Abstract
Purpose
In vivo, biomechanical stress plays an important role in tissue physiology and pathology, affecting cell and tissue behavior. Even though conventional outflow tissues are constantly exposed to dynamic changes in intraocular pressure, effects of such biomechanical stressors on outflow function have not been analyzed. The purpose of the present study was to determine the effect(s) of ocular pulse on conventional outflow facility in perfused anterior segments.
Methods
The anterior segment perfusion model was used to investigate the impact of ocular pulsation on human and porcine outflow facility. To determine tissue viability of human anterior segments, three complementary techniques (postperfusion morphology and cell density of outflow tissues plus central corneal thickness measurements over time of perfusion) were used.
Results
A consistent decrease in outflow facility was observed in response to cyclic intraocular pressure in both porcine (−29.96% ± 6.56; P = 0.009) and human (−27.65% ± 8.26; P = 0.010) perfused anterior segments. Viability data showed no significant difference between control and experimental anterior segments, with respect to postperfusion histologic evaluations (P = 0.227) or change in central corneal thickness over time (P = 0.289). In contrast, the cellularity of the trabecular meshwork in experimental (cyclically pulsed) anterior segments (333.86 ± 22.15 nuclei/field of view) was greater than in the control eyes (290.47 ± 17.60, P = 0.05).
Conclusions
Decreased outflow facility in cyclically pulsed anterior segments is not a function of cell or tissue damage, but rather is an active response of the conventional outflow tissues to a biomechanical stimulus. In fact, the observation of increased cellularity in tissues exposed to cyclic stress suggests a physiological benefit of mechanical stress to outflow cells in organ culture.
Intraocular pressure (IOP) is generated and maintained within a narrow range as a result of the balance between aqueous humor secretion and aqueous humor outflow. In humans, the majority of aqueous humor exits the eye via the conventional outflow pathway, composed of trabecular meshwork (TM) and Schlemm's canal (SC). The conventional outflow pathway is pressure sensitive, actively responding to its microenvironment by regulating fluid flow.1,2 The exact mechanisms by which trabecular outflow structures sense and respond to biomechanical forces is not well understood.
In other tissues, mechanical stress is a critical regulator of cell behavior, altering functional and structural properties.3–6 For instance, endothelial cells respond differently to various types of stress and frequencies and are able to discern between static and dynamic stressors.7 In the conventional outflow pathway, studies have shown that resident cells respond to mechanical changes (i.e., increase in IOP) by altering their morphology.8,9 However, IOP is not a steady force but a dynamic stressor that continuously alters the biomechanical environment to which the conventional pathway tissues are exposed.10 For example, diurnal variations in IOP (circadian rhythm) are know to occur throughout the day with an approximate peak-to-peak magnitude of 5 mm Hg, with IOP reaching its highest point during the nocturnal period.11,12 In addition, blood pulsations with each heartbeat transmit waves that create transient, repetitive changes in IOP at a rate of approximately 2.7 mm Hg/s (ocular pulse).13 The effect that these cyclic changes in IOP have in regulating resistance to aqueous humor flow through the conventional pathway is unknown. The goal of the present study was to investigate the effect of cyclic biomechanical stress on outflow facility through the conventional outflow pathway. We hypothesized that anterior segments perfused in the presence of a dynamic stress (i.e., cyclic IOP) differ from those cultured under static conditions.
Materials and Methods
Donor Eye Tissues
Enucleated human eyes were obtained from the National Disease Research Interchange (Philadelphia, PA), the Life Legacy Foundation (Tucson, AZ), the Donor Network of Arizona (Phoenix, AZ), and Sun Health Research Institute (Sun City, AZ), in accordance with the guidelines of the Declaration of Helsinki for research involving human tissue. Enucleated eyes were free of any known ocular disease and were stored in a moist chamber at 4°C until dissection.
Porcine anterior segments were obtained from the University of Arizona's meat science laboratory within 5 hours of death. Enucleated eyes were stored in phosphate-buffered saline at 4°C and were used within 10 hours of death.
