Summary
Based on their characteristics and function – migration, neural protection, proliferation, axonal guidance and trophic effects – glial cells may be regarded as probably the most versatile cells in our body. For many years, these cells were considered as simply support cells for neurons. Recently, it has been shown that they are more versatile than previously believed – as true stem cells in the nervous system – and are important players in neural function and development. There are several glial cell types in the nervous system: the two most abundant are oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Although both of these cells are responsible for myelination, their developmental origins are quite different. Oligodendrocytes originate from small niche populations from different regions of the central nervous system, while Schwann cells develop from a stem cell population (the neural crest) that gives rise to many cell derivatives besides glia and which is a highly migratory group of cells.
Keywords: Glia, Oligodendrocyte, Schwann cell
Historical background
The glia are the cells in the nervous system (NS) that for many years were thought to only provide support, protection and nutrition and facilitate conduction for the neurons they surround. However, after more detailed studies – and especially in the past 20 years or so – glial cells have also been implicated in important developmental mechanisms, such as guiding migration of neurons in early development, forming the necessary scaffold for neuronal architecture, being critical participants in synaptic transmission, as key regulators of neurotransmitter release, as well as other functions.
The differentiated cells of the NS arise from either a multipotential neuroepithelial cell population that originates from the ectoderm, or from ectodermal specialized cells that form the placodes (Baker and Bronner-Fraser, 1997). Early in development, the ectodermal cells are induced by factors such as Wnts, Pax7, and others to become the neuroectoderm and undergo neurulation (Basch et al., 2006). One important feature of these stem cells is that they become more restricted as they differentiate into more committed cells, until finally they mature into neurons and glia.
When studying glial cell biology, one interesting aspect that has been largely overlooked in neural development is that brains of higher organisms are composed of mostly – nearly 90% – glial cells, rather than neurons; even though neurons are the key elements in NS transmission of information. The common neuron-centered view underlies the assumption that glial cells simply provided support for neurons. The change towards a more accurate understanding of glial cells started in the early 1980s with seminal papers showing their role in neuron–glia interaction (Vernadakis, 1988), midline axon guidance (Klambt and Goodman, 1991), axon-myelin formation (Bunge, 1987; Ranscht et al., 1987), neuronal migration, immunity (Frohman et al., 1989; Stitt et al., 1991), development of organized neuropilar structures (Oland and Tolbert, 1989) and their role in forming the blood brain barrier by perinodal astrocytes (Black and Waxman, 1988).
Glial cells were discovered around the mid-1800s by a group of scientists including, Rudolf Virchow, Theodor Schwann and Robert Remak. It was the pathologist Rudolf Virchow that coined the term neuro-glia (nerve-cement) in 1856 in his book “Cellular Pathology” (Jacobson, 1991). However, his pre-eminence in the field of glial biology is contested by another scientist, Henrich Muller who made the first glial cells drawings and descriptions in the very same year as Virchow"s book was published (the glia were what we now know as Muller cells in the retina). In addition, none other than Camillo Golgi described astrocytes and oligodendrocytes in a book in 1871. It was Michael von Lenhossek who in 1893 introduced the term astrocyte in describing the star-shaped cells in the central nervous system (CNS). For an excellent review on the history of the glial cell, see (Kettenmann and Ransom, 2005).
Probably, the best known of all glial functions is the formation of myelin, the membranous sheath covering axons and facilitating rapid communication between neurons. It was Robert Remak who started to look at the peripheral nervous system (PNS) and its “medullated fibers”; but it was Virchow again who coined the term myelin in 1853, when referring to the fatty sheath surrounding some axons (Jacobson, 1991). The Schwann cell (SC) was termed as such by Louis Ranvier in 1871, and Ramon y Cajal established this name in his monumental work on the NS. Finally, it was Pio del Rio-Hortega who distinguished two other types of non-neuronal cells: oligodendrocytes and microglia. The term oligodendrocyte arose because they had fewer and smaller branches than astrocytes. Rio-Hortega also proposed that these cells, as well as SCs, make myelin; although this theory was not fully accepted until proved by electron microscopy (Jacobson, 1991).
Glial cell development
The development of glial cells is a fascinating field, especially because what seems to be their intrinsic “stem cell-ness” (Bonfanti and Peretto, 2007; Doetsch, 2003a; Gotz, 2003). That is to say: glial cells can give rise to a variety of other neural cell types (Doetsch, 2003b). Nevertheless, there is a clear glial cell lineage for both oligodendrocytes and SCs (Jessen and Mirsky, 2005; Miller, 2002; Wood and Bunge, 1991). The cell lineage of astrocytes is less clearly understood (Liu et al., 2002).
Several critical steps have led to a better understanding of glial cell biology. The first was having good animal model organisms. The most important animal model has been Drosophila melanogaster, a prime genetic model organism with a good number of glial mutant phenotypes. The glial phenotype gcm (“glial cell missing”) has been especially informative. The gcm gene encodes for a primary regulator of glial cell determination in Drosophila, showing that the division between glial or neuronal fates can be under the control of one single molecule. In gcm mutant embryos, presumptive glial cells are transformed into neurons and when gcm is ectopically expressed, presumptive neurons become glia. This makes gcm a binary switch between neurons and glia (Freeman et al., 2003). The other primary animal model for study of glial cells is the mouse. Currently, there are many strains of mice that carry mutations that range from mild to severe glial phenotypes. The advantage of using mice in glial studies is that it is closer to humans than Drosophila and that can be manipulated by directed mutations that range from a simple rescue-phenotype to the conditional Cre-Lox mutants.
The second critical approach in studying glial cells has been the use of classical in vitro studies with precursor cells. These studies took advantage of using controlled media conditions that favor the development of one or the other cell type and of the existence of specific cell makers that allowed researchers to distinguish precursors from immature and/or mature cells (Barres et al., 1996; Morrison et al., 1999; Shi et al., 1998). Based on such studies, as well as on other developmental studies, we now know of the existence of the progenitor cells for glia (Chandross et al., 1999). Many of these studies had until recently relied heavily on known specific markers and lineage tracing (Bhattacharyya et al., 1991; Brockes et al., 1977; Guerci et al., 1986; Hooghe-Peters et al., 1979; Pernber et al., 2002; Raff et al., 1978).
From such in vivo and in vitro studies, few major subpopulations of committed glioblasts have been defined: the oligodendrocyte precursor cell (OPC), astroblasts, and radial glia cell precursors (Asakura et al., 1996; Cameron-Curry and Le Douarin, 1995; Skoff and Knapp, 1991) in the CNS and the Schwann cell precursor (SCP) in the PNS (Bhattacharyya et al., 1991; Blanchard et al., 1996; Schneider et al., 2001).
Schwann cell development
SCs are the main glia of the PNS, originating from the neural crest. Like the oligodendrocyte, the SC is a highly specialized cell that has evolved into a myelinating cell. Unlike the oligodendrocyte, it is also a guidance partner for growing axons during peripheral development (Thompson and Buettner, 2006; Wanner et al., 2006a; Wanner and Wood, 2002).
