by the MS. Mean values within each column with different letters were significantly different (a < b < c < d < e) (p < 0.05), and values with more than one letter were not significantly different from means sharing either of the letters.
Anti-inflammatory Activity and Cytotoxicity of NCRPIS Hp Accession Extracts
An initial screen was conducted using the NCRPIS Soxhlet ethanol and chloroform Hp accession extracts at the concentration extracted from 6 g of dried plant material. None of the extracts significantly reduced the level of PGE2 produced without LPS (data not shown). No differences between light-activated and dark treatments were observed, and data were pooled across this variable for presentation. The accession Elixir (8 μg/mL) Soxhlet chloroform extract reduced LPS-induced PGE2 levels (43% reduction) at the lowest concentration observed in this screening (Table 3). All Soxhlet ethanol extracts of Hp accessions were able to significantly reduce LPS-induced PGE2 levels, albeit at higher concentrations than in the Soxhlet chloroform extracts. Next, we tested both the Soxhlet chloroform and Soxhlet ethanol extracts of four Hp accessions at 8 μg/mL (Figure 1). Accession Elixir reduced LPS-induced PGE2 levels in both the Soxhlet chloroform and Soxhlet ethanol extracts. The other accessions exhibited little anti-inflammatory activity at this dose.
Table 3.
Anti-inflammatory Activity and Cytotoxicity of NCRPIS Accessions of Dried Hp Plant Material Tested To Represent the Relative Amount Extracted from 6 g of Plant Material
| chloroform
|
ethanol
|
|||||
|---|---|---|---|---|---|---|
| accession | [μ g/mL] testeda | % reduction in LPS-induced PGE2b (95% CI) | % reduction in cell viabilityc (± SE) | [μ g/mL] testeda | % reduction in LPS-induced PGE2b (95% CI) | % reduction in cell viabilityc (± SE) |
| Common | 14 | 50 (7–74)* | 35 ± 4* | 73 | 91 (87–94)** | 38 ± 5* |
| PI 325351 | 15 | 66 (36–82)* | 41 ± 7** | 147 | 93 (83–93)** | 40 ± 6* |
| PI 371528 | 12 | 80 (62–89)** | 49 ± 3** | 65 | 93 (84–93)** | 41 ± 7** |
| ‘Helos’ | 16 | 73 (49–85)* | 49 ± 3** | 181 | 93 (85–93)** | 35 ± 2* |
| Elixir | 8 | 43 (35–51)* | 23 ± 11 | 122 | 89 (75–89)** | 36 ± 4* |
| ‘Topas’ | 29 | 85 (73–92)** | 32 ± 3* | 110 | 92 (83–93)** | 41 ± 4* |
The concentration tested in μ g/mL represents the final concentration of the extract in the media. Anti-inflammatory activity
[mean percent reduction in LPS-induced PGE2 levels as compared to media + LPS + DMSO control (95% confidence intervals)] and cytotoxicity
(mean percent reduction in cell viability as compared to media + DMSO control-treated cells ± standard error) of Hp extracts (n = 8 for each). Data represent light-activated and dark treatments combined as there were no significant differences between the light-activated and dark treatments for any of the extracts. Addition of LPS to the culture media + DMSO control increased the level of PGE2 36-fold over media + DMSO control alone (0.16 ± 0.03 ng/mL for media + DMSO, 2.9 ± 0.22 ng/mL for media + DMSO + LPS). Extracts in the culture media without LPS did not affect the concentration of PGE2 as compared to the media + DMSO control.
, p value < 0.05 as compared to control.
, p value < 0.0001 as compared to control.
Figure 1.