Anterior Segment Dissections
Dissections were performed as previously described.14,15 In brief, eyes were hemisected at the equator and vitreous and lens were removed. The iris was trimmed from the root, and the ciliary processes were excised, but the longitudinal ciliary muscle bundles and the conventional outflow tissues were left undisturbed. In porcine anterior segments, the pectinate ligaments were carefully detached.
Pulsatile System Design
To isolate and study the resistance generated by the conventional outflow pathway in response to different biomechanical conditions, a modified version of the anterior segment perfusion model was custom built (Fig. 1). In addition to a syringe pump (PHD 2000 Programmable Syringe Pump; Harvard Apparatus, Holliston, MA) generating a constant inflow rate of 2.5 μL/min, a positive piston displacement pump (Pulsatile Blood Pump; Harvard Apparatus) was used in tandem to generate IOP oscillations that simulated the ocular pulse found in vivo. An additional real-time pressure transducer (Research Grade Blood Pressure Transducer; Harvard Apparatus) was located in parallel to the original pressure transducer (Pressure Sensor 142PC01G; Honeywell, Golden Valley, MN) to monitor and adjust peak-to-peak magnitude of intraocular pulsations.
Figure 1.
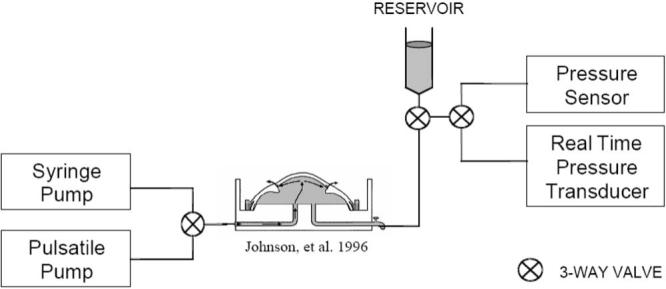
Simplified schematic diagram of the anterior perfusion model, modified to introduce IOP oscillations.16
Anterior Segment Pulsatile Perfusions
Postmortem paired human eyes were perfused at a constant inflow rate (2.5 μL/min) until they reached a stable baseline facility within a physiological range. When outflow facility had been stable for ≥8 hours, the real-time pressure transducer and the pulsatile blood pump were connected to the system. After the outflow facility recovered to baseline, IOP pulsations were introduced with a peak-to-peak magnitude of 2.7 mm Hg at a frequency of 1 Hz. Peak-to-peak pressure was monitored and manually adjusted until the desired magnitude was obtained. In the meantime, average intrachamber pressure was kept undisturbed by means of a column reservoir. The pressure sensor (Honeywell), connected in series with the anterior segment, was used to record intrachamber pressure every 2 minutes throughout the experiment. Data were stored (Data Acquisition Logging Unit; Valitec, Dayton, OH) and later used to calculate outflow facility (inflow rate/pressure). During IOP oscillations, the transducer (Real-Time Transducer; Harvard Apparatus) was used to monitor pressure at a frequency of 100 to 500 Hz. One eye of each pair received pulsation while the contralateral eye underwent a standard (steady) perfusion.
Viability
Human anterior segment viability was analyzed by three different methods: determination of the change in central corneal thickness (CCT) over the time of experiments, postperfusion histology of outflow tissues, and cell density in outflow tissues by nuclear density counts.
Central corneal thickness (CCT) was measured throughout the experiment with an ultrasound pachymetry system (Handy Pachymeter SP-100; Tomey, Waltham, MA). Every CCT measurement represented an average of three to five individual readings. The first CCT measurement was taken before tissue dissection, and consecutive measurements were taken every subsequent day (for 2 to 6 days) until IOP fluctuations were introduced into the system. CCT slopes from each individual anterior segment were later calculated over time of perfusion and pooled together to obtain an average ± SE measure of rate of change of CCT for both control and experimental groups.
