Abstract
Objective
To assess clinical determinants of systemic inflammation in persons with chronic spinal cord injury (SCI).
Design
Cross-sectional survey.
Setting
Veterans Affairs medical center.
Participants
As part of an epidemiologic study assessing SCI-related health conditions, 63 men with chronic SCI provided a blood sample and information regarding locomotive mode and personal habits.
Interventions
Not applicable.
Main Outcome Measure
Plasma high-sensitivity C-reactive protein (CRP).
Results
The mean ± standard deviation age was 56±14y, and participants were assessed 21±13y after injury. Adjusting for heart disease, hypertension, and body mass index (BMI), the mean CRP in 12 motorized wheelchair users (5.11mg/L) was not significantly greater than 23 participants who used a manual wheelchair (2.19mg/L) (P=.085) but was significantly greater than the 17 who walked with an assistive device (1.41mg/L) (P=.005) and the 12 who walked independently (1.63mg/L) (P=.027). CRP was significantly greater in participants with obesity but was not related to age, smoking, or SCI level and severity. CRP was elevated in participants reporting a urinary tract infection (UTI) or pressure ulcer within a year, but adjustment for this did not account for the elevated CRP in motorized wheelchair users.
Conclusions
These results suggest that CRP in chronic SCI is independently related to locomotive mode, BMI, and a history of pressure ulcers and UTI. It is suggested that future studies in SCI investigate whether modifying these factors influence systemic inflammation and cardiovascular health.
Keywords: Cardiovascular diseases, Inflammation, Rehabilitation, Spinal cord injuries.
SYSTEMIC INFLAMMATION IS implicated in the development of atherosclerosis and coronary heart disease and recently has been related to greater mortality from cancer and chronic respiratory disease. C-reactive protein (CRP) is a blood marker of systemic inflammation that is produced by the liver in response to the proinflammatory cytokine interleukin 6. Elevated CRP levels predict future coronary events and other manifestations of cardiovascular disease in the able-bodied1-3 through mechanisms that are poorly understood. It is known that as an acute-phase reactant CRP facilitates the elimination of foreign pathogens and damaged cells by binding to phosphocholine and can also activate the complement system.4 CRP may also increase levels of plasminogen activator inhibitor 1, a marker of impaired fibrinolysis and atherothrombosis.5 CRP levels add predictive value to established cardiovascular risk factors. Relative risk categories have been established for tertile CRP values (low risk [<1mg/L], average risk [1−3mg/L], high risk [>3mg/L]).6
A recent study7 reported cardiovascular disease to be a leading cause of mortality in persons with chronic spinal cord injury (SCI). It is possible that elevated CRP levels may contribute to the development of cardiovascular disease in this population. SCI results in increased fat mass, increased frequency of urinary tract infections (UTIs) and pressure ulcers, and in some instances loss of function of major muscle groups resulting in decreased levels of physical activity. All of these conditions may result in elevated CRP levels. In this study, we conducted a preliminary investigation of clinical predictors of CRP in persons with SCI and with varying levels of mobility. We hypothesize that insofar as mobility mode reflects the general activity level in persons with chronic SCI that it may be predictive of CRP levels independent of a history of infections and obesity.
METHODS
Participants
Participants were selected from a larger epidemiologic study assessing health in persons with chronic SCI conducted at the VA Boston Healthcare System.7 Participants were recruited from veterans with SCI who had previously been treated at VA Boston and from participants in the community. Every 2 to 3 years, participants underwent pulmonary function testing, completed a health questionnaire, and, starting in October 2003, were asked to provide a blood sample. Testing was conducted when participants are in their usual state of health and not clinically ill. Each person completed a health questionnaire and answered the question, “How do you usually get around (usually means more than half the time)?” Responses were recorded as motorized wheelchair, hand-propelled wheelchair, walk with aid (crutch, cane, or similar aid), or walk without assistance. Blood samples were collected from 82 white men between October 2003 and June 2005 who were 2 or more years post-SCI and who were not using statins. Because of financial constraints, for this preliminary investigation, we selected 64 participants based on the mobility mode. All participants using a motorized wheelchair (n = 12), walking with an assistive device (n = 17), and walking independently were included (n = 12), and a random sample of the remaining participants who usually used a manual wheelchair (n = 23) was selected. After subject selection, 1 person was found to be 1.6 years post-SCI and 1 was black. All subjects gave informed consent, and the study was approved by our institutional review boards.
