Abstract
It is known that animals decerebrated at the premammillary level are capable of standing and walking without losing balance, in contrast to postmammillary ones which do not exhibit such behavior. The main goals of the present study were, first, to characterize the postural performance in premammillary rabbits, and, second, to activate the postural system in postmammillary ones by brainstem stimulation. For evaluation of postural capacity of decerebrated rabbits, motor and EMG responses to lateral tilts of the supporting platform and to lateral pushes were recorded before and after decerebration. In addition, the righting behavior (i.e., standing up from the lying position) was video recorded.
We found that, in premammillary rabbits, responses to lateral tilts and pushes were similar to those observed in intact ones, but the magnitude of responses was reduced. During righting, premammillary rabbits assumed the normal position slower than intact ones.
To activate the postural system in postmammillary rabbits, we stimulated electrically two brainstem structures, the mesencephalic locomotor region (MLR) and the ventral tegmental field (VTF). The MLR stimulation (prior to elicitation of locomotion) and the VTF stimulation caused an increase of the tone of hindlimb extensors, and enhanced their responses to lateral tilts and to pushes.
These results indicate that the basic mechanisms for maintenance of body posture and equilibrium during standing are present in decerebrated animals. They are active in the premammillary rabbits but need to be activated in the postmammillary ones.
Keywords: posture, balance, sensory-motor integration, decerebrated rabbit, mesencephalic locomotor region, ventral tegmental field
1. Introduction
In quadrupeds, the most common posture of the body, with its dorsal side oriented upward (basic posture), is maintained by the postural system. This closed-loop control system is driven by sensory feedback signals and compensates for deviations from the desired body orientation by producing corrective motor responses (for review see [5,11,21,22]).
Many motor centers, from the spinal cord to the motor cortex, seem to participate in the maintenance of body posture [11,13]. However, the distribution of postural functions between these centers and the specific role of each of them in the control of posture are not quite clear [17,18]. Nevertheless, one principal fact is well established - the animals (cats and rabbits) with more rostral decerebration are capable of standing and walking without losing balance [1,19]. This fact implies that a substantial part of the system controlling body posture and equilibrium is located below the decerebration level, that is, in the brainstem, cerebellum, and spinal cord.
The capacity of decerebrated animals to maintain equilibrium, that is, to react properly to different perturbations of posture, has not been systematically investigated, however. This issue was addressed in the present study with experiments on rabbits. We used this animal model because (i) the rabbit does not need special training for the postural task of keeping balance on the tilting platform, and (ii) the functional organization of postural control system in the rabbit was characterized in our previous studies (Beloozeriva et al. 2003).
The first specific aim of the present study was to characterize postural performance in the decerebrated rabbits. Since the level of decerebration is known to strongly affect the animal’s motor activity including the value of muscle tone, the intensity of reflex responses, and the spontaneous movements (see e.g., [1,28]), we examined postural performance in animals decerebrated at different rostro-caudal levels, premammillary and postmammillary ones (see below).
It is known that in intact quadrupeds, a lateral tilt of the supporting surface causes corrective movements, which restore the dorsal side up orientation of the trunk. The functional organization of the postural control system generating these corrective movements in rabbits and cats has been analyzed in our previous studies [2,4]. It was found that this system consists of two relatively independent sub-systems responsible for stabilization of the anterior and posterior part of the trunk, respectively. Each of the sub-systems is driven by somatosensory input from the limbs of the corresponding girdle. In the present study we evaluated operation of the hindlimb sub-system in decerebrated rabbits. For this purpose, we compared the motor and EMG responses to lateral tilts of the platform supporting the hindquarters before and after decerebration.
An important postural task is compensation for trunk displacements in the frontal plane caused by a lateral force applied to the body. Intact quadrupeds, when standing, easily correct for lateral pushes and do not fall even with application of rather large forces [16]. Recently, operation of the postural mechanisms of the hind- and forequarters in response to application of pushes to the posterior or to the anterior part of the trunk was examined in cats [15]. In the present study, we evaluated operation of the hindquarters mechanism of this system by comparing motor and EMG responses to lateral pushes in rabbits before and after decerebration.
When positioned on their side, quadrupeds exhibit a set of righting reflexes and rapidly assume the basic standing posture [19]. In the present study, we compared the capacity to stand up from the lying position in the rabbits before and after decerebration.
The postural control system usually interacts with other motor systems. In particular, it provides postural support for voluntary movements of the trunk and limbs [23]. Another important function of the postural system is maintenance of equilibrium at the onset of and during locomotion [25,29]. The second specific aim of the present study was to examine the effect of activation of the locomotor system on the postural system. For this purpose, we characterized the effects of electrical stimulation of the mesencephalic locomotor region (MLR) [14,31] on postural performance in the postmammillary rabbits.
An important condition for postural activity is sufficient tone of the antigravity (extensor) muscles (see e.g., [7,25]). An increase of the extensor tone in decerebrated animals can be caused by stimulation of the ventral tegmental field (VTF) in the medial brainstem [26]. The third specific aim of this study was to examine the effect of VTF stimulation on muscle tone and on the capacity to perform postural corrections in the postmammillary rabbits.
A brief account of part of this study has been published in abstract form [27].
2. Methods
2.1. Subjects
Experiments were carried out on 15 adult male New Zealand rabbits (weighting 2.5-3.0 kg). All experiments were conducted with the approval of the local ethical committee (Norra Djurforsoksetiska Namnden) in Stockholm.
2.2. Surgical procedures
Each animal was subjected to two surgeries. First, under Hypnorm-midazolam anesthesia, using aseptic procedures, bipolar EMG electrodes (0.2 mm flexible stainless steel Teflon-insulated wires) were implanted bilaterally into m. gastrocnemius (Gast, ankle extensor) and m. vastus (Vast, knee extensor). The wires were led sub-cutaneously toward the head and then through a small incision in the skin on the dorsal aspect of the neck. The wound was sutured so that the wires were fastened to the skin. A small connector was soldered to each wire at a distance of 2-3 cm from the skin.