After perfusion, DMEM was quickly exchanged for 3% paraformaldehyde at 10 to 15 mm Hg. Anterior segments were perfusion fixed for 1 hour at a constant inflow rate of 2.5 μL/min. Wedges from each quadrant were processed by standard histology, and 0.5-μm radial sections were cut and stained with toluidine blue. Tissue was evaluated in a masked fashion according to a previously described standard scoring system. In brief, a score of 0 represented a nonviable tissue in which the inner wall has been disrupted and cells are not present anywhere in the TM; 1 indicated that few cells were present in the TM and that the inner wall was intact; 2 designated tissue with a well populated-juxtacanalicular tissue (JCT) and an intact inner wall; 3 indicated that the corneoscleral meshwork as well as the JCT were well-populated with cells; and 4 corresponded to the tissue's being in excellent condition with healthy-appearing cells present everywhere in the outflow pathway.
To determine tissue cellularity, additional wedges were taken from each quadrant of previously perfusion-fixed tissue and stored in 2% paraformaldehyde solution. Tissue wedges were later embedded in optimal cutting temperature (OCT) compound and frozen sections were prepared (5 μm thick). Tissue sections were labeled using 4′-6-diamidino-2-phenylindole (DAPI) to stain nuclei. Sections were oriented so that the filtering region of the TM was visible in the field of view (FOV), using the scleral spur as a landmark. Two sequential tissue sections were analyzed for each quadrant (eight sections per eye) and nuclei were counted in a masked fashion at 400× magnification (IX70 microscope; Olympus, Tokyo, Japan). Microscopic FOVs represented an approximate TM area of 0.196 mm2. Results are expressed as the average number of nuclei of eight different FOVs per eye.
Because of the freshness of the tissue, the viability of porcine anterior segments was determined solely by analyzing facility traces over time. Anterior segments that did not show a characteristic washout17–19 and/or did not reach a stable baseline in the range of 0.3 to 0.5 μL/min/mm Hg within 5 days of perfusion were rejected.
Results
Twelve pairs of human eyes from donors of a mean ± SD age of 73 ± 13.3 years were perfused in organ culture, averaging (5.14 ± 1.5 hours) from time of death to time of enucleation and (23.45 ± 11.0 hours) from time of death to time to receipt of tissue in our laboratory. A summary of the results of all human anterior segment perfusions (including donor information, time from death to enucleation, time from death to tissue receipt, and outflow facilities before and after pulsatile IOP) is contained in Table 1. Outflow facility information for all porcine anterior segment perfusions is shown in Table 2.
Table 1.
Summary of Results from Human Anterior Segment Perfusions in Response to Intraocular Pressure Oscillations
|
FACILITY (μL/min/mm Hg) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | TOD-TOE (h) | TOD-TOR (h) | Age | Gender | Stable Baseline | New Baseline | Run Pulse Time (h) | Time Pulse-New Baseline (h) | % Facility Change | Histology Score | Nuclear Count (AVG/FOV) | CCT Slope (μm/h) |
| 5C | 7:40 | 25:20 | 54 | M | Unstable | 0.83 | 182.00 | N/A | ||||
| 6E | 0.408 | 0.364 | 48 | 61.0 | −10.80 | 1.20 | 198.25 | |||||
| 9C | 3:45 | 31:20 | 64 | M | 0.289 | 0.302 | 4.50 | 2.50 | 336.25 | −1.53 | ||