SCI Classification
Motor level and completeness of injury were assessed by physical examination. The level of injury was classified according to strength preservation in key muscle groups in the upper and lower extremities and reported regionally as tetraplegia or paraplegia. Injury completeness was reported according to guidelines suggested by the American Spinal Injury Association (ASIA).8 Participants were assigned as motor complete (equivalent to ASIA motor score of A or B, ie, no motor function below the neurologic level of injury), C (motor incomplete, motor function preserved below the neurologic level, and more than half the key muscles below the neurologic level are not strong enough to overcome gravity), or D (motor incomplete, preservation of motor function below the neurologic level, and more than half the key muscles below the neurologic level are strong enough to overcome gravity). The SCI level and the severity of injury were considered in 3 groups that included motor complete and ASIA grade C tetraplegia, motor complete and ASIA grade C paraplegia, and ASIA grade D tetraplegia or paraplegia. By using participants in each of these 3 impairment groups, we were able to examine the effect of SCI in participants ranging from profound neurologic impairment to minimal or no neurologic impairment.
Biochemical Analyses
Blood was drawn into an ethylenediaminetetracetic acid tube, stored with a cooler pack in an insulated container, and shipped overnight to the core blood laboratory. The samples were centrifuged for 15 minutes at 2600rpm (1459×g) at 4°C, and plasma was stored at − 80°C until batch analysis. High-sensitivity CRP was determined by using a high-sensitivity immunoturbidimetric assay with a sensitivity of .03mg/L. The day-to-day variability of the assay at concentrations of .91, 3.07, and 13.38mg/L is 2.81%, 1.61%, and 1.1%, respectively (Clinical & Epidemiologic Research Laboratory, Children's Hospital Boston, MA). A quality-control specimen was analyzed every tenth sample. The 6 CRP results were within .04mg/L and had a standard deviation of .016mg/L.
Clinical Data
Participants completed a health questionnaire based on the American Thoracic Society adult respiratory disease questionnaire.9 Smokers were defined as smoking 20 or more packs of cigarettes or using 336g (12oz) of tobacco or more in a lifetime or smoking 1 or more cigarettes a day for at least 1 year.
Current smokers reported cigarette use within 1 month of testing. Hypertension and diabetes were defined if previously diagnosed by a doctor; heart disease was defined as receiving treatment for heart trouble in the 10 years prior to blood draw. These definitions of disease were validated in an earlier study.7 Participants were asked about a history of urinary infections or a pressure ulcer in the past year and if they had a cold in the week before testing (available in 60 participants). As part of the study protocol, subject length was measured in 60 participants, was available by self-report in 2, and from SCI clinic notes in 2. In 57 participants, weight was measured, was available by self-report in 3, and was obtained from SCI clinic notes in 4 participants. Length and weight were used to calculate body mass index (BMI). For 3 participants whose health questionnaires were completed at a time other than the blood-draw date, SCI clinic progress notes were reviewed to confirm previously reported questionnaire responses.
Statistical Analysis
Because the distribution of CRP was skewed, natural log-transformation was used to normalize the distribution and stabilize variance. General linear modelsa were used to assess determinants of CRP. Variables in table 1 were assessed in univariate models and, if significant at the .1 level or less, were included in a multivariate model in participants with complete data available. The Tukey-Kramer test was used to adjust for multiple comparisons.
Table 1.