The neural crest is a group of cells that originates in the dorsal part of the neural tube, goes through an epithelial to mesenchymal transition, and later disperses – migrating extensively throughout the embryo and differentiating into a wide variety of cell types – giving rise to the PNS (Jessen and Mirsky, 1998).
The origin of the SC lineage involves three main developmental transitions in neural crest cells (Jessen and Mirsky, 1998). The first is the formation of SCPs from actively migrating neural crest cells. Secondly, the formation of immature SCs from the precursors. Finally, the differentiation of the embryonic SCs into a myelin and non-myelin-forming SC. This is illustrated in Figure 1.
Figure 1.
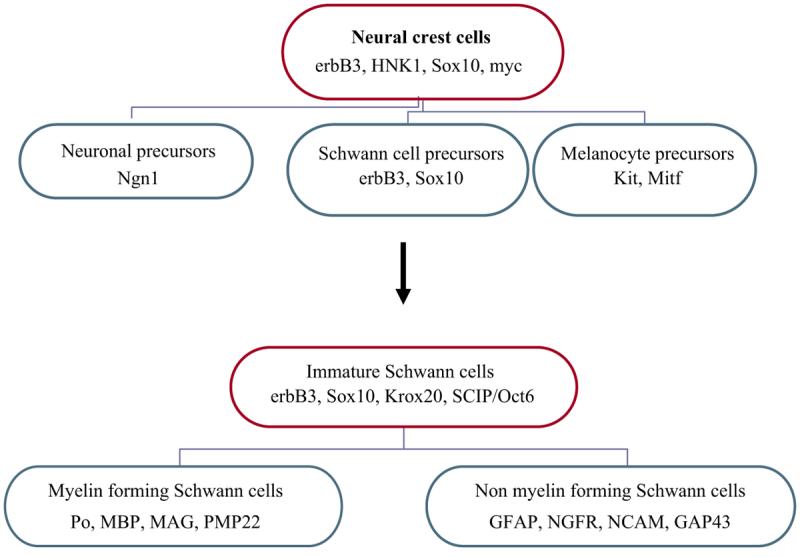
Schwann cell development. The diagram shows how Schwann cells develop from a pluripotent neural crest cell that can give rise to different cells, from which the immature and mature Schwann cells will originate. Mature Schwann cells can be either myelin or nonmyelinating type. Ngn1 (neurogenin-1), Mitf (microphthalmia transcription factor), myc (myelocytomatosis viral oncogene), GFAP (glial fibrillary protein), NGFR (nerve growth factor receptor), NCAM (Neural cell adhesion protein), GAP43 (growth-associated protein 43), Krox20, SCIP/Oct6 (Octamer-binding transcription factor 6).
Whether a SC has a myelin-forming or a nonmyelin-forming phenotype, both varieties of SCs are neural-crest derived and descend from a common precursor in the dorsal neural tube. This is quite a distinct developmental pathway from the oligodendrocyte, which originates from different populations, depending on the location of the precursor neuroblast. The regulation of the final cell fate of a SC is determined, to a certain extent, by the axons with which they find themselves in contact with. SC progenitors migrate and proliferate ahead, as well as along pre-existing axonal tracts, during embryonic development (Wanner et al., 2006b). What determines if a cell will become a myelinating one or not depends ultimately on axonal signals received during their close contact as they enter peripheral tissues.
The importance of SCs for neuron development is underscored by the neuropathies seen in mice or humans with mutations in SC or myelin genes. For example, “trembler” mice have a mutation in the peripheral myelin protein 22 (PMP22) and show sensory neuronal loss (Robertson et al., 1997). Patients with Charcot–Marie–Tooth neuropathy have also axonal damage, thus prompting the hypothesis that SCs provide a first line of axonal neuroprotection (Nave et al., 2007). This suggestion is important because SCs also are known to secrete inflammatory cytokines and neurotrophic factors critical in neuronal survival (Koski, 1997).
Differentiation and maturation
One of the most critical problems in the biology of the neural crest is what determines the fate of these cells and how soon these cells become committed to a specific fate. There is some evidence indicating that at least some neural crest cells enter the glial lineage quite early. Probably, the most relevant are the results from neuregulin, or their respective receptors ErbB2 and ErbB3, knockout mice. Neuregulins, a family of membrane-bound and diffusible extracellular proteins are required for the differentiation of the SC lineage, playing critical roles at multiple stages in SC development (Garratt et al., 2000; Lyons et al., 2005; Woldeyesus et al., 1999). Mice deficient in neuregulin-1 (NRG1) show a significant reduction in the number of SCP by embryonic day 10.5 (E10.5) (Meyer and Birchmeier, 1995), a phenotype that is shared by the ErbB2 and ErbB3 knockout animals (Riethmacher et al., 1997). Importantly, these findings are further supported by studies in vitro, which have shown that NRG1 biases the differentiation of neural crest stem cells toward a glial cell fate (Shah and Anderson, 1997; Shah et al., 1994). In addition to being a trophic and differentiation factor, NRG1 can also regulate myelin sheath thickness by inducing hypermyelination when over-expressed and hypomyelination when its expression is reduced (Michailov et al., 2004). Another piece of evidence of an early commitment to the glial cell lineage is the expression of a novel protein called Seraf (Wakamatsu et al., 2004) by a sub-population of neural crest cells from the earliest stages of their migrations until the most advanced ones (see Figure 2).
Figure 2.
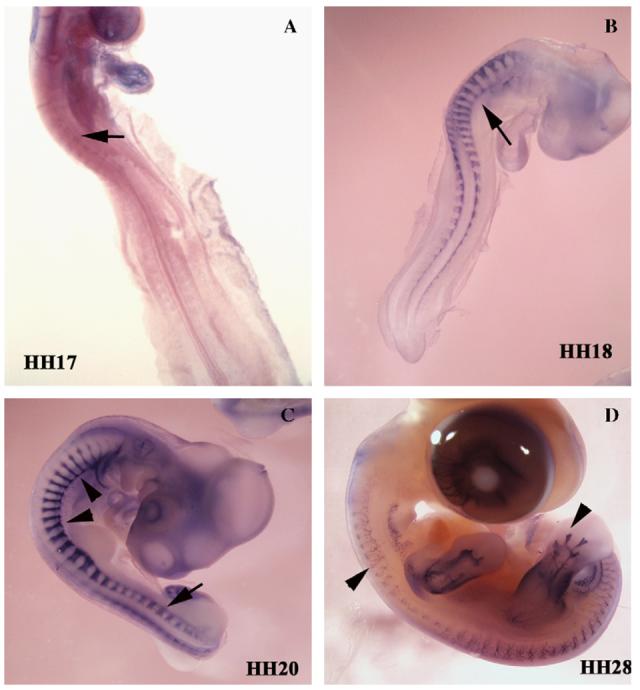
Schwann cell precursor during chicken development. Schwann cell precursors (SCP) were visualized by in situ hybridization using a probe specific to Seraf. SCP can be observed from the very early stages of neural crest development (arrows in A–C). At later stages of development (C, D), when mature Schwann cells are present, Seraf still labels these cells along forming nerves (arrowheads in C point to spinal nerves and in D points to hindlimb nerve plexus). Notice the distinct Seraf pattern in Figure 3C; arrows show migrating neural crest cells positive for Seraf, while arrowheads show staining of spinal nerve). HH = Hamburger and Hamilton developmental stages.