| 10E | 0.176 | 0.079 | 110 | 70.0 | −55.10 | 2.55 | 327.00 | −1.65 | ||||
| 21C | 6:02 | 8:00 | 90 | N/A | 0.129 | 0.136 | 5.40 | 1.63 | 346.75 | −1.10 | ||
| 22E | 0.124 | 0.091 | 30 | 12.0 | −26.20 | 1.25 | 463.63 | −1.17 | ||||
| 35E | 5:25 | 30:10 | 74 | M | 0.195 | 0.172 | 8/12 | 24.0 | −11.80 | 2.50 | 283.88 | −1.71 |
| 36C | 0.436 | 0.436 | 0.00 | 2.30 | 262.50 | −0.92 | ||||||
| 37C | 2:57 | 30:27 | 72 | F | 0.104 | 0.102 | −1.90 | 2.13 | 316.50 | −1.12 | ||
| 38E | 0.139 | 0.126 | 24 | 27.5 | −9.40 | 2.13 | 304.63 | −0.03 | ||||
| 39E | 5:00 | 33:00 | 76 | F | 0.151 | 0.132 | 10 | 12.0 | −12.60 | N/A | N/A | −1.46 |
| 40C | 0.089 | 0.094 | 5.60 | −2.05 | ||||||||
| 49E | 2:50 | 34:25 | 78 | F | 0.213 | 0.176 | 8 | 13.0 | −17.37 | 3.13 | 315.38 | −1.90 |
| 50C | 0.122 | 0.118 | −3.28 | 2.63 | 292.25 | −1.30 | ||||||
| 59C | 5:05 | 18:55 | 51 | F | 0.413 | 0.399 | −3.39 | 2.50 | 242.63 | −1.02 | ||
| 60E | 0.167 | 0.145 | 6 | 6.0 | −13.17 | 3.13 | 397.13 | −0.73 | ||||
| 63E | 6:35 | 37:00 | 74 | M | 0.283 | 0.050 | 8 | 25.0 | −82.33 | N/A | N/A | −0.72 |
| 64C | 0.247 | 0.238 | −3.64 | −0.25 | ||||||||
| 67E | 3:50 | 16:35 | 90 | F | 0.178 | 0.094 | 8 | 26.0 | −47.19 | 1.50 | 363.50 | −0.09 |
| 68C | Unstable | 1.50 | 322.38 | −1.02 | ||||||||
| 71E | 6:25 | 7:25 | 91 | F | 0.082 | 0.065 | 8 | 10.0 | −20.73 | 1.88 | 334.50 | −1.33 |
| 72C | Unstable | −1.74 | ||||||||||
| 77C | 6:05 | 8:45 | 64 | M | 0.101 | 0.101 | 0.00 | 2.25 | 313.00 | −3.04 | ||
| 78E | 0.115 | 0.091 | 8 | 20.5 | −20.87 | 2.13 | 350.75 | −1.63 | ||||
| Control | 0.37 ± 1.28 | 2.03 ± 0.20 | 290.47 ± 17.60 | −1.37 ± 0.22 | ||||||||
| Experimental | −27.65 ± 8.26 | 2.14 ± 0.22 | 333.86 ± 22.15 | −1.13 ± 0.20 | ||||||||
C, control; E, experimental (in bold); TOD, time of death; TOE, time of enucleation; TOR, time when tissue was received; AVG/FOV, average nuclei per field of view; N/A, not available. Summary data are mean ± SEM.
Table 2.
Summary of Results from Porcine Anterior Segment Perfusions in Response to Intraocular Pressure Oscillations
|
FACILITY |
|||||
|---|---|---|---|---|---|
| ID | Stable Baseline (5 h) | After Pulse (5 h) | % Change | Run Pulse Time (h) | Time (Pulse to Baseline) (h) |
| 10C | 0.435 | 0.424 | −2.61 | ||
| 11E | 0.413 | 0.314 | −23.79 | 10 | 49 |
| 12C | 0.449 | 0.476 | 6.01 | ||
| 13E | 0.450 | 0.258 | −42.67 | 8 | 76 |
| 16C | 0.506 | 0.438 | −13.55 | ||
| 17E | 0.482 | 0.254 | −47.34 | 10 | 34 |
| 18C | 0.333 | 0.358 | 7.74 | ||
| 19E | 0.498 | 0.379 | −23.96 | 8 | 16 |
| 22E | 0.466 | 0.410 | −12.01 | 8 | 11 |
| Control | −0.6 ± 4.87 | ||||
| Experimental | −29.96 ± 6.56 | ||||
Anterior Segment Perfusions
A decrease in outflow facility over time in response to IOP oscillations was observed in both the human and the porcine anterior segments. Average outflow facilities before normalization showed no significant difference between the control (0.21 μL/min/mm Hg) and the experimental (0.19 μL/min/mm Hg; P = 0.368) groups of human anterior segment perfusions. Data from the paired human anterior segments showed an average facility decrease of −27.7% ± 8.3% (mean ± SEM), whereas the control paired eyes showed no decrease in outflow facility (0.4% ± 1.3%). These results were remarkably similar to the average decrease in outflow facility (−27.3% ± 6.5%) observed in data pooled from all paired and unpaired human anterior segment perfusions.