Locomotive Mode and Participant Characteristics
| Characteristics | Motorized Wheelchair | Manual Wheelchair | Walks With an Aid | Walks Without an Aid | Total |
|---|---|---|---|---|---|
| Subjects | 12 | 23 | 16 | 12 | 63 |
| CRP (mg/L) | 5.22 (1.97, 13.57) | 1.53 (0.88, 4.66) | 1.10 (0.81, 2.96) | 1.41 (0.99, 2.56) | 1.63 (0.90, 4.03) |
| Age (y) | 58.6±13.5 | 51.0±12.2 | 60.7±17.2 | 54.2±13.5 | 55.5±14.3 |
| Duration of injury (y) | 24.2±12.7 | 22.5±9.8 | 22.6±16.8 | 15.2±9.5 | 21.5±12.5 |
| BMI (kg/m2) | 26.0±4.6 | 27.0±3.7 | 27.4±6.4 | 29.4±5.2 | 27.4±4.9 |
| BMI (kg/m2) | |||||
| Normal (<25) | 6 (50.0) | 8 (34.8) | 7 (43.8) | 4 (33.3) | 25 (40.0) |
| Overweight (25 to <30) | 4 (33.3) | 9 (39.1) | 4 (25.0) | 3 (25.0) | 20 (31.8) |
| Obese (≥30) | 2 (16.7) | 6 (26.1) | 5 (31.3) | 5 (41.7) | 18 (28.6) |
| Motor injury level and completeness | |||||
| Cervical complete and ASIA grade C | 4 (33.3) | 6 (26.1) | 1 (6.3) | 0 (0) | 11 (17.5) |
| Other complete and ASIA grade C | 4 (33.3) | 15 (65.2) | 1 (6.3) | 0 (0) | 20 (31.8) |
| All ASIA grade D | 4 (33.3) | 2 (8.7) | 14 (87.5) | 12 (100.0) | 32 (50.8) |
| Cigarette smoking | |||||
| Current | 1 (8.3) | 2 (8.7) | 3 (18.8) | 4 (33.3) | 10 (15.9) |
| Past | 9 (75.0) | 8 (34.8) | 7 (43.8) | 5 (41.7) | 29 (46.0) |
| Never | 2 (16.7) | 13 (56.5) | 6 (37.5) | 3 (25.0) | 24 (38.1) |
| Heart disease (past 10y) | 4 (33.3) | 2 (8.7) | 2 (12.5) | 2 (16.7) | 10 (15.9) |
| Diabetes | 2 (16.7) | 3 (13.0) | 3 (18.8) | 1 (8.3) | 9 (14.3) |
| High blood pressure | 3 (25.0) | 7 (30.4) | 4 (25.0) | 3 (25.0) | 17 (27.0) |
| Additional characteristics | 12 | 21 | 16 | 11 | 60 |
| UTI in the past year | 5 (41.7) | 12 (57.2) | 4 (25.0) | 2 (18.2) | 23 (38.3) |
| Pressure ulcer | 5 (41.7) | 8 (38.1) | 6 (37.5) | 0 (0) | 19 (31.7) |
| Cold (within 1wk blood draw) | 2 (16.7) | 2 (9.5) | 2 (12.5) | 0 (0) | 6 (10.0) |
NOTE. Values are n, median (25th and 75th percentile), mean ± standard deviation, or n (%).
RESULTS
Participant Characteristics
One participant who walked with an assistive device had a CRP value of 217mg/L and was considered an outlier and excluded from analysis. Of the remaining 63 participants (see table 1), the average age at the time of blood draw was 56±14y with an average of 21±13y since injury. Twenty-five percent of participants had CRP values of 4.03mg/L or above, and 6 participants (16%) had values greater than 10mg/L (maximum, 33.6). Of note, one third of the participants were in the low cardiovascular risk category based on CRP levels, one third in the average category, and one third in the high-risk category in tertiles similar to that described in the general population. The distribution of natural log CRP was continuous and normally distributed (Shapiro-Wilks test W = .98, P=.379) (fig 1). Specifically, there was no evidence that higher or lower values represented outliers, and all values were included in subsequent analyses. Other participant characteristics are presented based on mobility level (motorized wheelchair, hand-propelled wheelchair, walks with aid, walks without aid). Boxplots of natural log CRP based on mobility level are presented in figure 2 and show no obvious outliers. The median CRP level was 1.63mg/L and was greatest in motorized wheelchair users (5.22mg/L).
Fig 1.
Distribution of the natural log CRP (lnCRP).
Fig 2.
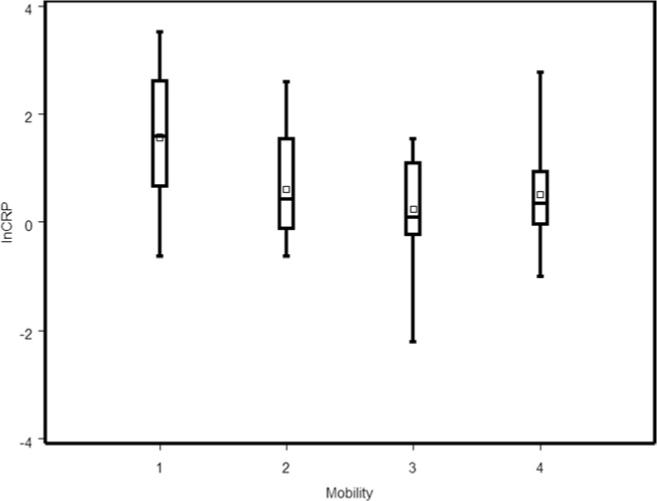
Boxplot of the natural log CRP by mobility group. The bars indicate the range of the data, the large box indicates the median and interquartile range, and the small square indicates the mean. Abbreviation: lnCRP, natural log CRP. Legend: mobility groups (x axis): 1, motorized wheelchair; 2, manual wheelchair; 3, walks with an aid; 4, walks without an aid.