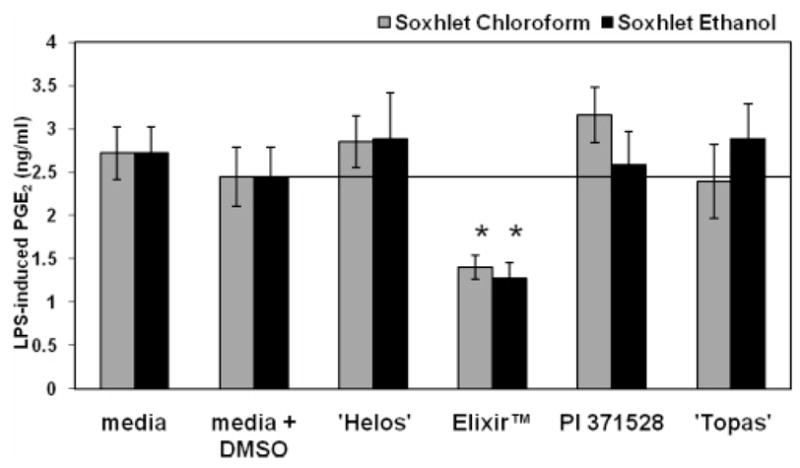
Anti-inflammatory activity was screened using the PGE2 assay (n = 8 for each). Data are presented as mean LPS-induced PGE2 level ± standard error. Data represent light-activated and dark treatments combined as there were no significant differences between light-activated and dark treatments for each extract. Addition of LPS to the culture media +DMSO control increased the level of PGE2 20-fold over media +DMSO control alone (0.1 ± 0.05 ng/mL for media + DMSO, 2.4 ± 0.3 ng/mL for media + LPS + DMSO). Extracts in the culture media without LPS did not affect the concentration of PGE2 as compared to the media + DMSO control. *, p < 0.05, as compared to media + DMSO control.
The cytotoxicity of the NCRPIS Hp extracts was also assessed. There was no significant difference between light-activated and dark treatments for any of the Hp extracts, and data were pooled across this variable. All Hp extracts tested at their highest concentration produced significant cytotoxicity as compared to the solvent control except for the Soxhlet chloroform Elixir extract at 8 μg/mL (Table 3). Reductions in PGE2 levels ranged from 89 to 93% and from 43 to 85% for Soxhlet ethanol and chloroform extracts, respectively, whereas the percent reductions in cell viability were 35–41 and 23–49%, respectively. Therefore, some of the reduction in PGE2 may have been due to cytotoxicity in the RAW264.7 macrophage cells. However, none of the Hp extracts assayed at 8 μg/mL produced statistically significant cytotoxicity (4–23% reduction in cell viability in chloroform extracts, 23–28% reduction in cell viability in ethanol extracts), suggesting that the anti-inflammatory activity of accession Elixir at 8 μg/mL (43 and 47% reductions for chloroform and ethanol, respectively) was not simply due to cytotoxicity of the cells (Table 4). To further support this, no reductions in LPS-induced PGE2 levels were observed with ‘Helos’, PI 371528, and ‘Topas’ at 8 μg/mL, despite 17–22% reduction in cell viability, suggesting that this range of cytotoxicity was not directly reflected in PGE2 levels in this assay (Table 4).
Table 4.
Cytotoxicitya of NCRPIS Soxhlet Ethanol Extracts of Accessions Common, PI 371528, Elixir, ‘Helos’, and ‘Topas’
| accession | extract | [μg/mL] testedb | % reduction in cell viabilityc (± SE) |
|---|---|---|---|
| Common | ethanol | 30 | 30 ± 6 |
| 15 | 20 ± 9 | ||
| 11.5 | 16 ± 13 | ||
| 8 | 17 ± 10 | ||
| PI 371528 | ethanol | 30 | 44 ± 8* |
| 15 | 42 ± 6* | ||
| 11.5 | 34 ± 13* | ||
| 8 | 22 ± 3 | ||
| PI 371528 | chloroform | 8 | 22 ± 10 |
| Elixir | ethanol | 30 | 37 ± 3* |
| 15 | 24 ± 8 | ||
| 11.5 | 25 ± 9 | ||
| 8 | 23 ± 10 | ||
| 5 | 18 ± 6 | ||
| 1 | 15 ± 11 | ||
| Elixir | chloroform | 8 | 23 ± 11 |
| ‘Helos’ | ethanol | 8 | 23 ± 10 |
| ‘Helos’ | chloroform | 8 | 4 ± 8 |
| ‘Topas’ | ethanol | 8 | 27 ± 11 |
| ‘Topas’ | chloroform | 8 | 22 ± 10 |
Cytotoxicity
(mean percent reduction in cell viability as compared to medium + DMSO control-treated cells ± standard error) of Hp extracts (n = 8 for each).
The concentration tested in μg/mL represents the final concentration of the extract in the media. Data represent light-activated and dark treatments combined as there were no significant differences between the light-activated and dark treatments for any of the extracts.
, p value < 0.05 as compared to control.