Similarly, outflow facilities of porcine anterior segments showed no significant difference between the control (0.43 μL/min/mm Hg) and experimental (0.46 μL/min/mm Hg, P = 0.56) groups before normalization to their stable baselines. Like the human anterior segments, the porcine anterior segments showed an average decrease in outflow facility (−29.96% ± 6.56%) in the experimental group in response to IOP oscillations, whereas no significant change in outflow facility (−0.6% ± 4.87%) was observed in the paired control group.
A biphasic decrease in normalized outflow facility was observed in both species of anterior segments in response to physiologically relevant cyclic mechanical stress (pulsatile IOP for durations of 8 to 10 hours). A significant difference between control and experimental groups was recorded 8 hours (P = 0.048) after pulsatile pressure stopped (Fig. 2) in the human anterior segments and 19 hours (P = 0.025) after IOP oscillations in the porcine anterior segments (Fig. 3).
Figure 2.

Summary of facility data obtained from nine pairs of human anterior segments in organ culture. Experimental anterior segments received IOP pulsations for 8 to 10 hours (mean ± SEM). Dashed lines: the beginning and end of pressure oscillations (*P < 0.05).
Figure 3.

Summary of facility data obtained from four porcine anterior segments receiving IOP pulsations for 8 to 10 hours (mean ± SEM). Dashed lines: the beginning and end of pressure oscillations (*P < 0.05, **P < 0.01).
Viability
Studies20 from our laboratory have shown that a decrease in CCT over time correlates with an increase in viability of outflow tissues cultured using the anterior segment perfusion model. Based on these previous experiments, CCT was used as a real-time viability indicator from the beginning of perfusion until IOP oscillations were introduced to the system. The average slopes of the CCT versus time curves showed no significant difference between control (slope: −1.37 μm/h) and experimental (slope: −1.13 μm/h, P = 0.289) human eyes (Fig. 4).
Figure 4.
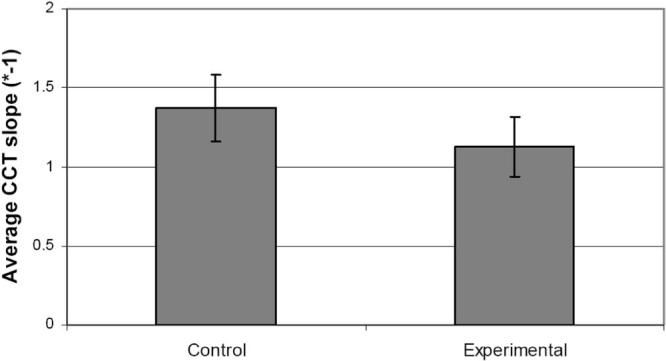
Comparison of average (±SEM) change in central corneal thickness (CCT) of human anterior segments in organ culture over time of perfusion.
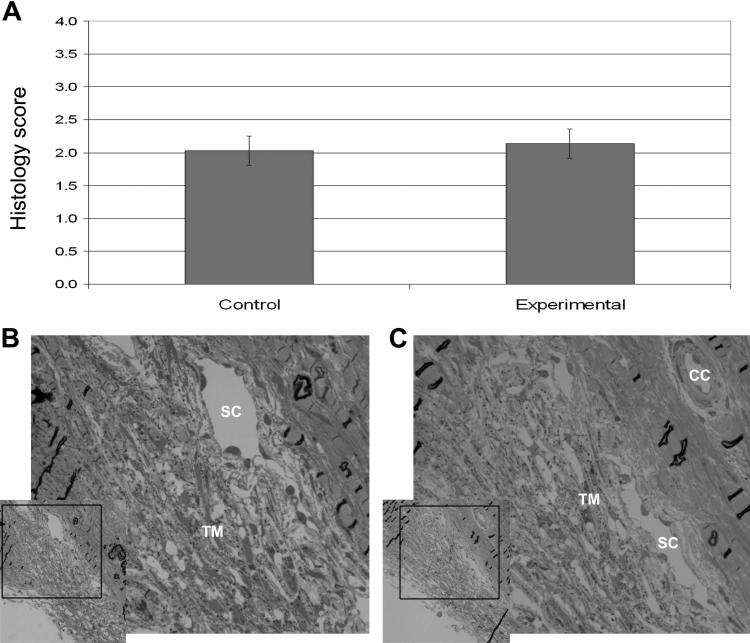
Second, tissue viability was assessed in the traditional manner by examining morphologic appearance of outflow tissues after perfusion. Figures 5B and 5C illustrate representative histologic sections of an experimental and a control human anterior segment. There were no obvious differences in tissue structure or cellularity between tissues exposed to pulsatile IOP and the paired controls. After we examined all tissues, we found that the mean histologic scores were not significantly different between the control (2.03) and experimental (2.14, P = 0.227) human anterior segments (Fig. 5A).