Predictors of CRP
In univariate regression models, age, duration of injury, SCI level and completeness of injury, history of smoking, diabetes, UTI history, or a cold in the preceding week were not associated with CRP. Univariate predictors of CRP included locomotive mode (P=.013), BMI (P=.049), heart disease (P=.084), hypertension (P=.029), and pressure ulcer in the past year (P=.021) (table 2). In a multivariate model (table 3), locomotive mode, BMI (normal, overweight, obese), heart disease, and hypertension were significant predictors of CRP. CRP in motorized wheelchair users (5.11mg/L) was not significantly greater than in participants who used a manual wheelchair (2.19mg/L, P=.085); however, it was significantly greater than those who walked with an assistive device (1.41mg/L, P=.005) or walked independently (1.63mg/L, P=.027). Differences among the other mobility groups were not significant (P>.492). Compared with participants with a normal BMI, those who were obese had significantly greater CRP values (3.7mg/L, P=.009). However, CRP values in obese participants were not significantly greater than those who were overweight (2.08mg/L, P=.173), and there was no significant difference in CRP levels between normal and over-weight participants (P=.501) (see table 3). Subjects with a history of hypertension had lower levels of CRP than those without (P=.004). SCI level and completeness added to the multivariate model in table 3 was not a significant predictor of CRP (P=.39), and locomotive mode remained a significant predictor (P=.004).
Table 2.
Univariate Predictors of Natural Log CRP
| Predictors | Coefficient (β) | e(β) | Model P |
|---|---|---|---|
| Age (mg/L per y) | 0.016 | 1.02 | .113 |
| Years since injury (mg/L per y) | 0.017 | 1.02 | .147 |
| BMI (mg/L per kg/m2) | 0.057 | 1.06 | .049 |
| Mean Natural Log CRP | e(In CRP) mg/L | Model P | |
|---|---|---|---|
| Motor injury level and completeness | .631 | ||
| Cervical complete and ASIA grade C | 0.971 | 2.64 | |
| Other complete and ASIA grade C | 0.662 | 1.94 | |
| All grade D | 0.586 | 1.80 | |
| Locomotive mode | .013 | ||
| Motorized wheelchair | 1.573 | 4.82 | |
| Manual wheelchair | 0.603 | 1.83 | |
| Walk with aid | 0.238 | 1.27 | |
| Walk without aid | 0.509 | 1.66 | |
| BMI (kg/m2)* | .133 | ||
| Normal (<25) | 0.447 | 1.56 | |
| Overweight (25 to <30) | 0.562 | 1.75 | |
| Obese (≥30) | 1.125 | 3.08 | |
| Cigarette smoking | .547 | ||
| Current smoker | 0.657 | 1.93 | |
| Past smoker | 0.839 | 2.31 | |
| Never smoker | 0.491 | 1.63 | |
| Heart disease (past 10y) | 1.246 | 3.48 | .084 |
| No heart disease | 0.570 | 1.77 | |
| Diabetes | 0.584 | 1.79 | .793 |
| No diabetes | 0.693 | 2.00 | |
| High blood pressure | 0.167 | 1.18 | .029 |
| No high blood pressure | 0.866 | 2.37 | |
| UTI in the past year* | 0.925 | 2.52 | .205 |
| No UTI* | 0.539 | 1.71 | |
| Pressure ulcer* | 1.181 | 3.26 | .021 |
| No pressure ulcer* | 0.458 | 1.58 | |
| Cold (within 1wk blood draw)* | 0.351 | 1.42 | .452 |
| No cold* | 0.724 | 2.06 |
NOTE. Sample size is 63 unless indicated by an asterisk (n=60).
Table 3.