As development proceeds, axon bundles are progressively subdivided and segregated by premyelinating SC processes, and SC and axon establish a 1:1 ratio relationship prior to myelination (Reynolds and Woolf, 1993; Yao et al., 1990). In the rodent sciatic nerve during the first week of postnatal life, pre-myelinating SCs begin to myelinate their associated axons, together with a high level transcription of myelin-specific genes and a down-regulation of immature SC genes (Trapp et al., 1988; Zorick et al., 1996). This is in contrast with non-myelinating SCs, which are not differentiated from immature SCs until the second postnatal week, and even then continue to express genes in a manner characteristic of immature cells.
The formation of the SC lineage involves diverse transcription factors that orchestrate their different stages of development (Mitchell et al., 1991). There are three main ones: Krox-20, Oct-6 and Sox10. It is clear from knockout mice studies that the zinc finger protein Krox-20, the Sox10 and the POU domain protein Oct-6, are very important for the transition of immature SCs to myelin-forming ones, since knockout mice for these three genes shows severe alterations of myelin: lack of myelination when Krox-20 is knocked out (Topilko et al., 1997), severe deficits in the number of SC when Sox10 is knocked out (Herbarth et al., 1998; Topilko et al., 1994) or severely delayed myelination when Oct-6 is knocked out (Bermingham et al., 1996). Oct-6 mRNA and protein can be detected in SCPs, rising to a peak in early postnatal life (Blanchard et al., 1996). In contrast, Krox-20 levels rise sharply between E13 and E15 in mouse nerves – the point at which the transition from precursors to SCs is occurring. The POU transcription factor Oct-6, like Krox-20, is also responsible for myelination. The key difference between the two transcription factors is that Krox-20 is found only in myelinating cells, whereas Oct-6 is found in all SCs (Jessen and Mirsky, 2002; Jessen and Mirsky, 2005). Krox-20 expression continues in myelin-forming cells, but not in mature non-myelin-forming cells, which express a different zinc finger transcription factor, Krox-24. Krox-24 and Krox-20 play antagonistic roles during the development of the SC lineage (Topilko et al., 1997). Krox-24 is strongly activated in most SCs at around the time of birth. During myelination of the PNS, Krox-24 is down-regulated in myelinating SCs, while it is maintained in nonmyelinating cells. In contrast, Krox-20 expression is maintained in myelinating cells (Decker et al., 2006; Leblanc et al., 2005). In summary, Krox-20 expression is observed only in mature SCs and its continued expression requires axonal signaling. However, Krox-24 is expressed initially in SCPs and expression continues after birth.
Of the known transcription factors involved in neural crest and glia development, Sox10 is probably the most important because of its role in regulating development of peripheral glial cells. Sox10 is expressed by the migrating neural crest from the moment they delaminate from the neural tube and expression remains later on in the SCs only (Britsch et al., 2001; Kuhlbrodt et al., 1998). Sox10 expression is required for early survival and migration of neural crest and the differentiation of melanocytes and SCs (Hagedorn et al., 2000; Paratore et al., 2001). Because Sox10 regulates ErbB3 expression, neuregulin signaling may be affected at the level of the receptor (Britsch et al., 2001).
The myelin-forming SCs synthesize a set of adhesive molecules that allows the formation of compact myelin (myelin-associated glycoprotein (MAG), protein zero, (Po), myelin basic protein (MBP)). In contrast, non-myelin-forming SC invest multiple small axons and express glial fibrillary acidic protein (GFAP), low-affinity nerve growth factor receptor (NGFR), neural cell adhesion molecule (NCAM) and growth-associated protein 43 kd (GAP-43), but none of the myelin-specific markers (Mirsky et al., 2001; Mirsky et al., 1996). SC can regulate their own survival because both myelinating and non-myelinating SCs can de-differentiate into immature SCs after axonal interaction ceases (Bhatheja and Field, 2006). Mature SCs can survive in the absence of neurons, whereas immature SCs depend on axonal signals (Jessen and Mirsky, 2005). Mature SCs are able to block apoptosis by participating in an autocrine circuit by releasing growth factors, including insulin-like growth factors (Cheng et al., 2000), platelet derived growth factor-BB (PDGF-BB) (Eccleston et al., 1990) and neurotrophin-3 (NT-3) (Bhatheja and Field, 2006; Britsch et al., 2001; Meier et al., 1999; Porter et al., 1987). This autocrine circuit is not seen in precursor or immature SCs (Jessen and Mirsky, 2005).
The main role of SCs, like the oligodendrocytes in the CNS, is to make myelin. PNS myelin is mostly dependent on Po. A member of the immunoglobulin superfamily, Po is the major myelin membrane protein in the mammalian PNS. Po is present in a sub-population of migrating neural crest cells in chick (Bhattacharyya et al., 1991) and rodents (Lee et al., 1997). This myelin protein was considered a myelin-restricted protein, made by myelin-forming cells alone, as a part of a glial-specific response to myelinating-inducing axonal signals (Bray et al., 1981; Lemke and Chao, 1988). However, research with better antibodies and transgenic mice carrying a reporter gene for Po showed that it is expressed not only by immature SCs, but also by the SCP (Bhattacharyya et al., 1991) and neural crest cells (Hagedorn et al., 1999; Morrison et al., 1999).
The relationship between Po expression in neural crest cells and as a true marker of entry to other crest lineages has not been fully examined. This research is important because studies looking for neural crest stem cells have shown both a Po-positive and a Po-negative population of stem cells (Hagedorn et al., 1999; Morrison et al., 1999). There are thus stem cells that display markers of true glial cells while being true stem cells. Similar kinds of cells have been found in the subventricular zone (SVZ) (Doetsch, 2003b); thus, it is not improbable that peripheral glia, which originate from a true stem cell population (the neural crest) can give rise to a stem cell population with peripheral fate restrictions. Nevertheless, the question remains as to whether these cells are true glial cells that can revert to a stem cell phenotype, or are stem cells that happen to also express a glial cell marker. We will not be able to answer this question properly until we fully understand what makes stem cells different, in their proteome, from other cells.
Oligodendrocytes development
Oligodendrocytes are the myelin-forming cells in the CNS (Figure 3). These cells originate mainly from two types of precursors: the OPCs nestled in the SVZ in the brain and a group of Sox10/Olig1-positive cells in the ventral spinal cord (Liu et al., 2002; Stolt et al., 2006; Timsit et al., 1995).
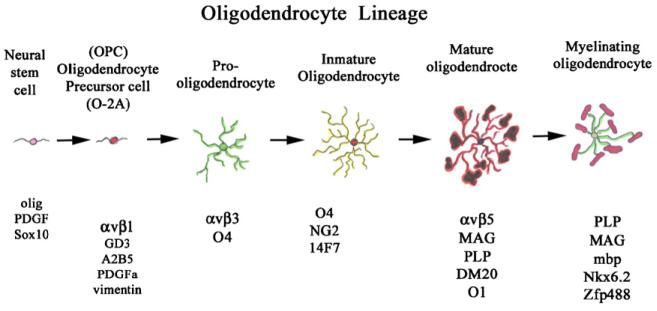
Figure 3.