Figure 5.
(A) Comparison of average histologic scores (±SEM) from human anterior segments in organ culture. Also shown are representative histologic sections from control segment 59 (B) and experimental segment 60 (C). (B, C) A low magnification image is shown on the left with a region delimited with a black box that is shown at higher magnification on the right. SC, Schlemm's canal; TM, trabecular meshwork; CC, collector channel.
Last, outflow tissue cellularity was examined. The groups of anterior segments were pooled and averaged to give the mean (±SEM) of nuclei per FOV in each group (control and experimental). Altogether, anterior segments exposed to IOP oscillations showed an average nuclear count per FOV of 333.86, whereas their paired controls averaged 290.47 nuclei per FOV. Control and experimental groups were significantly different (P = 0.05) in the cellularity of the conventional outflow tissues (Fig. 6).
Figure 6.
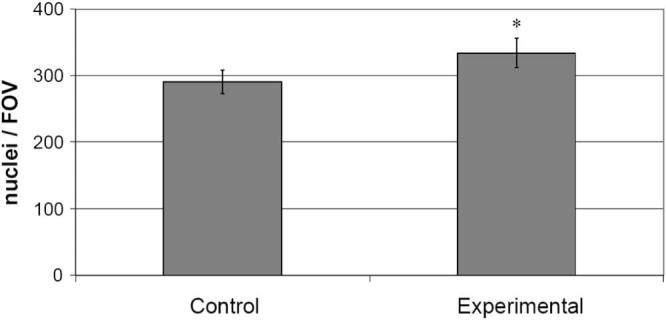
Comparison of the average (±SEM) number of nuclei per microscopic field of view (FOV) from paired human anterior segments in organ culture (*P = 0.05).
Discussion
The primary goal was to examine the capacity of conventional outflow tissues to respond to a cyclic biomechanical stress. Both human and porcine anterior segments exposed to pulsatile IOP responded with a consistent decrease in outflow facility, having remarkably similar magnitudes and patterns of response. Using three complementary techniques to evaluate viability of outflow tissues, we established that the observed decrease in outflow facility was not a consequence of tissue damage, suggesting instead an active response of outflow tissues to the cyclic biomechanical stress.
Mechanical stress impacts normal tissue physiology and plays a role in several diseases.3,21–24 Conventional outflow tissues (in vivo) are exposed to a variety of mechanical forces (static and dynamic) that may impact their function and morphology. Since past research has primarily focused on how a static increase in IOP can affect outflow tissues, little is known about the effect of cyclic or dynamic stresses applied to these tissues. Using the anterior segment perfusion system, we successfully introduced and studied the effects of a physiologically relevant pulsatile stress on the trabecular outflow tissues. Our results show a biphasic decrease in outflow facilities of human and porcine anterior segments. Because the anterior segment model physiologically isolates the conventional outflow pathway, decreases in outflow facility can be directly linked to an increased resistance through this pathway.
Of note, despite the physiological and anatomic differences that are known to exist among species, the porcine and human anterior segments showed similar behavior in response to cyclic biomechanical stress. Although a significant decrease was observed earlier in human anterior segments, both species showed a biphasic behavior in outflow facility that was characterized by two consecutive decreases in outflow facility over time. A short-term decrease was observed in both species during the pulse period and was followed by a second decrease in outflow facility starting approximately 9 hours (human) and 15 hours (porcine) after pulsation. Although differences in onset of responses may be related to disparities in the anatomy of conventional outflow pathway or difference in freshness of tissue preparations, the pattern of responses was remarkably similar, suggesting that the decreases in outflow facility observed are not species-specific, but instead are an intrinsic cellular reaction to a physiological stress. If true, it is likely that such a mechanism is integral to the regulation of outflow facility.