Multivariate Predictors of Natural Log CRP
| Characteristics | Mean Natural Log CRP | e(In CRP) (mg/L) | Model P |
|---|---|---|---|
| Mobility | .007 | ||
| Motorized wheelchair* | 1.631 | 5.11 | |
| Manual wheelchair | 0.785 | 2.19 | |
| Walk with aid | 0.345 | 1.41 | |
| Walk without aid | 0.489 | 1.63 | |
| BMI (kg/m2) | .013 | ||
| Normal (<25) | 0.397 | 1.49 | |
| Overweight (25−<30) | 0.731 | 2.08 | |
| Obese (≥30)† | 1.308 | 3.70 | |
| Heart disease (past 10y) | 1.182 | 3.26 | .037 |
| No heart disease | 0.443 | 1.56 | |
| High blood pressure | 0.377 | 1.46 | .004 |
| No high blood pressure | 1.247 | 3.48 |
P<.05 for motorized wheelchair compared with walk with an aid or walk without an aid; P=.085 for motorized wheelchair compared with manual wheelchair.
P<.05 for obese compared with normal BMI.
The Effect of a Pressure Ulcer or UTI
A history of a pressure ulcer or UTI in the prior year was available in 60 participants, and, when included separately in the multivariate model, each was associated with higher levels of CRP (P=.026, P=.05, respectively) (table 4); the effects of locomotive mode remained significant in each model (P=.007). When a history of a pressure ulcer or UTI in the prior year were both included in the model, the effect of a UTI was no longer significant (P=.112). Locomotive mode remained significant (P=.006) and compared with motorized wheelchair users (CRP=5.7mg/L); users of manual wheel-chairs and participants who walked with an assistive device had significantly lower CRP values (CRP=2.05mg/L, P=.023; CRP=1.61mg/L, P=.005, respectively). In participants who walked independently, the CRP level was lower but not significantly (CRP=2.29mg/L, P=.118).
Table 4.
CRP in Participants With and Without a History of UTI or Pressure Ulcer in the Previous Year or a Cold in the Week Before the Blood Draw, Adjusting for Locomotive Mode, BMI, Heart Disease, and Hypertension (n=60)
| Characteristics | Mean Natural Log CRP | e(In CRP) (mg/L) | P |
|---|---|---|---|
| UTI | 0.588 | 1.80 | .050 |
| No UTI | 1.138 | 3.12 | |
| Pressure ulcer | 1.226 | 3.41 | .026 |
| No pressure ulcer | 0.586 | 1.80 | |
| Cold | 0.612 | 1.84 | .644 |
| No cold | 0.813 | 2.25 |
DISCUSSION
In this cross-sectional study in persons with chronic SCI, our results suggest that locomotive mode is independently related to circulating levels of CRP adjusting for BMI, a history of heart disease, and hypertension. Among wheelchair users, those using motorized wheelchairs had the highest CRP levels. Compared with motorized wheelchair users, those who walked with an assistive device or walked independently had significantly lower CRP levels. Pressure ulcers and UTIs have both been shown to elevate CRP and other inflammatory markers in persons with SCI.10,11 Although we did not assess each participant for small pressure ulcers or exclude a UTI by culture, we used self-report of these conditions in the previous year in a subset of 60 participants in whom this information was available as an indicator of participants at risk for these conditions. These conditions were each associated with a greater CRP, and when included in the multivariate model, locomotive mode was still a significant predictor of CRP. Although comparisons between motorized wheelchair users and each mobility group did not achieve conventional levels of statistical significance in every regression model, taken together, the pattern observed is highly suggestive of a greater CRP in motorized wheelchair users. As in the able-bodied, a history of heart disease and a greater BMI were associated with greater CRP levels. Unexpectedly, a history of hypertension was associated with a lower CRP. In large epidemiologic studies in the able-bodied, positive relationships between measured blood pressure and CRP have been observed,12 and it is hypothesized that hypertension may promote vascular inflammation.13 In our study, we did not measure blood pressure, and it is possible that this effect is caused either by changes in vascular tone after SCI or the result of antihypertensive treatment that may cause blood pressure to be normalized or lower than preinjury values. Of the 17 participants reporting hypertension, 12 were using antihypertensive medications. An additional 9 participants were using medication that could also be used to treat hypertension.