To further evaluate the activities of these Hp extracts, dose–response studies were conducted for Soxhlet ethanol extracts of accessions Common, PI 371528, and Elixir. Soxhlet ethanol extracts of accessions PI 371528 and Elixir showed a dose-dependent inhibition of PGE2 at higher concentrations (Figure 2). At lower concentrations, only accession Elixir was able to significantly reduce PGE2. Significant cytotoxicity was observed for PI 371528 at 11.5, 15, and 30 μg/mL and for Elixir at 30 μg/mL (Table 4). Thus, some of the reductions in PGE2 levels in these Soxhlet ethanol Hp accession extracts could be due to cytotoxicity.
Figure 2.

Anti-inflammatory activity was screened using the PGE2 assay (n = 8 for each). Data are presented as mean LPS-induced PGE2 level ± standard error. Controls were the same for each accession tested and are represented as a single bar. Elixir at 5 and 1 μg/mL did not significantly reduce PGE2 levels as compared to control with values of 1.7 ± 0.3 and 2.1 ± 0.5, respectively (data not shown). Data represent light-activated and dark treatments combined as there were no significant differences between light-activated and dark treatments for each extract. Addition of LPS to the culture media + DMSO control increased the level of PGE2 13-fold over media + DMSO control alone (0.1 ± 0.02 ng/mL for media + DMSO, 1.7 ± 0.2 ng/mL for media + LPS + DMSO). Extracts in the culture media without LPS did not affect the concentration of PGE2 as compared to the media + DMSO control. *, p < 0.05, as compared to media + DMSO control.
Anti-inflammatory Activity and Cytotoxicity of Constituents Identified within Hp Extracts
The anti-inflammatory activity of constituents identified within Hp extracts (hyperforin, quercetin, quercitrin, isoquercitrin, rutin, hyperoside, amentoflavone, chlorogenic acid, pseudohypericin, and hypericin) was studied. Because there was no significant difference between dark and light-activated treatments for most constituents, data were pooled across this variable in Table 5. Pseudohypericin and hypericin were the only constituents that displayed differences between dark and light-activated treatments and are displayed in Figure 3. Hyperforin significantly decreased PGE2 levels at 40 and 80 μM. Quercetin significantly reduced PGE2 at 5–40 μM (Table 5). Quercitrin and isoquercitrin reduced PGE2 levels at 5–20 μM. Rutin was the only flavonoid that did not significantly reduce LPS-induced PGE2 levels at the doses tested. Amentoflavone significantly reduced PGE2 levels at 10 μM. Chlorogenic acid did not reduce PGE2 levels at concentrations up to 40 μM. The range of concentrations of the individual constituents tested for bioactivity spanned the amounts detected in the extracts as tested at the highest concentration for each extract (Table 2). Constituents that were not detected within the extracts or detected at levels too low to quantify were not included in this concentration range. Although amentoflavone concentrations in the Hp extracts at their highest concentration ranged from 0.2 to 2 μM, because 10 μM amentoflavone was needed to significantly reduce PGE2 levels, amentoflavone was not tested at concentrations below 1 μM.