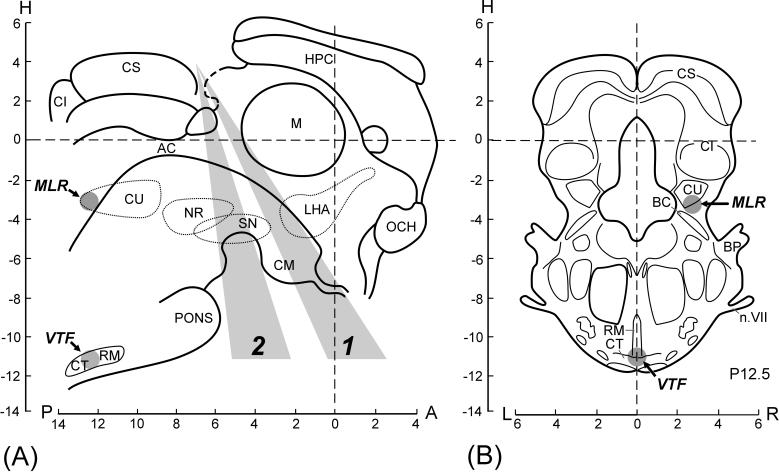
In 1-2 days, when the animal had recovered completely from the first surgery, its postural capacity was tested (see below), and then the second surgery was performed. The animal was injected with propofol (average dose 10 mg / kg i.v.) for induction of anesthesia, which was continued on isoflurane (1.5-2.5%) delivered in O2. The trachea was cannulated. The animal was then decerebrated. The scalpel entered the brainstem at the precollicular level, but the angle of cutting varied between different subjects. In Fig. 1A, all transections made in the present study are divided into two categories, the premammillary ones (1) and postmammillary ones (2), strongly differing in their effect on postural performance (see below). After decerebration, the anesthesia was discontinued. During the experiment, the rectal temperature and mean blood pressure of the animal were continuously monitored and were kept at 37-38°C and at greater than 90 mmHg, respectively. Recordings in decerebrated animals were started no less than 1 h after cessation of anesthesia.
Fig. 1.
Levels of decerebration and sites of brainstem stimulation. (A) Midsagittal plane. (B) Frontal plane (Horsley-Clarke coordinates). The brainstem transections were performed at the precollicular-premammillary level (the plane of transections was situated within the sector 1) or precollicular-postmammillary level (the sector 2). Brain structures: AC, aquaeductus cerebri; BC, brachium conjunctivum; BP, brachium pontis; CI, colliculus inferior; CM, corpus mammillaris; CS, colliculus superior; CT, corpus trapezoides; CU, area cuneiformis; HPC, hippocampus; LHA, lateral hypothalamic area; M, massa intermedia; n.VII, nervus facialis; NR, nucleus ruber; OCH, optic chiasma; SN, substantia nigra; RM, nucleus raphe magnus. Structures delineated by interrupted lines are located in parasagittal planes (LR 1-4). (Based on the stereotaxic atlases of the rabbit brain [8,30]). MLR, mesencephalic locomotor region; VTF, ventral tegmental field. Gray circles indicate effective sites of the brainstem stimulation.
2.3. Experimental design
Two types of experiments were carried out on decerebrated rabbits. First, the animals were not restrained (Fig. 2A). The hindlimbs were standing on the tilting platform (P), whereas the forelimbs had a stationary position. Second, to stimulate brainstem structures (MLR and VTF), the animals were restrained by fixing the head in a stereotaxic device (Fig. 2B). The hindlimbs were standing on the tilting platform, whereas the forequarters were suspended.
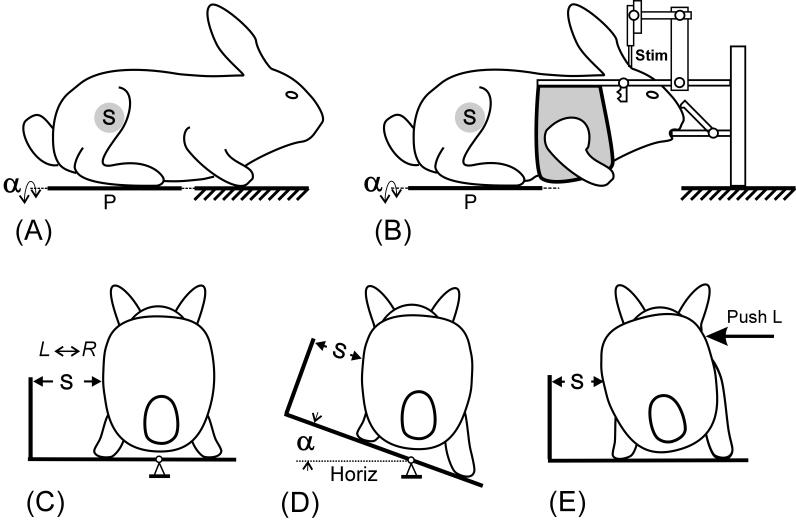
Fig. 2.
Two types of experiments, with the animal not restrained (A), and with the head fixed in a stereotaxic device (B). (A-C) Hindlimbs of the rabbit were standing on the platform P, which could be tilted in the transverse plane (α, platform tilt angle), whereas forelimbs either had a stationary position (A) or were suspended (B). (D and E) Two tests for postural reactions during standing were used: (D) lateral tilts of the platform for ±20°, and (E) lateral pushes in the lumbar region. Mechanical sensor S, positioned at a half-height of the caudal part of the body (C), measured lateral displacements of this part in relation to the platform during lateral tilts (D) and lateral pushes (E). Schematic body configurations in A-E were based on video recordings.
In both types of experiments, two tests for postural reactions during standing were used: lateral tilts and lateral pushes. The tilts of the platform had a peak-to-peak value of 40° (Fig. 2D). Two types of tilt trajectories were used: a sine-like trajectory (Fig. 9B) with a frequency of 1 Hz, and a trapezoidal trajectory (Fig. 5A and B, Fig. 7, Fig. 9D) with transition between stationary (extreme) positions taking 0.5-1.5 s and with each position being maintained for 1.5-3 s. In both sinusoidal and trapezoidal tests, tilts were symmetrical in relation to the horizontal position.
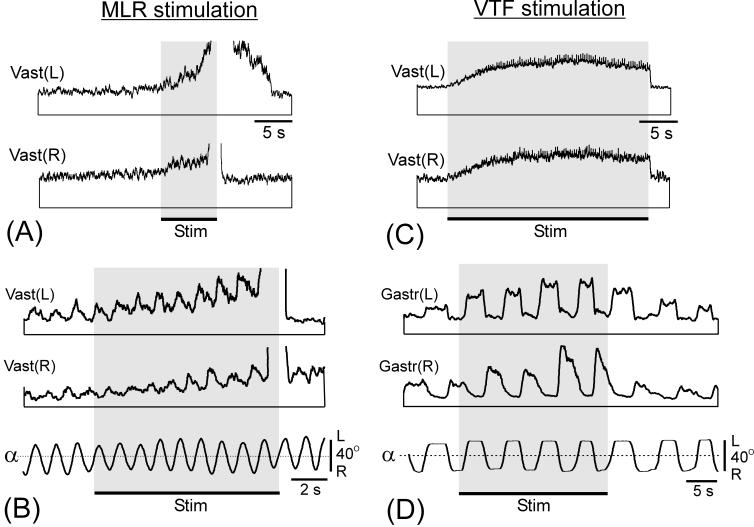
Fig. 9.