Although there have been improvements in survival after SCI, the average life expectancy is still reduced.14 Respiratory diseases were previously reported to be the most common cause of death, but more recent data support the emergence of cardiovascular diseases as an important cause of death. In SCI, physical adaptations occur that appear to promote systemic inflammation and promote the development of cardiovascular disease. There is a decrease in lean tissue mass and an even greater increase in fat mass. There is an increase in central (truncal) fat and an increase in fat in the limbs below the level of injury, and some studies15-17 have even shown an increase in fat in nonparalyzed limbs above the level of injury. The ability to ambulate independently is reduced, and many use either manual or motorized wheelchairs. Studies in able-bodied populations have shown that greater adiposity and a lesser degree of physical activity and fitness (ie, a sedentary lifestyle) are independently associated with elevated basal levels of CRP.
Many studies in the able-bodied18-21 have found higher levels of CRP to be associated with lower levels of physical activity. However, in studies in which the effects of specific training programs were assessed,22,23 CRP levels were not consistently reduced by exercise training. In the current study, we consider that locomotive mode may be reflective of usual physical activity, and those who are ambulatory or use a manual wheelchair may be less sedentary than those who are motorized wheelchair users. It is generally agreed that SCI is a risk factor for decreased physical activity and therefore reduced cardiovascular fitness24-26 because of the loss of muscle function. Although we assume that physical activity accounts for the difference in CRP based on mobility level, this finding needs to be confirmed. The effect of exercise training on elevated CRP levels in chronic SCI patients also needs to be determined with additional studies.
Alternatively, the higher levels of CRP in motorized wheel-chair users could be more related to chronic and/or subacute infections than to exercise capacity in this population. Individuals with SCI also develop a neurogenic bladder that predis-poses them to UTIs and related illnesses (renal and bladder stones) that promote additional infections. Also, pressure ulcers may occur because of a lack of sensation in dependent areas. In studies that included relatively few participants, therefore limiting the assessment of multiple risk factors, elevated CRP levels were related to slowly healing pressure ulcers10 and indwelling bladder catheters.11 Although it was previously considered that infections result in falsely elevated CRP levels, there is recent evidence suggesting that chronic or recurrent infection may increase cardiovascular disease risk. Evidence that chronic or recurrent infection affects cardiovascular disease in the able-bodied comes from positive associations with gingivitis and poor dental health27 and a relationship between periodontal disease and carotid intima-media thickness.28 More recently, an increased incidence of myocardial infarction and stroke was also reported after a systemic respiratory tract infection or UTI.29,30 Previous guidelines31 have suggested that people with elevated levels of CRP (>10mg/L) be evaluated for a noncardiac cause (eg, infection). However, recent evidence32 suggests that people with persistently elevated levels are at a particularly increased risk for future cardiovascular events. In the current study, the distribution of CRP levels in tertiles similar to the general population argues against a bias in CRP levels because of unrecognized acute infection, and there was no evidence that these elevated CRP values represented outliers other than 1 extreme value of CRP equal to 217mg/L.
Study Limitations
Our study was originally designed to be a preliminary investigation of the relationships between locomotive mode and other personal risk factors with systemic inflammation in chronic SCI. Although weaknesses of the current study include the small sample size and the inclusion of relatively few motorized wheelchair users, the results are remarkably robust and consistent with the known impact of infection, obesity, and physical activity on chronic systemic inflammation in the able-bodied. In both able-bodied men and women, elevated CRP levels independently predict the risk of cardiovascular events and add to the predictive value of lipid profiles.31,33-36
CONCLUSIONS
The concept of chronic SCI as an extreme model of chronic systemic inflammation secondary to immobility, greater adiposity, and recurrent or chronic infection with systemic consequences is novel and may have important implications for understanding disease pathways that contribute to cardiovascular disease in the years after SCI and possibly in others with chronic disability. It is suggested that future studies in a larger SCI cohort investigate the relationship between modification of these risk factors, longitudinal changes in CRP, and various health outcomes. Associations with CRP in persons with chronic SCI may not only be pertinent for cardiovascular risk assessment but also for other diseases because in the able-bodied elevations in CRP have also been related to mortality from chronic lung disease and cancer.37-41
Acknowledgments
Supported by the Research and Development Service, Department of Veterans Affairs, VA Boston Healthcare System Boston; National Institute of Child Health and Human Development, National Institutes of Health (grant no. RO1 HD42141), Massachusetts Veterans Epidemiology Research and Information Center, VA Cooperative Studies Program, and the Foundation for PM&R.