Table 5.
Anti-inflammatory Activitya and Cytotoxicity of Constituents Identified within Hp Extracts
| class | constituent | concn (μM) | % reduction in LPS- induced PGE2b (95% CI) | % reduction in cell viabilityc (± SE) |
|---|---|---|---|---|
| phloroglucinol | hyperforin | 0.02, 2, 5, 10 | 0 (0–36) | 3 ± 8 |
| 20 | 14 (0–43) | 22 ± 6 | ||
| 40 | 48 (22–66)* | 28 ± 4 | ||
| 80 | 62 (43–75)* | 34 ± 11 | ||
| flavonoid | quercetin | 0.2, 2 | 0 (0–52) | 0 ± 4 |
| 5 | 66 (21–85)* | 1 ± 13 | ||
| 10 | 87 (78–92)** | 14 ± 15 | ||
| 20 | 93 (84–97)** | 29 ± 12 | ||
| 40 | 96 (90–98)** | 33 ± 11 | ||
| quercitrin | 0.02, 0.2 | 0 (0–60) | 0 ± 4 | |
| 2 | 36 (0–72) | 3 ± 8 | ||
| 5 | 54 (11–77)* | 9 ± 9 | ||
| 10 | 67 (35–83)* | 18 ± 20 | ||
| 20 | 71 (22–87)* | 33 ± 15 | ||
| isoquercitrin | 0.02 | 0 (0–47) | 0 ± 5 | |
| 0.2 | 9 (0–53) | 0 ± 12 | ||
| 2 | 31 (0–64) | 13 ± 7 | ||
| 5 | 60 (8–83)* | 22 ± 12 | ||
| 10 | 62 (23–85)* | 23 ± 13 | ||
| 20 | 66 (43–94)* | 40 ± 8* | ||
| rutin | 0.2, 2, 5 | 0 (0–41) | 0 ± 12 | |
| 10 | 7 (0–57) | 17 ± 8 | ||
| 20 | 17 (0–59) | 18 ± 7 | ||
| 40 | 24 (10–60) | 19 ± 16 | ||
| hyperoside | 0.2 | 8 (0–33) | 22 ± 20 | |
| 2 | 27 (0–63) | 29 ± 10 | ||
| 5 | 42 (0–70) | 33 ± 17 | ||
| 10 | 51 (5–75)* | 37 ± 13 | ||
| 20 | 56 (15–78)* | 35 ± 6* | ||
| biflavonoid | amentoflavone | 1 | 38 (0–73) | 8 ± 4 |
| 5 | 53 (0–79) | 13 ± 5 | ||
| 10 | 77 (46–90)* | 15 ± 14 | ||
| other | chlorogenic acid | 1, 5, 10, 20, 40 | 0 (0–54) | 0 ± 6 |
Anti-inflammatory activity
[mean percent reduction in LPS-induced PGE2 level as compared to media + LPS + DMSO control (95% confidence intervals)] and cytotoxicity
(mean percent reduction in cell viability as compared to media + DMSO control-treated cells ± standard error) (n = 8 for anti-inflammatory treatments; n = 8 for cytotoxicity treatments) of pure compounds identified within Hp extracts on RAW264.7 macrophage cells. Data represent light-activated and dark treatments combined as there was no significant difference between light-activated versus dark treatments. Pure compounds in the culture media without LPS did not affect the concentration of PGE2 as compared to the media + DMSO control. Addition of LPS to the culture media + DMSO control increased the level of PGE2 15–35-fold over media + DMSO control alone.
, p value < 0.05 as compared to control.
, p value < 0.0001 as compared to control.
Figure 3.
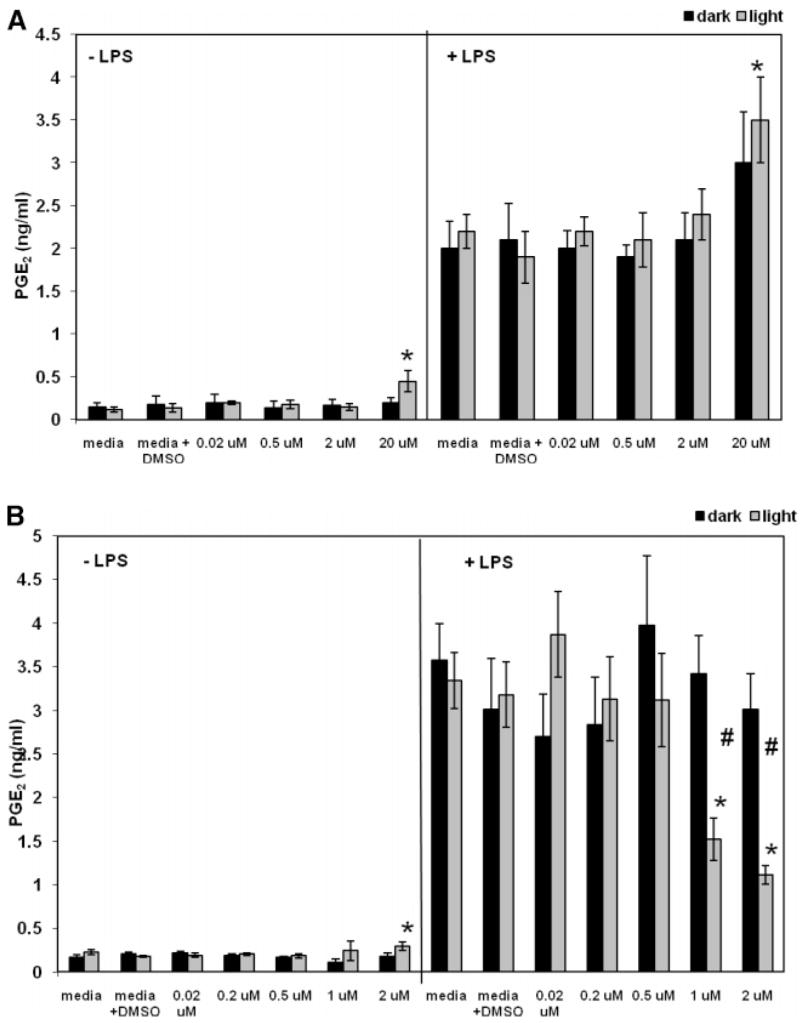
Anti-inflammatory activity (mean PGE2 level ± standard error) of hypericin and pseudohypericin was screened using the PGE2 assay (n = 4). Addition of LPS to the culture media + DMSO control increased the level of PGE2 12-fold over media + DMSO control alone (0.18 ± 0.09 ng/mL for media + DMSO, 2.1 ± 0.3 ng/mL for media + LPS + DMSO) for pseudohypericin and 18-fold (0.17 ± 0.02 ng/mL for media + DMSO, 3.0 ± 0.6 ng/mL for media + DMSO + LPS) for hypericin. *, p < 0.05, as compared to media + DMSO or media + LPS + DMSO control. #, p < 0.05, significant difference between light-activated and dark treatments for corresponding dose.