A summary of the best-known markers for the different stages of development of the oligodendrocyte. Not all researchers agree on the arbitrary stages of development shown here, but this combined figure highlights most of the current research on their development. OPC (oligodendrocyte precursor cell); O-2A (oligodendrocyte-astrocyte type 2A precursor); Olig (oligodendrocyte marker-1 and 2); PDGF (platelet derived growth factor); αvβ1 (integrin alpha v and beta-1 subunits); GD3 (ganglioside 3); A2B5; αvβ3; O4 (oligodendrocyte marker-4); NG2 (chondroitin sulfate proteoglycan); 14F7 (for an antibody with that nomenclature); αvβ5; MAG (myelin-associated glycoprotein); PLP/DM20 (proteolipid protein); O1 (oligodendrocyte marker-1); MBP (myelin basic protein).
Oligodendrocyte precursors develop through a series of stages that have been characterized by their distinct cell morphology and the expression of cell surface proteins: the migratory OPC is typically a PSA-NCAM-positive cell that also expresses a ganglioside recognized by the A2B5 antibody (Gard and Pfeiffer, 1990; Shi et al., 1998). These precursor cells are extremely dependent on the presence of PDGF for proliferation and can be identified because they express high levels of the PDGF receptor (Baron et al., 2002; Barres et al., 1992) while surrounding neurons secrete PDGF (Ellison et al., 1996). The importance of this neurotrophin in oligodendrocyte development has been corroborated by the results from PDGF knockout mice, which show a severe reduction in the numbers of OPCs (Fruttiger et al., 1999). Eventually, the OPCs reach their target regions and mature into myelinating cells and start expressing classic myelin proteins, proteolipid protein (PLP), MBP and MAG.
For many years, the origin of the spinal cord oligodendrocyte remained a mystery because of the lack of early oligodendrocyte-specific markers. Two major steps came from three labs: first, Bill Richardson showed in the early 1990s that a small group of PDGF positive cells gave rise to OPC (Pringle et al., 1992). Then David Anderson at Caltech and David Rowitch at Harvard simultaneously reported that a small group of cells in the presumptive motor neuron area, are positive for the bHLH factor olig2 (Rowitch et al., 2002). This group of cells divides and migrates radially to cover the spinal cord later in development (Talbott et al., 2005; Zhou and Anderson, 2002). That olig genes are critical for oligodendrocyte development was further supported by the absence of spinal cord oligodendrocytes in the olig1/olig2 double knockout mice (Zhou and Anderson, 2002).
Probably the best-known OPC population is the one initially known as the oligodendrocyte-astrocyte type-2 (O-2A) progenitor cells, originally isolated from the postnatal rat optic nerve and subsequently from the postnatal cerebellum, cortex, brain stem and spinal cord (Lee et al., 2000). These OPC cells were observed to have a default pathway of differentiation into oligodendrocytes and this differentiation could be modulated by growth factors (Collarini et al., 1992; McMorris and McKinnon, 1996). For many years, it was believed that they could also give rise to astrocytes type-2 glial cells (Raff et al., 1983). However, because upon transplantation these OPC cells differentiate only into myelinating oligodendrocytes (Groves et al., 1993), most scientists had assumed that the type-2 astrocyte generated in such studies was very likely an in vitro artifact induced on the precursor by the trophic factors present in the media (Franklin et al., 1995; Sawamura et al., 1995). What is more important regarding these precursors is that the cells formerly known as O-2A will not differentiate into neurons under any culture conditions tested; in other words, OPC is a true glial precursor.
The current view is that cortical oligodendrocytes in rodents are born from the cortical subventricular zone (SVZ) after birth; however, recent data from Richardson group suggest that many forebrain oligodendrocyte progenitor cells (OPCs) are specified much earlier (between E9.5 and E13.5 in the mouse) in the ventricular zone of the ventral forebrain under the control of sonic hedgehog (Shh) and then migrate into the cortex (Ivanova et al., 2003) and that even caudal OPC can compensate loss of cortical oligodendrocytes (Kessaris et al., 2006).
As stated for SCs, oligodendrocytes are more than just a myelinating cell. There are quite a number of mutations of genes affecting oligodendrocyte development and differentiation that cause serious neurological disorders. Probably the best known is Pelizaeus–Merzbacher disease, which is a rare, progressive, degenerative CNS disorder in which coordination, motor abilities and intellectual function deteriorate. The best model is a mouse strain referred as the “Jimpy” mice that carry a mutation in PLP protein (Nave et al., 1987). PLP also acts in a paracrine mode to regulate neuronal survival. Co-culture of neurons with non-glial cell lines that overexpress native PLP but not DM20 shortens survival of neurons (Boucher et al., 2002). This finding is mimicked in vivo, where either modest over-expression of native PLP or its absence leads to axonal abnormalities and neuronal death (Griffiths et al., 1998).
Differentiation and maturation
The ability of multipotent cells to differentiate into committed glioblasts and further differentiation of these glioblasts into differentiated/mature glial cells is mediated by transcription factors that act at specific stages in the developmental process (Kinameri and Matsuoka, 2003). These transcription factors are induced by signaling from receptors stimulated by another group of specific trophic factors and/or morphogens (Augustine et al., 1993; Mehler et al., 1997).
PDGF has been identified as an important growth factor for both the proliferation of glial precursors and differentiation of oligodendrocytes (Collarini et al., 1992; Raff et al., 1988; Shi et al., 1998). Triiodothyronine, Shh, BMPs, cAMP retinoic acid and neuregulin are other factors that can induce/generate oligodendrocytes (Canoll et al., 1996; Mehler et al., 2000; Orentas et al., 1999). While neuregulin is required for maintenance and survival of oligodendrocytes, both cAMP and retinoic acid regulate the differentiation of oligodendrocyte precursors into more mature stages (Noll and Miller, 1994).
The development of oligodendrocytes is orchestrated by an extremely complex chain and interdependence of transcription factors (see Nicolay et al., 2007 for an excellent review). Adding more complexity to this picture is the fact that the function of most of these transcription factors varies substantially by brain region. In other words, not all oligodendrocytes seem to follow the same path in their development (Menn et al., 2006). This phenomenon probably explains the discrepancy in the conclusions by glial researchers in the past 15 years over which factors, are required, and where and when, for oligodendrocyte development and maturation.
Part of the complexity derives from research in recent years, which revealed a complex regulatory network of transcription factors in oligodendrocyte development. Thus, neural basic helix–loop–helix (HLH; i.e., Olig1 and Olig2, Mash1), associated inhibitory HLH (i.e., Id2 and Id4), high-mobility group domain (i.e., Sox10), and homeodomain (i.e., Nkx2.2) transcription factors have all been directly implicated in oligodendrocyte development; from lineage specification through their progressive stages of maturation until myelination. (Gokhan et al., 2005; Stolt et al., 2006). Probably, the best known of this group are two families of transcription factors critical for oligodendrocyte development: one is the Sox group (Sox8, Sox9 and Sox10) and the other is the Olig genes (Olig1 and Olig2) (Liu et al., 2007).