Activation of postural mechanisms in a postmammillary rabbit by MLR or VTF stimulation. (A and B) MLR stimulation caused an increase of the extensor muscle tone (A) and augmentation of the responses to tilts (B). This increase preceded the bout of locomotor activity (abrupt upward deflection of EMG curves). (C and D) VTF stimulation caused an increase of the extensor muscle tone (C) and of the responses to tilts (D). EMGs of the left (L) and right (R) gastrocnemius (Gastr) and vastus (Vast) are shown.
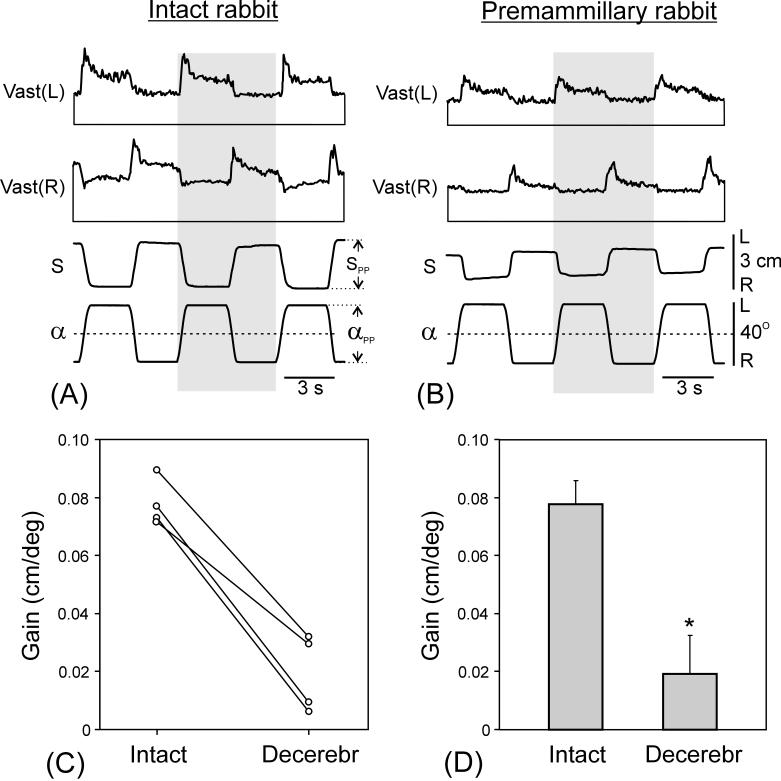
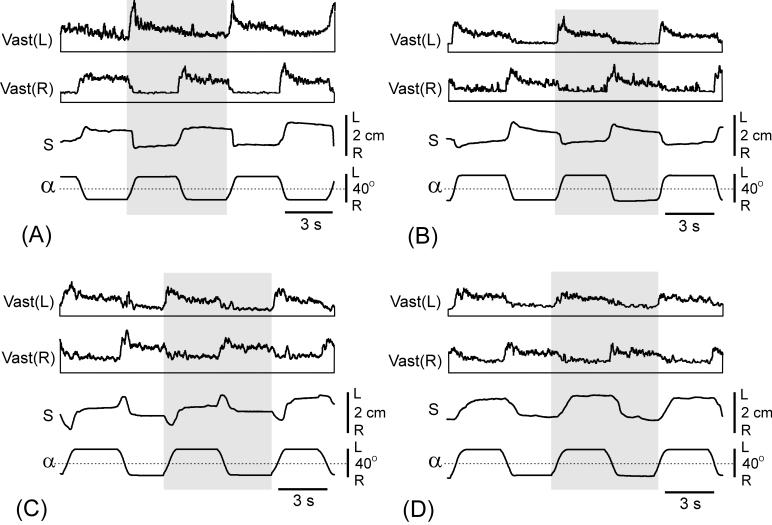
Fig. 5.
Comparison of postural responses to tilts in intact and premammillary rabbits. (A and B) Postural responses to tilts before (A) and after decerebration (B). EMGs of the left (L) and right (R) vastus (Vast) are shown. Shaded columns highlight one of the tilt cycles to facilitate comparison between curves. Designations as in Fig. 2. (C and D) Gain of postural corrections before and after decerebration in 4 individual rabbits (C) and averaged (mean ± SE) over these animals (D). In (A) and (B), amplification in EMG channels was the same.
Fig. 7.
Spontaneous changes of motor and EMG responses to tilts in a premammillary rabbit. Sequential recordings of responses in (A-D) were separated by several minutes. Designations as in Fig. 2 and 5. In (A-D), amplification in EMG channels was the same. Note the gradual decrease of postural corrections (S) and EMG responses, reflecting a spontaneous decrease of excitability of the animal. Also note that body displacements (S) were in anti-phase with tilts in (A,B) and in-phase in (D).
Lateral pushes were applied in the medial-lateral direction to the trunk in the lumbar region (Fig. 2E) using a pusher (cylindrical tool 1.5 cm in diameter and 10 cm in length) with a force sensor on the side facing the rabbit. This sensor was used to characterize timing of push application. Each push lasted for 150-200 ms. During the push, an experimenter displaced the trunk by a few centimeters in the medio-lateral direction, after which the pusher was rapidly withdrawn.
A mechanical sensor (S), positioned at a half-height of the caudal part of the body (Fig. 2), measured lateral displacements of this body area in relation to the supporting platform. These displacements allowed characterizing postural corrections generated in response to lateral pushes; in unrestrained animals they show postural corrections caused by platform tilts (Fig. 2D) [2]. The EMGs of hindlimb muscles were recorded during postural tests along with the data from the mechanical sensors.
To characterize the body configuration of the standing rabbit, the side view and view from below (using a transparent horizontal platform and a mirror) were simultaneously video recorded (25 frames/s). To characterize the righting behavior, the rabbit was positioned on its side by the experimenter and then released. The sequence of movements aimed at assuming the standing posture was video recorded. In addition, a sequence of locomotor movements was video recorded.
The intact rabbits, intended for decerebration, were used as controls. Before decerebration, they were tested (by lateral tilts and pushes) in the non-restrained condition. In addition, the basic body configuration and righting were video recorded.