The cytotoxicity of the constituents identified within Hp extracts was also assessed. No significant differences between light-activated and dark treatments were observed for constituents except for pseudohypericin and hypericin (Table 6 and Figure 3A). Significant cytotoxicity was observed with 20 μM isoquercitrin and hyperoside (40 and 35% reductions in cell viability, respectively) (Table 5). However, 20 μM isoquercitrin and hyperoside also significantly reduced PGE2 levels to a greater extent (66 and 56% reductions, respectively) (Table 6), suggesting that although there was cytotoxicity present at this dose, it probably did not account for all of the reduction in PGE2.
Table 6.
Cytotoxicitya of Hypericin and Pseudohypericin
| % reduction in cell viabilityb (± SE)
|
||||
|---|---|---|---|---|
| treatment | concn (μM) | dark | light-activated | light vs dark statistical significance |
| hypericin | 0.02 | 0 ± 6 | 4 ± 8 | – |
| 0.5 | 0 ± 12 | 8 ± 3 | – | |
| 2 | 8 ± 13 | 15 ± 4 | # | |
| 20 | 23 ± 5 | 86 ± 3** | ## | |
| pseudohypericin | 0.02 | 0 ± 13 | 7 ± 9 | – |
| 0.2 | 10 ± 8 | 12 ± 8 | – | |
| 0.5 | 22 ± 4 | 17 ± 11 | – | |
| 1 | 20 ± 5 | 30 ± 4* | # | |
| 2 | 26 ± 10 | 43 ± 7* | # | |
The cytotoxicity
(mean percent reduction in cell viability as compared to medium + DMSO control-treated cells ± standard error) of pseudohypericin and hypericin was tested in RAW264.7 macrophage cells in both light-activated and dark treatments (n = 4 for each).
, p value < 0.05 as compared to control.
, p value < 0.0001 as compared to control.
, p value < 0.05 when light and dark treatments were compared.
, p value < 0.0001 when light and dark treatments were compared.
Hypericin at 2 μM produced significantly greater cytotoxicity in light-activated treatments than in the dark, with no reductions in PGE2 (Table 6). Hypericin at 20 μM increased PGE2 levels both with and without LPS in the light-activated condition (Figure 3A). Hypericin at 20 μM produced significant cytotoxicity in light-activated conditions but not in the dark, and a significant difference between light-activated and dark treatments was observed (Table 6). Hypericin at 20 μM exhibited 86% reduction in cell viability while significantly increasing PGE2 levels; thus, the increase in PGE2 by 20 μM hypericin may have been attenuated by this cytotoxicity.
Pseudohypericin exhibited light-activated effects on both PGE2 levels and cytotoxicity. When light-activated at 2 μM, pseudohypericin slightly but significantly increased PGE2 levels as compared to control when LPS was not added (Figure 3B). Pseudohypericin at 1 and 2 μM significantly reduced LPS-induced PGE2 levels in light-activated but not dark treatments (Figure 3B). Pseudohypericin at 0.02, 0.2, and 0.5 μM did not alter PGE2 production without or with LPS. Pseudohypericin at 1 and 2 μM produced significant cytotoxicity in light-activated conditions, and there were significant differences between light-activated and dark treatments for 1 and 2 μM pseudohypericin (Table 6). The cytotoxicity of light-activated pseudohypericin may have contributed to the reduction in PGE2 production in the light-activated condition.