Development of myelin-forming oligodendrocytes in the CNS is dependent on Sox10. No myelin is generated upon transplantation of Sox10-deficient neural stem cells into wild-type hosts – showing a permanent, cell-autonomous role for Sox10 in oligodendrocyte differentiation and that Sox10 directly regulates myelin gene expression in these cells (Stolt et al., 2004; Woodruff et al., 2001). When looking at mice mutant for single or combined Sox genes, the picture that emerges is that Sox9 is involved in oligodendrocyte specification, whereas Sox10 is required for terminal differentiation. Meanwhile, Sox8-deficient mice, which exhibit only a delay in the oligodendrocyte terminal differentiation of oligodendrocytes, highlights the importance of redundancy of other Sox genes in glial development (Stolt et al., 2004).
The other group of critical glial genes, expressed from very early in the developing neural tube is the Olig genes. To answer the discrepant developmental questions regarding the role of Olig genes during development, Rowitch and co-workers generated null mutations of Olig1 and Olig2. Their results further confirmed that Olig genes are necessary during CNS development, especially for the generation of oligodendrocytes. However, observation of their knockout animals lead to the surprising conclusion that the two Olig genes, though structurally similar and coordinately expressed, encode proteins with quite distinct biological capabilities. Olig2, the “primordial” Olig gene, plays prominent roles in the developing spinal cord where it is essential for oligodendrocyte and motor neuron specification. Olig1 is not required for motor neuron development, though it promotes formation and maturation of oligodendrocytes. A striking feature of the Olig null phenotypes is that astrocyte formation proceeds normally in mice that fail to develop oligodendrocytes. This observation, together with fate mapping analysis of Olig-expressing cells, challenges the view that oligodendrocytes and astrocytes arise exclusively from a glial-restricted precursor (Lu et al., 2001, 2002).
Oligodendrocytes not only depend on the specific expression of certain transcription factors, but their development depends on the tightly controlled timing of expression of certain maturation inhibitors, like BMP or Id2 (See et al., 2004; Wang et al., 2001), the expression of specific receptors, like erbB4 (Sussman et al., 2005) or Notch. The story of Notch in oligodendrocyte development is still a controversial one. While Barres and co-workers found that oligodendrocyte differentiation is powerfully inhibited by activation of the Notch pathway (Wang et al., 1998), studies with zebrafish mutants showed that constitutive Notch activity could promote formation of excess OPCs (Appel et al., 2001; Park and Appel, 2003). The in vitro study determined that Notch receptor activation inhibits OPCs from differentiating into oligodendrocytes, although it does not affect the ability of the cells to survive and divide in response to stimulation (Wang et al., 1998), thus implying that Notch receptor activation is not mitogenic per se. Apparently, the signaling role of Notch acts to maintain subsets of ventral spinal cord precursors which are later specified to become oligodendrocyte (Park and Appel, 2003). The in vivo study found that zebrafish embryos deficient for Notch activity failed to maintain proliferative precursors and produced excess early born neurons at the expense of later-forming neurons and glia (Appel et al., 2001). The difference between both well-known studies is a classic example of apparent contradiction when comparing in vivo versus in vitro observations. Perhaps oligodendrocytes, like other cells, change their trophic need when placed in culture, thus explaining why Notch seem to be capable of keeping them in a non-dividing, non-differentiated stage in vitro, while stimulating them to divide in vivo.
Recently, another less well-known transcription factor Yin Yang 1 (YY1), has been shown to be yet another critical regulator of oligodendrocyte progenitor differentiation. Lack of YY1 will arrest differentiation of oligodendrocyte progenitors after they exit from the cell cycle, thus preventing their further maturation. Therefore, YY1 is an essential component of the transcriptional network regulating the transition of oligodendrocyte progenitors from cell cycle exit to differentiation (He et al., 2007).
In summary, oligodendrocytes do not seem to be as homogeneous as SCs in respect to the kind of molecular mechanisms that they use during development and differentiation. This is probably due to their diverse developmental origins: not all oligodendrocytes come from the same neuroblast populations and CNS regions. Thus, although they will all reach a final identical specificity – i.e. all mature oligodendrocytes seem to exhibit the same molecular profile – these cells are different. Their developmental mechanisms are distinct from each other, depending on where they originate and at what point they are along their differentiation pathway.
The myelin question
Despite their different developmental origin and biology, SCs and oligodendrocyte share one of the most interesting cell functions: myelin formation. Myelin is a complex structure consisting of consecutive layers of plasma membrane wrapped around an axon to insulate it. Synthesis depends on a delicate orchestration of molecules responsible for the build-up of each of its parts. Interestingly, it is in their myelin composition that oligodendrocytes and SCs take separate pathways. Thus, this distinction poses an interesting evolutionary question: how have two very different cells developed the same structure using different molecules?
Both SCs and oligodendrocytes have a different process for myelination. SCs initially wrap their membrane around several axons, and eventually each cell segregates to wrap around one axon segment. Oligodendrocytes extend cell processes that reach out to axons and wrap around them. Thus, each myelinating oligodendrocyte can wrap as many as 10–20 axonal segments, while SCs only wrap one axonal segment at a time.
SCs use the following myelin-specific proteins: Po glycoprotein, MBP, PMP22kd (PMP-22), MAG and cyclic nucleotide protease (known as CNPase) (Lemke, 1993; Lemke et al., 1990). Oligodendrocytes, like SCs, express MBP and MAG, but their main compact myelin component is PLP. The relative importance of each of these molecules has been further proven by studies with mice deficient in each of these molecules. Thus, as expected, lack of Po in humans and mice causes demyelination in the PNS (Giese et al., 1992; Su et al., 1993), while mutations of the Plp gene have been shown to cause Pelizaeus–Merzbacher disease, spastic paraplegia type 2 and demyelinating disorders in a range of animal species (Garbern et al., 1997; Griffiths et al., 1995; Shy et al., 2003). Both of these proteins are responsible for the compaction of the intraperiod line of myelin which corresponds to two extracellular layers of plasma membranes adhering in a zipper manner (Shapiro et al., 1996). The major dense line of myelin, which corresponding to two intracellular/cytoplasmic sides of plasma membrane removed of any cytoplasm, depends on MBP. This has been confirmed in “shiverer mice” which are mutant for MBP (Allinquant et al., 1991).
Studies looking at the evolution of myelin had focused on a variety of questions: where did myelin first appeared: PNS or CNS? Which structural molecules first appeared and provided proper membrane compaction? Or why is there a close association between the apparition of a hinged-jaw and the myelin sheath? Answers to these and other questions will help us understand better the many pathologies of myelin in humans.