No commercial party having a direct financial interest in the result of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Footnotes
Presented to the American Spinal Cord Injury Association, June 28, 2006, Boston, MA.
Supplier
PROC GLM, SAS version 9.2; SAS Institute, 100 SAS Campus Dr, Cary, NC 27513−2414.
References
- 1.Langenberg C, Bergstrom J, Scheidt-Nave C, Pfeilschifter J, Barrett-Connor E. Cardiovascular death and the metabolic syndrome: role of adiposity-signaling hormones and inflammatory markers. Diabetes Care. 2006;29:1363–9. doi: 10.2337/dc05-2385. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am J Cardiol. 2003;92:522–8. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 3.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 4.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 5.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 6.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 7.Garshick E, Kelley A, Cohen SA, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–16. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marino RJ, Barros T, Biering-Sorensen F, et al. ASIA Neurological Standards Committee 2002. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–6. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 9.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 10.Segal JL, Gonzales E, Yousefi S, Jamshidipour L, Brunnemann SR. Circulating levels of IL-2R, ICAM-1, and IL-6 in spinal cord injuries. Arch Phys Med Rehabil. 1997;78:44–7. doi: 10.1016/s0003-9993(97)90008-3. [DOI] [PubMed] [Google Scholar]
- 11.Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86:312–7. doi: 10.1016/j.apmr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–9. doi: 10.1161/01.CIR.0000104566.10178.AF. [published erratum in: Circulation 2007;115:e537]. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Alexander RW. Exacerbation of atherosclerosis by hypertension. Potential mechanisms and clinical implications. Arch Intern Med. 1996;156:1952–6. [PubMed] [Google Scholar]
- 14.Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–85. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Jones LM, Legge M, Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003;84:1068–71. doi: 10.1016/s0003-9993(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Maggioni M, Bertoli S, Margonato V, Merati G, Veicsteinas A, Testolin G. Body composition assessment in spinal cord injury subjects. Acta Diabetol. 2003;40(Suppl 1):S183–6. doi: 10.1007/s00592-003-0061-7. [DOI] [PubMed] [Google Scholar]
- 17.Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 18.Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports. 2007;17:580–7. doi: 10.1111/j.1600-0838.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 20.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–9. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 21.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huffman KM, Samsa GP, Slentz CA, et al. Response of high-sensitivity C-reactive protein to exercise training in an at-risk population. Am Heart J. 2006;152:793–800. doi: 10.1016/j.ahj.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006;55:1500–7. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman MD. Cardiorespiratory fitness and training in quadriplegics and paraplegics. Sports Med. 1986;3:312–30. doi: 10.2165/00007256-198603050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34:727–51. doi: 10.2165/00007256-200434110-00003. [DOI] [PubMed] [Google Scholar]
- 26.Phillips WT, Kiratli BJ, Sarkarati M, et al. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998;23:641–716. doi: 10.1016/s0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- 27.Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systemic review and meta-analysis. J Periodontal. 2007;12:2289–2302. doi: 10.1902/jop.2007.070140. [DOI] [PubMed] [Google Scholar]
- 28.Desvarieux M, Demmer RT, Rundek T, et al. Periodontal micro-biota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111:576–82. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims JB, de Lemos JA, Maewal P, Warner JJ, Peterson GE, McGuire DK. Urinary tract infection in patients with acute coronary syndrome: a potential systemic inflammatory connection. Am Heart J. 2005;149:1062–5. doi: 10.1016/j.ahj.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 30.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Val-lance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–9. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Haughie P. Prospective studies of C-reactive protein as a risk factor for cardiovascular disease. J Investig Med. 1998;46:391–5. [PubMed] [Google Scholar]
- 35.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 37.Il'yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 38.Kony S, Zureik M, Driss F, Neukirch C, Leynaert B, Neukirch F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax. 2004;59:892–6. doi: 10.1136/thx.2003.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sin DD, Man SF, McWilliams A, Lam S. Progression of airway dysplasia and C-reactive protein in smokers at high risk of lung cancer. Am J Respir Crit Care Med. 2006;173:535–9. doi: 10.1164/rccm.200508-1305OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomiyama H, Koji Y, Yambe M, et al. Elevated C-reactive protein augments increased arterial stiffness in subjects with the metabolic syndrome. Hypertension. 2005;45:997–1003. doi: 10.1161/01.HYP.0000165018.63523.8a. [DOI] [PubMed] [Google Scholar]
- 41.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–4. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]