DISCUSSION
Many studies have assessed the anti-inflammatory activity of Hp extracts both in cell culture and in vivo (4–8). These previous studies usually examined one Hp extract made from one extraction procedure and from a single accession of Hp. The present paper expands upon the earlier studies; however, because our work involved assessing anti-inflammatory activity by only one assay, LPS-induced PGE2 production, other anti-inflammatory endpoints must be studied to further extend these observations. The identification of accessions and extraction procedures that exhibit greater anti-inflammatory activity with less cytotoxicity may lead to improved Hp botanical supplements. Furthermore, although cytotoxicity and antiviral studies are often performed in light-activated and dark treatment conditions, the light dependence of the anti-inflammatory activity of Hp extracts had not been studied. To our knowledge, this is the first study demonstrating that the anti-inflammatory activity of Hp extracts is light-independent.
The Hp accessions tested displayed different anti-inflammatory activities at the highest concentrations tested with the greatest inhibition of PGE2 production (93% reduction) with Soxhlet ethanol accessions of PI 325351, PI 371528, and ‘Helos’, which may reflect the genetic background or developmental differences among accessions. Accession Elixir clearly exhibited the greatest anti-inflammatory activity at lower concentrations in both Soxhlet chloroform and Soxhlet ethanol extractions. It is interesting that no differences were observed between light-activated and dark treatments for anti-inflammatory activity, contrary to previously reported results for antiviral and other bioactivities (1, 12, 13, 19), suggesting that the constituents present within Hp extracts that exerted anti-inflammatory activity were not dependent on light activation. Furthermore, both Soxhlet ethanol and Soxhlet chloroform Elixir extracts exhibited similar anti-inflammatory activities at 8 μg/mL, suggesting that the compounds responsible for anti-inflammatory activity may be extracted in both ethanol and chloroform. However, the only common constituent that was detected by LC-MS-UV analysis in both of the Elixir extracts was hypericin. Hypericin tested as a pure constituent did not reduce PGE2 levels, suggesting that unknown compounds within these two extracts may explain the greater anti-inflammatory activity of Elixir.
Because hypericin has been shown to inhibit 12-lipoxygenase (20) and inhibit the release of arachidonic acid in human granulocytes (21), treatments with hypericin were performed to determine the effect of light activation on anti-inflammatory activity. Hypericin showed no reduction in LPS-induced PGE2 levels as compared to controls in light-activated or dark treatments up to concentrations of 20 μM. Because hypericin concentrations between 0.05 and 0.2 μM would be present in the highest concentration of extract tested, it is unlikely that hypericin present within the Hp extract was reducing PGE2 levels. The concentration of pure hypericin needed to observe an effect was 20 μM, but this was a pro-inflammatory effect, and this dose was significantly cytotoxic to the cells. This is the first report of hypericin’s effect on PGE2 levels in LPS-induced RAW264.7 macrophages.
Pseudohypericin displayed light-activated properties. Pseudohypericin reduced PGE2 with LPS and increased PGE2 without LPS. In a lipoxygenase activity assay, pseudohypericin inhibited 12-lipoxygenase, although light conditions were not described (20). These results support the role of pseudohypericin as an anti-inflammatory compound in stimulated cells, although data from Hp extracts presented here suggest that compounds responsible for the anti-inflammatory activity of extracts were not light-activated. The concentration of pseudohypericin needed to observe an anti-inflammatory effect was 1 μM, whereas the concentration of pseudohypericin present in the highest concentration of extract tested was 0.3–1 μM; thus, it is possible that pseudohypericin contributed at least to some extent to the anti-inflammatory activity of Hp extracts.
Hyperforin inhibited LPS-induced PGE2 levels at 40 and 80 μM. The hyperforin concentration in Hp extracts at the highest concentration tested was 1–70 μM. Therefore, higher concentrations of hyperforin may be contributing to the anti-inflammatory activity of Hp extracts. Albert et al. showed that hyperforin suppressed COX-1 product formation with an IC50 of 0.3 μM for thrombin-stimulated or of 3 μM for ionophore-stimulated human monocytic MM6 (Mono Mac 6) cells, but hyperforin had no effect on COX-2 protein levels in LPS-stimulated MM6 cells (22). This is the first report of hyperforin’s effects in RAW264.7 macrophage cells.
Similar to our results with flavonoids, 40 and 80 μM quercetin significantly decreased LPS-induced PGE2 levels in RAW264.7 macrophage cells while decreasing COX-2 protein at 80 μM (23). Furthermore, rutin had no effect on LPS-induced PGE2 levels or COX-2 protein levels in RAW264.7 macrophages at 40 and 80 μM (23). Chlorogenic acid at 1–40 μM had no significant effect on LPS-induced PGE2 levels, and 10 μM chlorogenic acid would be present in the Hp extracts at the highest concentration tested. Chlorogenic acid had no effect on LPS-induced PGE2 levels in J774 macrophages up to 100 μM (24).