Several facts underscore the importance of studying this major evolutionary step: (1) in elasmobranches, oligodendrocytes in the CNS use Po protein instead of PLP as their main myelin adhesive protein (Yin et al., 2006). (2) Lower vertebrates, like earthworms, lampreys and hagfish have structures that resemble myelin: glial cells loosely wrapped around axons. It is not known how efficient these structures are in insulating the neurons, but they are present in these organisms and likely pose an advantage to them, though they do not increase significantly nerve conduction (Bullock et al., 1984; Zalc and Colman, 2000). (3) Copepods, which are invertebrates, have myelin (Davis et al., 1999). Thus, it seems that what evolved first was the “structure” per se of plasma membrane wrapping an axon, and this was followed by the “method” of using different adhesive molecules that make possible the tight adhesion between two plasma membrane interfaces.
Conclusion
The glial cells are probably the most versatile cells in our body based on their characteristics and function: migration, neural protection, proliferation, axonal guidance and trophic effect. In addition, SCs (or their precursors) have recently been suggested to have stem-cell-like properties (Morrison et al., 2000). The importance of this newly discovered stem cell population is underscored by the physical symptoms of patients suffering from glial pathologies such as. Multiple sclerosis, Charcot–Marie–Tooth syndrome and gliomas. In order to alleviate or prevent these conditions, it is critical that we understand their development and biology.
Acknowledgments
We are grateful to Vivian Lee for her helpful comments during the preparation of this review.
References
- Allinquant B, Staugaitis SM, D"Urso D, Colman DR. The ectopic expression of myelin basic protein isoforms in Shiverer oligodendrocytes: implications for myelino-genesis. J Cell Biol. 1991;113:393–403. doi: 10.1083/jcb.113.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Givan LA, Eisen JS. Delta-notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Dev Biol. 2001;1:13. doi: 10.1186/1471-213X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura K, Miller DJ, Murray K, Bansal R, Pfeiffer SE, Rodriguez M. Monoclonal autoantibody SCH94.03, which promotes central nervous system remyelination, recognizes an antigen on the surface of oligodendrocytes. J Neurosci Res. 1996;43:273–81. doi: 10.1002/(SICI)1097-4547(19960201)43:3<273::AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Augustine K, Liu ET, Sadler TW. Antisense attenuation of Wnt-1 and Wnt-3a expression in whole embryo culture reveals roles for these genes in craniofacial, spinal cord, and cardiac morphogenesis. Dev Genet. 1993;14:500–20. doi: 10.1002/dvg.1020140611. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. The origins of the neural crest. Part II: An evolutionary perspective. Mech Dev. 1997;69:13–29. doi: 10.1016/s0925-4773(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Baron W, Shattil SJ, ffrench-Constant C. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 2002;21:1957–66. doi: 10.1093/emboj/21.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, et al. Cell death in the oligodendrocyte lineage. J Neurobiol. 1992;23:1221–30. doi: 10.1002/neu.480230912. [DOI] [PubMed] [Google Scholar]
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci. 1996;8:146–56. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–22. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Scherer SS, O"Connell S, Arroyo E, Kalla KA, Powell FL, et al. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–62. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–9. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Frank E, Ratner N, Brackenbury R. P0 is an early marker of the Schwann cell lineage in chickens. Neuron. 1991;7:831–44. doi: 10.1016/0896-6273(91)90285-8. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. The perinodal astrocyte. Glia. 1988;1:169–83. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- Blanchard AD, Sinanan A, Parmantier E, Zwart R, Broos L, Meijer D, et al. Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20, and Pax-3. J Neurosci Res. 1996;46:630–40. doi: 10.1002/(SICI)1097-4547(19961201)46:5<630::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P. Radial glial origin of the adult neural stem cells in the subventricular zone. Prog Neurobiol. 2007;83:24–36. doi: 10.1016/j.pneurobio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Boucher SE, Cypher MA, Carlock LR, Skoff RP. Proteolipid protein gene modulates viability and phenotype of neurons. J Neurosci. 2002;22:1772–83. doi: 10.1523/JNEUROSCI.22-05-01772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GM, Rasminsky M, Aguayo AJ. Interactions between axons and their sheath cells. Annu Rev Neurosci. 1981;4:127–62. doi: 10.1146/annurev.ne.04.030181.001015. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. A surface antigenic marker for rat Schwann cells. Nature. 1977;266:364–6. doi: 10.1038/266364a0. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–8. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Tissue culture observations relevant to the study of axon–Schwann cell interactions during peripheral nerve development and repair. J Exp Biol. 1987;132:21–34. doi: 10.1242/jeb.132.1.21. [DOI] [PubMed] [Google Scholar]
- Cameron-Curry P, Le Douarin NM. Oligodendrocyte precursors originate from both the dorsal and the ventral parts of the spinal cord. Neuron. 1995;15:1299–310. doi: 10.1016/0896-6273(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–43. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Chandross KJ, Cohen RI, Paras P, Jr, Gravel M, Braun PE, Hudson LD. Identification and characterization of early glial progenitors using a transgenic selection strategy. J Neurosci. 1999;19:759–74. doi: 10.1523/JNEUROSCI.19-02-00759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Steinway M, Delaney CL, Franke TF, Feldman EL. IGF-I promotes Schwann cell motility and survival via activation of Akt. Mol Cell Endocrinol. 2000;170:211–5. doi: 10.1016/s0303-7207(00)00324-5. [DOI] [PubMed] [Google Scholar]
- Collarini EJ, Kuhn R, Marshall CJ, Monuki ES, Lemke G, Richardson WD. Down-regulation of the POU transcription factor SCIP is an early event in oligodendrocyte differentiation in vitro. Development. 1992;116:193–200. doi: 10.1242/dev.116.1.193. [DOI] [PubMed] [Google Scholar]
- Davis AD, Weatherby TM, Hartline DK, Lenz PH. Myelin-like sheaths in copepod axons. Nature. 1999;398:571. doi: 10.1038/19212. [DOI] [PubMed] [Google Scholar]
- Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci. 2006;26:9771–9. doi: 10.1523/JNEUROSCI.0716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003a;6:1127–34. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003b;13:543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Eccleston PA, Collarini EJ, Jessen KR, Mirsky R, Richardson WD. Schwann cells secrete a PDGF-like factor: evidence for an autocrine growth mechanism involving PDGF. Eur J Neurosci. 1990;2:985–92. doi: 10.1111/j.1460-9568.1990.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Ellison JA, Scully SA, de Vellis J. Evidence for neuronal regulation of oligodendrocyte development: cellular localization of platelet-derived growth factor alpha receptor and A-chain mRNA during cerebral cortex development in the rat. J Neurosci Res. 1996;45:28–39. doi: 10.1002/(SICI)1097-4547(19960701)45:1<28::AID-JNR3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Bayley SA, Milner R, Ffrench-Constant C, Blakemore WF. Differentiation of the O-2A progenitor cell line CG-4 into oligodendrocytes and astrocytes following transplantation into glia-deficient areas of CNS white matter. Glia. 1995;13:39–44. doi: 10.1002/glia.440130105. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–80. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Frohman EM, van den Noort S, Gupta S. Astrocytes and intracerebral immune responses. J Clin Immunol. 1989;9:1–9. doi: 10.1007/BF00917121. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–67. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Cambi F, Tang XM, Sima AA, Vallat JM, Bosch EP, et al. Proteolipid protein is necessary in peripheral as well as central myelin. Neuron. 1997;19:205–18. doi: 10.1016/s0896-6273(00)80360-8. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4- and O4+GalC-) under different mitogenic control. Neuron. 1990;5:615–25. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the Schwann cell precursor pool. J Cell Biol. 2000;148:1035–46. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–76. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Gokhan S, Marin-Husstege M, Yung SY, Fontanez D, Casaccia-Bonnefil P, Mehler MF. Combinatorial profiles of oligodendrocyte-selective classes of transcriptional regulators differentially modulate myelin basic protein gene expression. J Neurosci. 2005;25:8311–21. doi: 10.1523/JNEUROSCI.1850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M. Glial cells generate neurons – master control within CNS regions: developmental perspectives on neural stem cells. Neuroscientist. 2003;9:379–97. doi: 10.1177/1073858403257138. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–3. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Schneider A, Anderson J, Nave KA. Transgenic and natural mouse models of proteolipid protein (PLP)-related dysmyelination and demyelination. Brain Pathol. 1995;5:275–81. doi: 10.1111/j.1750-3639.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Groves AK, Barnett SC, Franklin RJ, Crang AJ, Mayer M, Blakemore WF, et al. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–5. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Guerci A, Monge M, Baron-Van Evercooren A, Lubetzki C, Dancea S, Boutry JM, et al. Schwann cell marker defined by a monoclonal antibody (224–58) with species cross-reactivity. I. Cellular localization. J Neurochem. 1986;46:425–34. doi: 10.1111/j.1471-4159.1986.tb12986.x. [DOI] [PubMed] [Google Scholar]
- Hagedorn L, Paratore C, Brugnoli G, Baert JL, Mercader N, Suter U, et al. The Ets domain transcription factor Erm distinguishes rat satellite glia from Schwann cells and is regulated in satellite cells by neuregulin signaling. Dev Biol. 2000;219:44–58. doi: 10.1006/dbio.1999.9595. [DOI] [PubMed] [Google Scholar]
- Hagedorn L, Suter U, Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development. 1999;126:3781–94. doi: 10.1242/dev.126.17.3781. [DOI] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, et al. The transcription factor yin yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–30. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans-Borgmeyer I, Puliti A, et al. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci USA. 1998;95:5161–5. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghe-Peters EL, Fowlkes BJ, Hooghe RJ. A new neuronal marker identified by phosphorylcholine-binding myeloma proteins. Nature. 1979;281:376–8. doi: 10.1038/281376a0. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Nakahira E, Kagawa T, Oba A, Wada T, Takebayashi H, et al. Evidence for a second wave of oligodendrogenesis in the postnatal cerebral cortex of the mouse. J Neurosci Res. 2003;73:581–92. doi: 10.1002/jnr.10717. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Developmental neurobiology. ix. Plenum Press; New York: 1991. p. 776pp. [Google Scholar]
- Jessen KR, Mirsky R. Origin and early development of Schwann cells. Microsc Res Technol. 1998;41:393–402. doi: 10.1002/(SICI)1097-0029(19980601)41:5<393::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–76. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–9. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. Neuroglia. xix. Oxford University Press; New York: 2005. p. 601pp. [Google Scholar]
- Kinameri E, Matsuoka I. Autocrine action of BMP2 regulates expression of GDNF-mRNA in sciatic Schwann cells. Brain Res Mol Brain Res. 2003;117:221–7. doi: 10.1016/s0169-328x(03)00326-7. [DOI] [PubMed] [Google Scholar]
- Klambt C, Goodman CS. Role of the midline glia and neurons in the formation of the axon commissures in the central nervous system of the Drosophila embryo. Ann N Y Acad Sci. 1991;633:142–59. doi: 10.1111/j.1749-6632.1991.tb15604.x. [DOI] [PubMed] [Google Scholar]
- Koski CL. Mechanisms of Schwann cell damage in inflammatory neuropathy. J Infect Dis. 1997;176(Suppl 2):S169–72. doi: 10.1086/513795. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc SE, Srinivasan R, Ferri C, Mager GM, Gillian-Daniel AL, Wrabetz L, et al. Regulation of cholesterol/lipid biosynthetic genes by Egr2/Krox20 during peripheral nerve myelination. J Neurochem. 2005;93:737–48. doi: 10.1111/j.1471-4159.2005.03056.x. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–21. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lee M, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, et al. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol Cell Neurosci. 1997;8:336–50. doi: 10.1006/mcne.1996.0589. [DOI] [PubMed] [Google Scholar]
- Lemke G. The molecular genetics of myelination: an update. Glia. 1993;7:263–71. doi: 10.1002/glia.440070402. [DOI] [PubMed] [Google Scholar]
- Lemke G, Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988;102:499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Lemke G, Kuhn R, Monuki ES, Weinmaster G. Transcriptional controls underlying Schwann cell differentiation and myelination. Ann N Y Acad Sci. 1990;605:248–53. doi: 10.1111/j.1749-6632.1990.tb42397.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, et al. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hu X, Cai J, Liu B, Peng X, Wegner M, et al. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev Biol. 2007;302:683–93. doi: 10.1016/j.ydbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lu QR, Cai L, Rowitch D, Cepko CL, Stiles CD. Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat Neurosci. 2001;4:973–4. doi: 10.1038/nn718. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, et al. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–24. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- McMorris FA, McKinnon RD. Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol. 1996;6:313–29. doi: 10.1111/j.1750-3639.1996.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–17. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22:74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19:3847–59. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–67. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Stewart HJ, Tabernero A, Bradke F, Brennan A, Dong Z, et al. Development and differentiation of Schwann cells. Rev Neurol (Paris) 1996;152:308–13. [PubMed] [Google Scholar]
- Mirsky R, Parkinson DB, Dong Z, Meier C, Calle E, Brennan A, et al. Regulation of genes involved in Schwann cell development and differentiation. Prog Brain Res. 2001;132:3–11. doi: 10.1016/S0079-6123(01)32060-5. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Timmons PM, Hebert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991;5:105–19. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–49. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Nave KA, Lai C, Bloom FE, Milner RJ. Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc Natl Acad Sci USA. 1987;84:5665–9. doi: 10.1073/pnas.84.16.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Sereda MW, Ehrenreich H. Mechanisms of disease: inherited demyelinating neuropathies – from basic to clinical research. Nat Clin Pract Neurol. 2007;3:453–64. doi: 10.1038/ncpneuro0583. [DOI] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–99. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- Noll E, Miller RH. Regulation of oligodendrocyte differentiation: a role for retinoic acid in the spinal cord. Development. 1994;120:649–60. doi: 10.1242/dev.120.3.649. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Patterns of glial proliferation during formation of olfactory glomeruli in an insect. Glia. 1989;2:10–24. doi: 10.1002/glia.440020103. [DOI] [PubMed] [Google Scholar]