2.4. Brainstem stimulation
Brainstem stimulation was used to activate the postural system in the animals with postmammillary decerebration. The stimulating bipolar electrode (two wires, dia = 150 μm, insulated except for the tips and separated by 0.5 mm) was inserted into the MLR or VTF area (Fig. 1A and B). The VTF electrode was inserted into the brainstem close to the midline, at the level of the superior colliculi, at an angle of 75-80° with respect to the horizontal plane, to a depth of 17-18 mm. The MLR electrode was inserted vertically, at 3 mm lateral to the midline, between the superior and inferior colliculi, to a depth of about 5 mm. The location of electrode tip was confirmed by histological examination.
2.5. Recording and data analysis
The signals from the EMG electrodes and from the position sensors were amplified, digitized with a sampling frequency of 5 kHz (EMGs) and 1 kHz (sensors), and recorded on a computer disk using data-acquisition and analysis software (Power-1401/Spike-2, Cambridge Electronic Design, Cambridge, UK). Then the EMG signals were rectified and smoothed (time constant, 50 ms). To evaluate the postural performance in tests with tilts, we calculated the gain of postural reflexes defined as G = SPP/αPP (cm/deg), where αPP is the peak-to-peak value of the platform tilt, and SPP is the peak-to-peak value of the postural corrections.
All quantitative data in this study are presented as mean ± SE. Student’s t-test was used to characterize the statistical significance when comparing different means. In the figures, differences with p<0.05, p<0.01, and p<0.001 are indicated by one, two and three asterisks, respectively.
2.6. Histological procedures
At the end of each experiment, electrolytic lesions were made by passing a short current pulse of 1 mA through the stimulating electrode. The brainstem was removed and placed in 10% Formalin. After fixation in Formalin, (i) the level of decerebration was determined macroscopically, and (ii) frozen sections of 40 μm thickness were cut frontally and then stained for Nissl substance with cresyl violet. The location of the electrode tip was determined microscopically, with reference to the stereotaxic atlases of the rabbit brain [8,30].
3. Results
3.1. Postural performance in premammillary rabbits
In 10 rabbits, the brainstem transection was performed at the premammillary level (the plane of transection was situated within the sector 1 in Fig. 1A). A characteristic feature of premammillary rabbits was that they exhibited spontaneous periodical changes in the level of their excitability, which were reflected in alterations of their motor activity including postural and locomotor ones (see e.g., [28]). This periodicity was better pronounced in the animals with more rostral transections. We studied postural performance during periods of increased excitability.
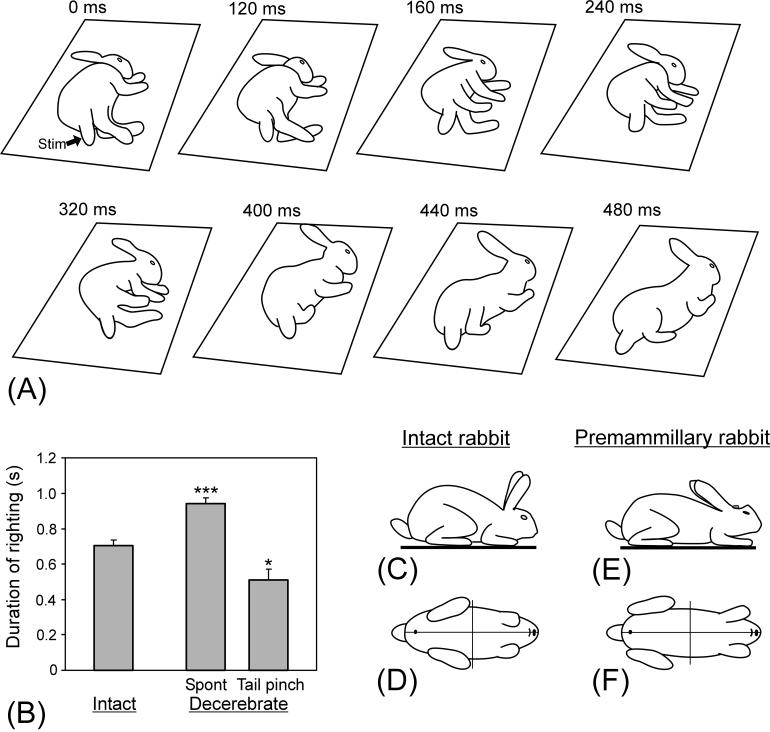
Premammillary rabbits were able to stand up from the lying position through a sequence of righting reflexes. One of the possible patterns of righting is shown in Fig. 3A. The sequence started with a ventral flexion of the trunk accompanied by a movement of the upper limbs towards each other (frame 160 ms). The head gradually turned towards its normal position (160-320 ms). Then the trunk erected and turned toward the back up position (400-440 ms). The righting movements terminated when the rabbit occurred at the normal standing position (480 ms).
Fig. 3.
Righting behavior. (A) Sequential positions of a premammillary rabbit that, starting from the lying position, acquired the dorsal side-up position. (B) Duration of righting in intact (N=10) and decerebrated (N=8) rabbits (mean ± SEM). (C-F) Typical body configuration in the standing intact rabbit (C and D) and premammillary rabbit (E and F). Side view (C and E) and view from below (D and F) are shown. (A) and (C-F) are drawings from video recordings.
A similar pattern of righting was observed in intact rabbits (not illustrated). The duration of righting in the intact animals was, on average, shorter than in the decerebrated ones. However, the righting in decerebrated animals could be considerably speeded up by exteroceptive stimulation, e.g., by pinching the tail (Fig. 3B), suggesting that the nervous mechanisms for this type of postural reactions can function normally after decerebration if sufficient excitatory drive is provided.
When standing, the postural configuration of premammillary rabbits was mostly similar to that of intact rabbits, as illustrated in Fig. 3C-F. The differences were: (i) a larger distance between the fore and hind limbs (mainly because of caudal deflection of the hindlimbs), which resulted in a more prostrating position of the trunk, and (ii) a decrease of the angles of the feet in relation to the trunk. These differences suggest some distortions in the distribution of the postural tone among different leg and trunk muscles in premammillary rabbits.