Although cytotoxicity was observed at the highest concentration tested for all the Hp extracts except for the Soxhlet chloroform Elixir, no significant cytotoxicity was observed at 8 μg/mL for the Hp accession extracts. However, the extracts did possess some moderate cytotoxicity that was not statistically significant. The reduction in PGE2 for both the Hp extracts and constituents cannot be explained by cytotoxicity alone as evidenced by the Hp extracts at lower doses and the constituents, and in agreement with Schmitt et al. the Hp extracts exhibited light-independent cytotoxicity, whereas the naphthodianthrones had light-dependent cytotoxicity (12).
In conclusion, Hp extracts possessed anti-inflammatory activity that varied with extraction solvent and accession. The profiles of known chemical constituents, flavonoids, biflavonoids, phloroglucinols, and naphthodianthrones, varied among the different accessions tested. Accession Elixir displayed the most anti-inflammatory activity at lower concentrations; both Soxhlet ethanol and Soxhlet chloroform extracts were active at a concentration of 8 μg/mL. The cytotoxicity of Elixir at 8 μg/mL was not statistically significant and cannot solely account for the reductions in PGE2. Finally, flavonoids, biflavonoids, phloroglucinols, and pseudohypericin were present within the extracts and possessed significant anti-inflammatory activity; however, the concentrations of these constituents in the Hp extracts at the highest concentration tested were far less than the concentration of pure constituent needed to observe a significant anti-inflammatory effect, with the exception of light-activated pseudohypericin at 1 μM in two of the Hp extracts and hyperforin at 70 μM in one of the Hp extracts. Thus, the anti-inflammatory activity of Hp extracts cannot be explained by the presence of these constituents alone. Because Hp extracts showed light-independent anti-inflammatory activity, it is likely that interactions among identified and unidentified compounds account for the diverse activities seen in different accessions of Hp.
Acknowledgments
We thank members of the Iowa Center for Research on Botanical Dietary Supplements, especially Norma Leyva and Man-Yu Yum for statistical support and Zili Zhai and Dr. Joan Cunnick for endotoxin analysis of the extracts. We acknowledge the gift of Hp plant material from Frontier Natural Products Co-op, Norway, Iowa, and the efforts of Fredy Romero and members of the Organic Agriculture Program and the NCRPIS in growing the six Hp accessions at Iowa State University, Ames, IA.
This publication was made possible by Grant P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), National Institutes of Health (NIH), and Grant 9P50AT004155-06 from the National Center for Complementary and Alternative Medicine (NCCAM) and ODS, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCCAM, or NIH.
ABBREVIATIONS USED
- Hp
Hypericum perforatum
- PI
Plant Introduction
- FNPC
Frontier Natural Products Co-op
- NCRPIS
North Central Regional Plant Introduction Station
- PGE2
prostaglandin E2
- LPS
lipopolysaccharide
Footnotes
SAFETY
Organic solvents, such as hexane and chloroform, are toxic chemicals and should be properly handled in a fume hood.