Both real time (CCT) and histologic scoring methods of viability evaluation showed no significant differences between segments exposed to pressure oscillations and their paired controls that were cultured under nonpulsatile conditions. Results suggest that the decrease in outflow facility observed in response to cyclic IOP oscillations was not a function of damage to cells or structures in the conventional outflow pathway, but rather an active cellular response to the mechanical stimulus. Even though CCT and histologic evaluations were not different between control and experimental groups, we unexpectedly observed an increased cellularity in the experimental group. The increased number of nuclei per FOV in response to cyclic stress suggests that some stressed cells (due to postmortem time and manipulations before perfusion) may have recovered in response to cyclic mechanical stress. Such an observation is congruent with the hypothesis that in vivo cells are exposed to a variety of mechanical forces and therefore have an affinity for being stressed.
Previous studies that examine endothelial cell behavior suggest that contractility plays an important role in tissue response to cyclic mechanical stress.25–27 Given that the TM is a smooth-muscle–like tissue with contractile properties and that drugs that affect contractility of TM influence facility,28–31 we speculated that the decrease in outflow facility observed in response to pulsatile IOP is related to TM contractility and associated with calcium signaling through stretch-activated cation channels. Opening of stretch channels may lead to cell depolarization and L-type calcium channel activation. With this line of reasoning, introduction of a calcium channel inhibitor (i.e., nifedipine) to our novel model system may provide a better understanding of the importance of calcium-dependent contractility in the TM in response to cyclic IOP.
Although our calcium hypothesis is plausible, the mechanism(s) responsible for the observed increased resistance through the outflow pathway in response to cyclic mechanical stress are presently speculative. Further studies are needed to determine the exact mechanism that underlies the response of outflow cells to cyclic stress. Regardless, given that outflow tissues are exposed to a unique biomechanical environment that plays a role in cellular distribution of filamentous (F)-actin23,32 (and now outflow facility), we hypothesize that the morphology and organization of F-actin fibers (and associated cell junction complexes) in response to cyclic stress will provide a better understanding of the role that biomechanics play in the regulation of conventional outflow.
Acknowledgments
The authors thank Ross Ethier for helpful advice during the construction of our experimental approach and for careful review of the manuscript, Ann Baldwin for lending us the pulsatile blood pump, and Zhou Wan for assistance with scoring of histological sections.
Supported in part by National Eye Institute Grants EY01687 and EY17007 and Research to Prevent Blindness Foundation.
Footnotes
Disclosure: R.F. Ramos, None; W.D. Stamer, None
References
- 1.Hashimoto JM, Epstein DL. Influence of intraocular pressure on aqueous outflow facility in enucleated eyes of different mammals. Invest Ophthalmol Vis Sci. 1980;19:1483–1489. [PubMed] [Google Scholar]
- 2.Johnstone MA. Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm's canal. Invest Ophthalmol Vis Sci. 1979;18:44–51. [PubMed] [Google Scholar]
- 3.Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- 4.Deng L, Fairbank NJ, Fabry B, Smith PG, Maksym GN. Localized mechanical stress induces time-dependent actin cytoskeletal remodeling and stiffening in cultured airway smooth muscle cells. Am J Physiol. 2004;287:C440–C448. doi: 10.1152/ajpcell.00374.2003. [DOI] [PubMed] [Google Scholar]
- 5.Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- 6.Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32:338–345. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- 7.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol. 1975;14:286–292. [PubMed] [Google Scholar]
- 9.Epstein DL, Rohen JW. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci. 1991;32:160–171. [PubMed] [Google Scholar]