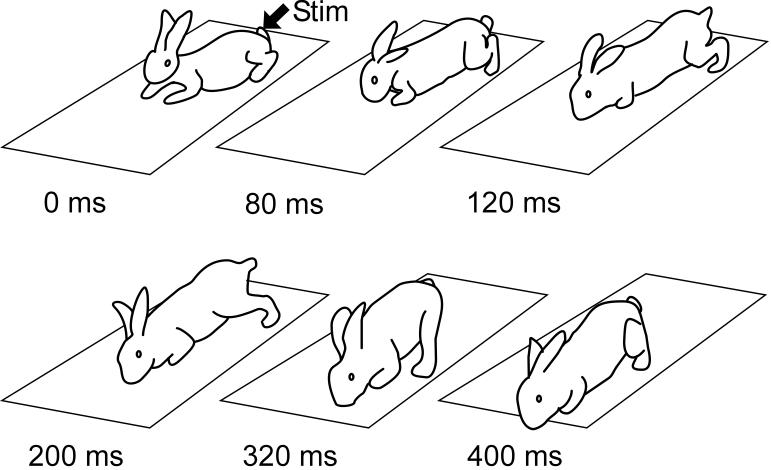
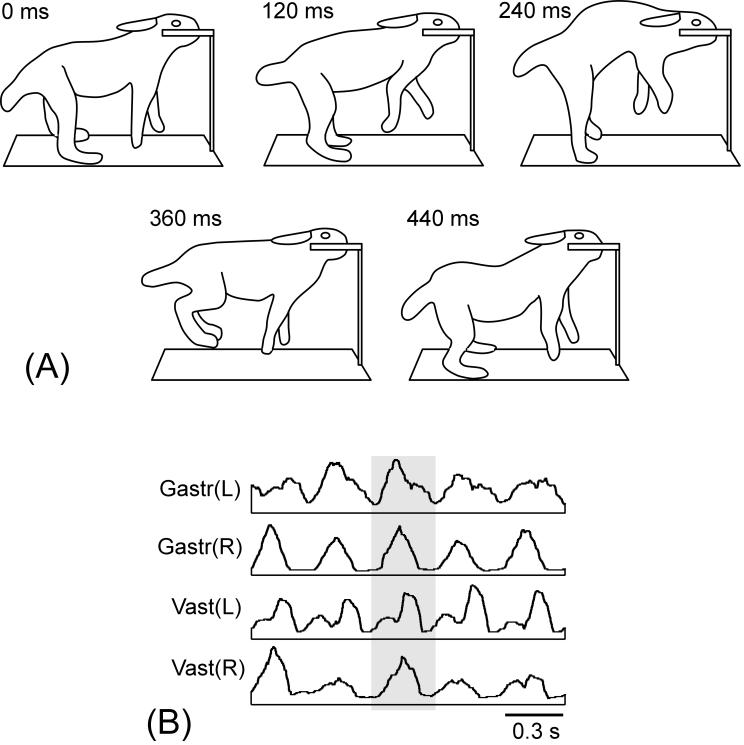
During the periods of spontaneously increased motor activity, premammillary rabbits exhibited bouts of locomotion, either spontaneously or in response to sensory stimuli. Figure 4 shows sequential positions of the rabbit during one cycle of locomotion elicited by pinching the tail. The rabbit exhibited gallop, with in-phase movements of both hindlimbs. From Fig. 4 it is also evident that, during progression, the rabbit maintained equilibrium. When rabbits encountered obstacles during locomotion, they could keep balance and change the direction of progression. This was characteristic for all premammillary rabbits. These observations suggest that, in these rabbits, the basic postural mechanisms operate during locomotion.
Fig. 4.
Locomotion of a premammillary rabbit. Sequential positions of the rabbit during one locomotor cycle are shown. Locomotor activity was evoked by pinching the tail. Note that the rabbit maintained equilibrium during progression. Drawings from video recording.
When positioned on the tilting platform (Fig. 2A and C), premammillary rabbits maintained equilibrium in the same way as intact ones [2]. Tilt of the platform caused extension of the limbs on the side moving downward and flexion of the limbs on the opposite side (as shown schematically in Fig. 2D). These limb movements led to the trunk displacement in the direction opposite to tilt (postural correction, S). Figure 5 shows postural corrections and EMG responses in the intact rabbit (A) and in the same rabbit after decerebration (B). In both cases, body displacements (S) were in anti-phase to tilts (α), indicating presence of postural corrections. The amplitude of corrections was considerably reduced in the decerebrated animal, however. The EMG responses of Vast in decerebrated rabbit were also correctly phased (activation with ipsilateral tilt), but they were considerably reduced as compared to the intact rabbit.
We calculated the gain of postural reflexes (see Methods) for 4 rabbits tested before and after decerebration. After decerebration, the gain was considerably reduced in individual animals (Fig. 5C) and in the whole group (Fig. 5D). The difference was statistically significant.
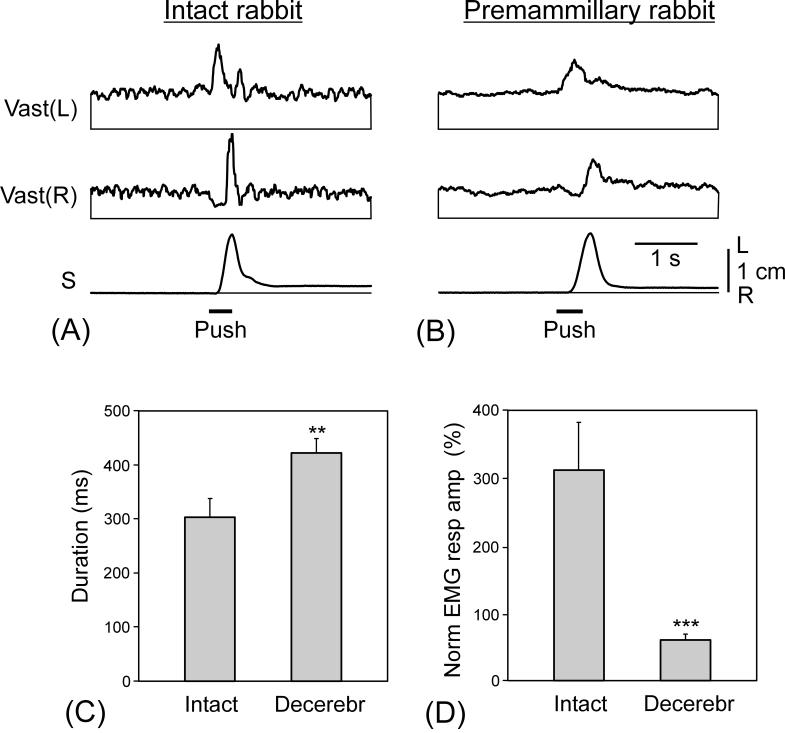
In the tests with lateral pushes, decerebrated rabbits maintained equilibrium in the same way as intact rabbits. Figure 6 shows the effect of pushes in the intact rabbit (A) and in the same rabbit after decerebration (B). In the two cases, the pushes to the left caused a leftward trunk displacement of 1.5 cm followed by a rapid return to the initial position (postural correction). The patterns of EMG responses were qualitatively similar, with sequential activation of the left and right Vast; the burst in the right Vast being preceded by inhibition. The burst of activity in the left Vast counteracted leftward displacement of the trunk and initiated the rightward corrective movement. This movement was terminated by the burst of activity in the right Vast. However, EMG responses in the decerebrated rabbit were smaller in amplitude and longer in duration. This was characteristic for all other animals of this group as well (Fig. 6C and D).