LITERATURE CITED
- 1.Bilia AR, Gallori S, Vincieri FF. St. John’s wort and depression: efficacy, safety and tolerability–an update. Life Sci. 2002;70(26):3077–3096. doi: 10.1016/s0024-3205(02)01566-7. [DOI] [PubMed] [Google Scholar]
- 2.St John’s wort Hypericum perforatum. American Herbal Pharmacopoeia and Therapeutic Compendum. 1997;25(2) [Google Scholar]
- 3.Serhan CN, Savill JN. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Raso GM, Pacilio M, Di Carlo G, Esposito E, Pinto L, Meli R. In-vivo and in-vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum. J Pharm Pharmacol. 2002;54(10):1379–1383. doi: 10.1211/002235702760345464. [DOI] [PubMed] [Google Scholar]
- 5.Herold A, Cremer L, Calugaru A, Tamas V, Ionescu F, Manea S, Szegli G. Hydroalcoholic plant extracts with anti-inflammatory activity. Roum Arch Microbiol Immunol. 2003;62(1–2):117–129. [PubMed] [Google Scholar]
- 6.Abdel-Salam OM. Anti-inflammatory, antinociceptive, and gastric effects of Hypericum perforatum in rats. Sci World J. 2005;8(5):586–595. doi: 10.1100/tsw.2005.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar V, Singh PN, Bhattacharya SK. Anti-inflammatory and analgesic activity of Indian Hypericum perforatum L. Indian J Exp Biol. 2001;39(4):339–43. [PubMed] [Google Scholar]
- 8.Shipochliev T, Dimitrov A, Aleksandrova E. Anti-inflammatory action of a group of plant extracts. Vet Med Nauki. 1981;18(6):87–94. [PubMed] [Google Scholar]
- 9.Meier B. Herbal medicinal products of St. John’s wort manufacturing and quality control. In: Ernst E, editor. Hypericum: the Genus Hypericum. 1. Vol. 31. Taylor and Francis; New York: 2003. pp. 106–136. [Google Scholar]
- 10.Schulz HU, Schurer M, Bassler D, Weiser D. Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin, and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a Hypericum extract containing tablet. Arzneimittelforschung. 2005;55(1):15–22. doi: 10.1055/s-0031-1296820. [DOI] [PubMed] [Google Scholar]
- 11.Lavie G, Mazur Y, Lavie D, Meruelo D. The chemical and biological properties of hypericin: a compound with a broad spectrum of biological activities. Med Res Rev. 1995;15:731–739. doi: 10.1002/med.2610150203. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt LA, Liu Y, Murphy PA, Birt DF. Evaluation of the light-sensitive cytotoxicity of Hypericum perforatum extracts, fractions, and pure compounds. J Agric Food Chem. 2006;54:2681–2890. doi: 10.1021/jf052344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt LA, Liu Y, Murphy PA, Petrich JW, Dixon PM, Birt DF. Reduction in hypericin-induced phototoxicity by Hypericum perforatum extracts and pure compounds. J Photochem Photobiol B: Biol. 2006;85:118–130. doi: 10.1016/j.jphotobiol.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.USDA, ARS, National Genetic Resources Program. National Germplasm Resources Laboratory; Beltsville, MD: [accessed June 16, 2006]. Germplasm Resources Information Network (GRIN) (online database) available at http://www.ars-grin.gov/cgi-bin/npgs/html/index.pl. [Google Scholar]
- 15.Richter’s Herb Specialists. http://www.richters.com/
- 16.Ganzera MJZIAK. Hypericum perforatum–chemical profiling and quantitative results of St. John’s wort products by an improved high-performance liquid chromatography method. J Pharm Sci. 2002;91(3):623–630. doi: 10.1002/jps.10057. [DOI] [PubMed] [Google Scholar]
- 17.Snedecor GW, Cochran WG, editors. Statistical Methods. 8. University Press; Ames, IA: 1989. [Google Scholar]
- 18.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 19.Carpenter S, Kraus GA. Photosensitization is required for inactivation of equine infectious anemia virus by hypericin. Photochem Photobiol. 1991;53(2):169–174. doi: 10.1111/j.1751-1097.1991.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 20.Bezakova L, Psenak M, Kartnig T. Effect of dianthrones and their precursors from Hypericum perforatum L. on lipoxygenase activity. Pharmazie. 1999;54(9):7–11. [PubMed] [Google Scholar]
- 21.Panossian AG, Gabriellan E, Manvelian V, Jurcic K, Wagner H. Immunosuppressive effects of hypericin on stimulated human leukocytes: inhibition of arachidonic acid release, leukotriene B4, and interleukin-1α production, and activation of nitric oxide formation. Phytomedicine. 1996;3(1):19–28. doi: 10.1016/S0944-7113(96)80005-5. [DOI] [PubMed] [Google Scholar]
- 22.Albert D, Zundorf I, Dingermann T, Muller WE. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem Pharmacol. 2002;64:1767–1775. doi: 10.1016/s0006-2952(02)01387-4. [DOI] [PubMed] [Google Scholar]
- 23.Shen CS, Lee WR, Lin HY, Huang HC, Ko CH, Yang LL, Chen YC. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E2 production. Eur J Pharmacol. 2002;44:187–194. doi: 10.1016/s0014-2999(02)01792-2. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A, Ligresti A, Longo R, Russo A, Borrelli F, Sautebin L. The inhibitory effect of propolis and caffeic acid phenethyl ester on cyclooxygenase activity in J774 macrophages. Phytomedicine. 2002;9(6):530–535. doi: 10.1078/09447110260573164. [DOI] [PubMed] [Google Scholar]