- 10.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- 11.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 12.Liu JH, Gokhale PA, Loving RT, Kripke DF, Weinreb RN. Laboratory assessment of diurnal and nocturnal ocular perfusion pressures in humans. J Ocul Pharmacol Ther. 2003;19:291–297. doi: 10.1089/108076803322279354. [DOI] [PubMed] [Google Scholar]
- 13.Kerr J, Nelson P, O'Brien C. Pulsatile ocular blood flow in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2003;136:1106–1113. doi: 10.1016/s0002-9394(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DH, Tschumper RC. The effect of organ culture on human trabecular meshwork. Exp Eye Res. 1989;49:113–127. doi: 10.1016/0014-4835(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DH, Tschumper RC. Human trabecular meshwork organ culture: a new method. Invest Ophthalmol Vis Sci. 1987;28:945–953. [PubMed] [Google Scholar]
- 16.Johnson DH. Human trabecular meshwork cell survival is dependent on perfusion rate. Invest Ophthalmol Vis Sci. 1996;37:1204–1208. [PubMed] [Google Scholar]
- 17.Yan DB, Trope GE, Ethier CR, Menon IA, Wakeham A. Effects of hydrogen peroxide-induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthalmol Vis Sci. 1991;32:2515–2520. [PubMed] [Google Scholar]
- 18.Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest Ophthalmol Vis Sci. 1990;31:2384–2388. [PubMed] [Google Scholar]
- 19.Scott PA, Overby DR, Freddo TF, Gong H. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007;84:435–443. doi: 10.1016/j.exer.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Z, Brigatti L, Ranger-Moore J, Ethier CR, Stamer WD. Rate of change in central corneal thickness: a viability indicator for conventional drainage tissues in organ culture. Exp Eye Res. 2006;82:1086–1093. doi: 10.1016/j.exer.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Lemus LA, Sun Z, Trache A, Trzciakowski JP, Meininger GA. Integrins and regulation of the microcirculation: from arterioles to molecular studies using atomic force microscopy. Microcirculation. 2005;12:99–112. doi: 10.1080/10739680590896054. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Xu Q. Mechanical stress-initiated signal transduction in vascular smooth muscle cells in vitro and in vivo. Cell Signal. 2007;19:881–891. doi: 10.1016/j.cellsig.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 24.Pearson JD. Tightening the barrier: mechanical forces and the control of endothelial permeability. Arteriosclerosis Thromb Vasc Biol. 2006;26:10–11. doi: 10.1161/01.ATV.0000197858.50074.c6. [DOI] [PubMed] [Google Scholar]
- 25.Wang JH, Goldschmidt-Clermont P, Yin FC. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann Biomed Eng. 2000;28:1165–1171. doi: 10.1114/1.1317528. [DOI] [PubMed] [Google Scholar]
- 26.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274:H1532–H1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 27.Naruse K, Sokabe M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol. 1993;264:C1037–C1044. doi: 10.1152/ajpcell.1993.264.4.C1037. [DOI] [PubMed] [Google Scholar]
- 28.Thieme H, Stumpff F, Ottlecz A, Percicot CL, Lambrou GN, Wiederholt M. Mechanisms of action of unoprostone on trabecular meshwork contractility. Invest Ophthalmol Vis Sci. 2001;42:3193–3201. [PubMed] [Google Scholar]
- 29.Lepple-Wienhues A, Stahl F, Wiederholt M. Differential smooth muscle-like contractile properties of trabecular meshwork and ciliary muscle. Exp Eye Res. 1991;53:33–38. doi: 10.1016/0014-4835(91)90141-z. [DOI] [PubMed] [Google Scholar]
- 30.Wiederholt M, Schafer R, Wagner U, Lepple-Wienhues A. Contractile response of the isolated trabecular meshwork and ciliary muscle to cholinergic and adrenergic agents. German J Ophthalmol. 1996;5:146–153. [PubMed] [Google Scholar]
- 31.Tian B, Gabelt BT, Peterson JA, Kiland JA, Kaufman PL. H-7 increases trabecular facility and facility after ciliary muscle disinsertion in monkeys. Invest Ophthalmol Vis Sci. 1999;40:239–242. [PubMed] [Google Scholar]
- 32.Ethier CR, Read AT, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J. 2004;87:2828–2837. doi: 10.1529/biophysj.103.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]