Fig. 6.
Comparison of postural EMG responses to pushes in intact and premammillary rabbits. (A and B) EMG responses to pushes before (A) and after decerebration (B). Designations as in Figs. 2 and 5. (C and D) Mean value (± SE) of the duration (C) and amplitude (D) of the EMG response in intact (N=4) and decerebrated (N=4) rabbits. The duration was calculated for the first excitatory component of the EMG response. The amplitude of the response was normalized to the background level. In (A) and (B), amplification in EMG channels was the same.
Spontaneous periodical alterations of excitability level in premammillary rabbits (see above) were reflected in their postural performance, as illustrated in Fig. 7. At the peak of activity, the rabbit well compensated for tilts, and lateral body displacements (S) were in anti-phase to tilts, indicating presence of postural corrections (Fig. 7A). Three minutes later, the static component of body displacement markedly decreased (Fig. 7B). A few minutes later, only weak, dynamic corrections were generated (Fig. 7C). Finally, when the activity of the rabbit occurred at its minimum, body displacements were in-phase with tilts (Fig. 7D). In this case, there were no postural corrections, and the body passively swayed with each tilt of the platform. From (A) to (D), one can also see a gradual decrease of EMG responses. However, in all cases, EMG responses were correctly phased (activation with ipsilateral tilt). Similar periodical changes of postural reactions (EMG and S values) were observed in all premammillary rabbits.
3.2. Postural performance in postmammillary rabbits; effects of MLR and VTF stimulation
Postmammillary rabbits (n=5) differed dramatically from premammillary ones. When lying on the side, they did not exhibit righting reflexes. When positioned with the dorsal side up, they were not able to keep standing and to maintain equilibrium. One can conclude that postural mechanisms do not function in the postmammillary rabbits. We tried to activate the postural mechanisms of the hindlimbs by stimulation of brainstem structures (MLR and VTF). For this purpose, the animal was restrained by fixing the head in a stereotaxic device, which allowed us to position the stimulating electrode in MLR or VTF (see Methods and Fig. 1). The hindlimbs were standing on the tilting platform, whereas the forelimbs were suspended (Fig. 2B). In postmammillary rabbits, the extensor tone in the hindlimbs was usually sufficient to support the body weight.
3.2.1. Stimulation of MLR
MLR was stimulated in four postmammillary rabbits. Initially, an optimal site for stimulation was found, in which well-coordinated locomotor movements were elicited by a minimal current. This is illustrated in Fig. 8A, which shows sequential positions of the rabbit during one locomotor cycle. Stimulation caused in-phase movements of the hindlimbs (gallop, Fig. 8A), with in-phase bursts in their extensors (Fig. 8B), and alternating movements of the forelimbs. Such a pattern was observed in all postmammillary rabbits; it is characteristic for intact rabbits as well [3].
Fig. 8.
Locomotion of a postmammillary rabbit. (A) Sequential positions of the rabbit during one locomotor cycle. The head of the rabbit was fixed in a stereotaxic device; the locomotor activity was evoked by MLR stimulation. (B) EMGs of the hindlimb extensors, vastus (Vast) and gastrocnemius (Gast), during locomotion. Note their in-phase bursting.
Locomotor movements were evoked by stimulation (30 Hz, 0.2 ms, 300-600 μA) in a restricted area below the inferior colliculus (Fig. 1). Inspection of brainstem slices has shown that the “mesencephalic locomotor region” in the rabbit was located approximately at Horsley-Clarke coordinates (P12.5, L3, H-3), and corresponded to nucleus cuneiformis according to the stereotaxic atlases of the rabbit’s brain [8,30].
The position of the stimulating electrode (optimal for elicitation of locomotion) was then used in postural tests. The strength of stimulation, however, was reduced by a factor of 1.5-2, which allowed us to considerably prolong the period preceding the initiation of locomotion (up to 10-15 s) and perform several postural tests (lateral tilts or pushes) before the locomotion started.
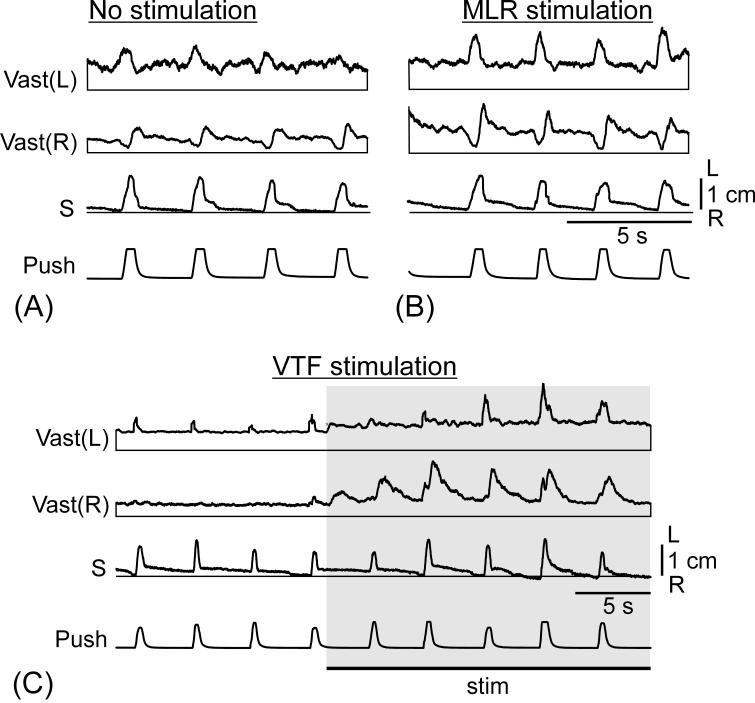
In most trials, MLR stimulation caused a gradual increase of the tone of limb extensors that preceded locomotion, as illustrated in Fig. 9A. We terminated the locomotor bouts by turning off the stimulation. In about a half of the trials, a gradual decay of extensor activity was observed after the locomotor bout, as in Vast(L) in Fig. 9A.