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–29. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–61. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- Park HC, Appel B. Delta-Notch signaling regulates oligodendrocyte specification. Development. 2003;130:3747–55. doi: 10.1242/dev.00576. [DOI] [PubMed] [Google Scholar]
- Pernber Z, Molander-Melin M, Berthold CH, Hansson E, Fredman P. Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. J Neurosci Res. 2002;69:86–93. doi: 10.1002/jnr.10264. [DOI] [PubMed] [Google Scholar]
- Porter S, Glaser L, Bunge RP. Release of autocrine growth factor by primary and immortalized Schwann cells. Proc Natl Acad Sci USA. 1987;84:7768–72. doi: 10.1073/pnas.84.21.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–51. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Raff MC, Mirsky R, Fields KL, Lisak RP, Dorfman SH, Silberberg DH, et al. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978;274:813–6. [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–6. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–5. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Ranscht B, Wood PM, Bunge RP. Inhibition of in vitro peripheral myelin formation by monoclonal anti-galactocerebroside. J Neurosci. 1987;7:2936–47. doi: 10.1523/JNEUROSCI.07-09-02936.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. Reciprocal Schwann cell–axon interactions. Curr Opin Neurobiol. 1993;3:683–93. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–30. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Robertson AM, King RH, Muddle JR, Thomas PK. Abnormal Schwann cell/axon interactions in the Trembler-J mouse. J Anat. 1997;190(Part 3):423–32. doi: 10.1046/j.1469-7580.1997.19030423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, Lu QR, Kessaris N, Richardson WD. An “oligarchy” rules neural development. Trends Neurosci. 2002;25:417–22. doi: 10.1016/s0166-2236(02)02201-4. [DOI] [PubMed] [Google Scholar]
- Sawamura S, Sawada M, Ito M, Nagatsu T, Nagatsu I, Suzumura A, et al. The bipotential glial progenitor cell line can develop into both oligodendrocytes and astrocytes in the mouse forebrain. Neurosci Lett. 1995;188:1–4. doi: 10.1016/0304-3940(95)11378-a. [DOI] [PubMed] [Google Scholar]
- Schneider S, Bosse F, D"Urso D, Muller H, Sereda MW, Nave K, et al. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci. 2001;21:920–33. doi: 10.1523/JNEUROSCI.21-03-00920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun SB, Mamontov P, Golden JA, et al. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–92. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shah NM, Anderson DJ. Integration of multiple instructive cues by neural crest stem cells reveals cell-intrinsic biases in relative growth factor responsiveness. Proc Natl Acad Sci USA. 1997;94:11369–74. doi: 10.1073/pnas.94.21.11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–60. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Doyle JP, Hensley P, Colman DR, Hendrickson WA. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron. 1996;17:435–49. doi: 10.1016/s0896-6273(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–36. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy ME, Hobson G, Jain M, Boespflug-Tanguy O, Garbern J, Sperle K, et al. Schwann cell expression of PLP1 but not DM20 is necessary to prevent neuropathy. Ann Neurol. 2003;53:354–65. doi: 10.1002/ana.10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff RP, Knapp PE. Division of astroblasts and oligodendroblasts in postnatal rodent brain: evidence for separate astrocyte and oligodendrocyte lineages. Glia. 1991;4:165–74. doi: 10.1002/glia.440040208. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Gasser UE, Hatten ME. Molecular mechanisms of glial-guided neuronal migration. Ann N Y Acad Sci. 1991;633:113–21. doi: 10.1111/j.1749-6632.1991.tb15602.x. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131:2349–58. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, et al. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell. 2006;11:697–709. doi: 10.1016/j.devcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Su Y, Brooks DG, Li L, Lepercq J, Trofatter JA, Ravetch JV, et al. Myelin protein zero gene mutated in Charcot–-Marie-tooth type 1B patients. Proc Natl Acad Sci USA. 1993;90:10856–60. doi: 10.1073/pnas.90.22.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman CR, Vartanian T, Miller RH. The ErbB4 neuregulin receptor mediates suppression of oligodendrocyte maturation. J Neurosci. 2005;25:5757–62. doi: 10.1523/JNEUROSCI.4748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott JF, Loy DN, Liu Y, Qiu MS, Bunge MB, Rao MS, et al. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Buettner HM. Neurite outgrowth is directed by schwann cell alignment in the absence of other guidance cues. Ann Biomed Eng. 2006;34:161–8. doi: 10.1007/s10439-005-9013-4. [DOI] [PubMed] [Google Scholar]
- Timsit S, Martinez S, Allinquant B, Peyron F, Puelles L, Zalc B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J Neurosci. 1995;15:1012–24. doi: 10.1523/JNEUROSCI.15-02-01012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–9. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Topilko P, Levi G, Merlo G, Mantero S, Desmarquet C, Mancardi G, et al. Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res. 1997;50:702–12. doi: 10.1002/(SICI)1097-4547(19971201)50:5<702::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Hauer P, Lemke G. Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells. J Neurosci. 1988;8:3515–21. doi: 10.1523/JNEUROSCI.08-09-03515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernadakis A. Neuron–glia interrelations. Int Rev Neurobiol. 1988;30:149–224. [PubMed] [Google Scholar]
- Wakamatsu Y, Osumi N, Weston JA. Expression of a novel secreted factor, Seraf indicates an early segregation of Schwann cell precursors from neural crest during avian development. Dev Biol. 2004;268:162–73. doi: 10.1016/j.ydbio.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla A, Johnson JE, Yokota Y, Barres BA. A role for the helix–loop–helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29:603–14. doi: 10.1016/s0896-6273(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell–cell interaction in rat Schwann cells. J Neurosci. 2002;22:4066–79. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Guerra NK, Mahoney J, Kumar A, Wood PM, Mirsky R, et al. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia. 2006a;54:439–59. doi: 10.1002/glia.20390. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Mahoney J, Jessen KR, Wood PM, Bates M, Bunge MB. Invariant mantling of growth cones by Schwann cell precursors characterize growing peripheral nerve fronts. Glia. 2006b;54:424–38. doi: 10.1002/glia.20389. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, et al. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–48. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PM, Bunge RP. The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte. Glia. 1991;4:225–32. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Tekki-Kessaris N, Stiles CD, Rowitch DH, Richardson WD. Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int J Dev Neurosci. 2001;19:379–85. doi: 10.1016/s0736-5748(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Yao JK, Windebank AJ, Poduslo JF, Yoshino JE. Axonal regulation of Schwann cell glycolipid biosynthesis. Neurochem Res. 1990;15:279–82. doi: 10.1007/BF00968672. [DOI] [PubMed] [Google Scholar]
- Yin X, Baek RC, Kirschner DA, Peterson A, Fujii Y, Nave KA, et al. Evolution of a neuroprotective function of central nervous system myelin. J Cell Biol. 2006;172:469–78. doi: 10.1083/jcb.200509174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalc B, Colman DR. Origins of vertebrate success. Science. 2000;288:271–2. doi: 10.1126/science.288.5464.271c. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol Cell Neurosci. 1996;8:129–45. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]