In postmammillary rabbits, the EMG responses to tilts were weak, but they could be increased by MLR stimulation. This is illustrated in Fig. 9B. Stimulation of MLR, during the period preceding the locomotor bout, resulted in a gradual increase of EMG responses to tilts. The temporal pattern of these responses was characteristic for postural reactions to tilts-activation of extensors with ipsilateral tilts. In some of the trials, increased responses to tilts were observed also for 10-20 s after termination of locomotion (not illustrated).
Also, EMG responses to lateral pushes in the postmammillary rabbits were very weak. The MLR stimulation considerably enhanced these responses (Fig. 10A and B). The temporal pattern of these responses was characteristic for postural reactions to pushes (c.f. Fig. 6A).
Fig. 10.
Activation of postural mechanisms in a postmammillary rabbit by MLR or VTF stimulation. (A and B) MLR stimulation caused an increase of EMG postural responses to pushes. (C) VTF stimulation caused an increase of EMG postural responses to pushes. Designations as in Fig. 2 and 5.
3.2.2. Stimulation of VTF
VTF was stimulated in 3 postmammillary rabbits. The effective area was located at the depth of 17-18 mm from the dorsal surface of the brainstem; its Horsley-Clarke coordinates were (P12.5, LR0, H-11). This area corresponded to the nucleus raphe magnus [8,30].
Electrical stimulation of VTF (50-100 Hz, 0.2 ms, 100-300 μA) considerably increased the tone of extensor muscles, as illustrated in Fig. 9C. In the postural tests on the tilting platform, VTF stimulation considerably enhanced the EMG responses to tilts (Fig. 9D); the timing of responses being typical for postural reactions - activation of extensors with ipsilateral tilts. Also, the EMG responses to lateral pushes considerably increased with VTF stimulation (Fig. 10C). The temporal pattern of these responses was characteristic for postural reactions to pushes (c.f. Fig. 6A).
4. Discussion
4.1. Postural capacity of premammillary rabbits
It has been known for a long time that quadrupeds (e.g., cats and rabbits) decerebrated at the premammillary (or more rostral) level are capable of standing and walking without losing balance. By contrast, postmammillary animals cannot stand and keep balance [1,19]. These facts mean that an essential part of the mechanisms responsible for maintenance of the basic, dorsal-side-up body posture persists in the premammillary animals. Postural performance in these animals, however, has not been characterized in any detail. In particular, it remained unclear if decerebration caused specific postural deficits, or if postural corrections in decerebrated animals were weaker and/or slower than in the intact ones. This knowledge is important for better understanding of the functional organization of postural control, as well as for characterization of the distribution of postural functions in the CNS.
In standing quadrupeds, the most demanding postural task is that of maintaining lateral stability, because the base of support in the transverse plane is usually more restricted than in the sagittal plane (Fig. 3D). Equilibrium in the transverse plane (stabilization of the dorsal side-up body posture) is maintained by a closed-loop control system. In normal subjects, this system compensates for a wide range of perturbations of posture [5,11]. Functional organization of this system was analysed in our previous experiments on rabbits and cats [2,4]. It was found that the dorsal side-up position of the fore- and hindquarters is maintained by two different mechanisms (driven by somatosensory inputs from the corresponding limbs), which, under certain conditions, can operate independently of each other.
To characterize operation of the postural system in decerebrated rabbits, in the present study we used three different tests (postural perturbations). To examine postural mechanisms of the hindlimbs, we used lateral tilts and lateral pushes applied to the hindquarters, whereas positioning of the rabbit on its side allowed us to characterize a complex sequence of righting reflexes which require coordinated activity of the fore- and hindlimbs.
We have found that typically, in all these tests, the premammillary rabbits were able to compensate for perturbations of body posture. However, even during the periods of higher excitability, postural performances in the premammilary rabbits were not as good as in the intact ones: (i) In the test with lateral tilts, the gain of postural reflexes was reduced fourfold as compared to control (Fig. 5D). (ii) In the test with lateral pushes, the value of EMG response was reduced fivefold (Fig. 6D). (iii) In the test with righting, the time needed to assume the normal position was longer than in control by 25 %. However, this time could be considerably reduced and made even shorter than in control when exteroceptive stimulation, i.e., pinching the tail, was used to initiate righting (Fig. 3B).
These findings taken together suggest that, after decerebration at the premammillary level, the postural mechanisms maintaining the lateral stability remained mostly undamaged, but the level of their activity was considerably reduced. It seems likely that an excitatory drive, which they receive from brainstem structures, is insufficient to fully activate them. Also, the premammilary rabbits were able to maintain equilibrium during locomotion (Fig. 4), suggesting presence of the dynamic control of equilibrium, and close interaction of postural and locomotor mechanisms [24]. This issue was not specifically addressed in the present study, however.
An intriguing feature of premammillary rabbits was a periodical change in the level of excitability, similar to that described in premammillary cats [28]. The nature of this phenomenon remains unclear, however. This periodicity in the level of excitability could be caused by fluctuation of input to postural and locomotor centres from some unknown source. One of the possible candidates are the neurons from substantia nigra pars reticulata projecting to MLR and to postural centres [33,34]. However, experimental evidences about periodical activity of these neurons in premammillary animals are absent.
4.2. Activation of postural mechanisms in postmammillary rabbits
We have confirmed the early findings (see e.g., [19,31]) that, in postmammillary quadrupeds, the postural system does not function. Postmammillary rabbits did not exhibit righting reflexes, were not able to maintain balance during standing, and to generate corrective movements in response to lateral pushes and tilts. This could be caused by two main reasons: (i) The brain structures located between the two levels of decerebration, pre- and postmammillary, contain specific nervous mechanisms (components of postural reflex chains), which are necessary for operation of the closed-loop postural control mechanisms. (ii) These structures provide the postural mechanisms with unspecific, tonic excitatory drive, which is necessary for their activation. Presence of weak EMG responses to tilts and pushes, with the temporal pattern similar to that in control, supports the second hypothesis. To test these hypotheses, in the present study we stimulated tonically two brainstem areas, which are known to affect the postural system, the MLR and VTF [10,25,26], and examined postural performance by applying lateral tilts and lateral pushes to the posterior part of the body. It was found that MLR stimulation, during the period preceding the onset of locomotion, caused an increase of the extensor muscle tone, as well as an augmentation of the EMG responses to tilts and pushes. Similarly, VTF stimulation caused an increase of the extensor tone and augmentation of responses to tilts and pushes (Figs. 9 and 10). In these experiments, however, the head and forequarters were fixed (Fig. 2B), which led to a distortion of the caudal trunk movements caused by platform tilts, and did not allow us to answer the question if the EMG responses to tilts were sufficient to produce the anti-phase (corrective) movement of the body, or they only reduced the tilt-related body sway.
These findings strongly suggest that an essential part of postural mechanisms of the hindquarters, compensating for lateral tilts and lateral pushes during standing, persists in the postmammillary rabbits and can be activated by a tonic drive. In this respect, the postural system has an important feature in common with the locomotor system, in which removal of the structures between the pre- and postmammillary levels also causes a dramatic effect, that is, a complete disappearance of spontaneous locomotor behavior, but locomotion can be evoked by MLR stimulation [14,29,31].
At present, it is not clear which of the structures located between the two levels are responsible for activation of the postural and locomotor systems. Two structures in this area - the red nucleus and substantia nigra - are known to be related to motor function (see e.g., [20,33,34]). In particular, experimental evidences suggest that substantia nigra pars reticulata takes part in the control of muscle tone and in initiation of locomotion [33,34].
Normally, operation of the closed-loop postural mechanisms is based mainly on the somato-sensory information coming from limb mechanoreceptors rather than on vestibular and visual inputs [2,6,12,32]. The corresponding limb afferents give rise to two postural reflex loops, spinal and spino-supraspinal [5,9,11,13,17]. The latter loop includes the brainstem and forebrain mechanisms. Results of the present study strongly suggest that the loop involving the forebrain structures is not necessary for the feedback control of the dorsal side-up body posture.
Acknowledgements
This work was supported by grants from NIH R01 NS-049884, the Swedish Research Council (no. 11554), and Gösta Fraenckels Foundation to TGD.
References
- 1.Bard P, Macht MB. The behavior of chronically decerebrate cats. In: Wolstenholme GEW, O’Connor CM, editors. Neurological basis of behavior. Churchill; London: 1958. pp. 55–71. [Google Scholar]
- 2.Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003;90:3783–3793. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- 3.Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J. Physiol. 2006;573:211–224. doi: 10.1113/jphysiol.2006.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology. 2006;21:216–225. doi: 10.1152/physiol.00001.2006. [DOI] [PubMed] [Google Scholar]
- 6.Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Edgerton VR, de Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fifkova E, Marsala J. Stereotaxic atlases for the cat, rabbit and rat. Electrophysiological Methods in Biological Research, 653-731, Prague, Acad Sci. 1967 [Google Scholar]
- 9.Fung J, Macpherson JM. Attributes of quiet stance in the chronic spinal cat. J Neurophysiol. 1999;82:3056–3065. doi: 10.1152/jn.1999.82.6.3056. [DOI] [PubMed] [Google Scholar]
- 10.Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group 1b pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- 11.Horak F, Macpherson J. Postural orientation and equilibrium. In: Shepard J, Rowell L, editors. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Sect. 12. Oxford University Press; New York: 1996. pp. 255–292. [Google Scholar]
- 12.Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol. 1995;73:1181–1191. doi: 10.1152/jn.1995.73.3.1181. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007 doi: 10.1007/s00702-007-0657-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan LM. Initiation of locomotion in mammals. Ann NY Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 15.Karayannidou A, Orlovsky GN, Sirota MG, Beloozerova IN, Deliagina TG. Compensation for lateral perturbations of body orientation in the standing and walking cat. Soc Neurosci Abstr. 2007 [Google Scholar]
- 16.Kato M, Murakami S, Hirayama H, Hikino K. Recovery of postural control following chronic bilateral hemisections at different spinal cord levels in adult cats. Exp Neurol. 1985;90:350–364. doi: 10.1016/0014-4886(85)90024-x. [DOI] [PubMed] [Google Scholar]
- 17.Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol. 2005;94:3677–3690. doi: 10.1152/jn.00538.2005. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson JM, Fung J, Lacobs R. Postural orientation, equilibrium, and the spinal cord. In: Seil FJ, editor. Advances in Neurology, vol. 72. Neuronal Regeneration, Reorganization, and Repair. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 227–232. [PubMed] [Google Scholar]
- 19.Magnus R. Körperstellung. Springer; Berlin: 1924. [Google Scholar]
- 20.Massion J. The mammalian red nucleus. Physiol Rev. 1967;47:383–436. doi: 10.1152/physrev.1967.47.3.383. [DOI] [PubMed] [Google Scholar]
- 21.Massion J. Postural control system. Curr Opin Neurobiol. 1994;4:877–888. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 22.Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;2:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Massion J, Dufosse M. Coordination between posture and movement: why and how? News Physiol Sci. 1988;3:88–93. [Google Scholar]
- 24.Misiaszek JE. Control of frontal plane motion of the hindlimbs in the unrestrained walking cat. J Neurophysiol. 2006;96:1816–1828. doi: 10.1152/jn.00370.2006. [DOI] [PubMed] [Google Scholar]
- 25.Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987;28:161–196. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Kawahara K, Sakamoto T, Aoki M, Tomiyama T. Setting and resetting of postural muscle tone in the decerebrate cat by stimulation of the brain stem. J Neurophysiol. 1982;48:737–748. doi: 10.1152/jn.1982.48.3.737. [DOI] [PubMed] [Google Scholar]
- 27.Musienko PE, Orlovsky GN, Zelenin PV, Lyalka VF, Deliagina TG. Postural performance in decerebrate rabbit. Soc Neurosci Abstr. 2006 doi: 10.1016/j.bbr.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlovsky GN. Spontaneous and induced locomotion of the thalamic cat. Biophysics. 1969;14:1154–1162. [Google Scholar]
- 29.Orlovsky GN, Deliagina TG, Grillner S. From mollusc to man. Oxford UP; Oxford: 1999. Neuronal control of locomotion. [Google Scholar]
- 30.Sawyer CH, Everett JW, Green JD. The rabbit diencephalon in stereotaxic coordinates. J Comp Neurol. 1954;101:801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]
- 31.Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mid-brain. Biophysics. 1966;11:756–765. [PubMed] [Google Scholar]
- 32.Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci. 2002;22(14):5803–5807. doi: 10.1523/JNEUROSCI.22-14-05803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neuroscience Research. 2004;50:137–151. doi: 10.1016/j.neures.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion; A new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]