Abstract
Purpose
Aurora-A and p16 play a major role in cell cycle checkpoint regulation. Both of them are important in the maintenance of centrosome duplication. Therefore, we hypothesized that polymorphisms in the two genes may interact or work together to influence the finely tuned mechanisms of cell cycle regulation that these proteins regulate. The purpose of this study was to investigate the association of the Aurora-A (T91A), and p16 (C540G and C580T) polymorphisms with age at diagnosis of pancreatic cancer.
Experimental Design
We genotyped 148 Caucasian patients with a diagnosis of pancreatic cancer for the Aurora-A and p16 polymorphisms using pyrosequencing. We tested the association between age at diagnosis and the Aurora-A and p16 genotypes by comparing Kaplan-Meier curves, evaluating the homogeneity of the curves using the log-rank test. We used Cox proportional hazard regression analysis to estimate the association between time to diagnosis and genotype, adjusting for gender.
Results
Patients with the Aurora-A polymorphic genotypes had a median age at diagnosis with pancreatic cancer that was 2.8 years earlier than those with the wild-type genotype [log-rank, P = 0.015; hazard ratio (HR), 1.55; 95% confidence intervals (95% CI), 1.09–2.20]. There was no significant association between the p16 genotypes and age at diagnosis. However, the Aurora-A and p16 C580T polymorphisms combined had a synergistic effect on age-associated risk for early diagnosis of pancreatic cancer. Compared with patients with wild-type genotypes for both genes, the median age at diagnosis for patients with one or two polymorphic alleles for both genes was 12.6 years earlier (log-rank, P = 0.0002; HR, 3.88; 95% CI, 1.94–7.76). No significant associations between the polymorphisms and the cancer metastatic status or survival after diagnosis were found.
Conclusions
Our findings suggest that the Aurora-A polymorphism contributes to a significantly earlier age at diagnosis of pancreatic cancer, and that Aurora-A and p16 C580T polymorphisms synergistically contribute to an earlier age at diagnosis of pancreatic cancer.
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States, with 33,370 deaths predicted to occur from the disease in 2007 (1). Because of the asymptomatic onset of pancreatic cancer, most patients already have metastatic or locally advanced disease at the time of diagnosis, resulting in poor prognoses. Thus, novel approaches leading to the earlier diagnosis and treatment of this deadly disease are needed. More importantly, identifying the genetic risk factors associated with pancreatic cancer could lead to strategies to prevent it.
Uncontrolled cell proliferation is a characteristic of tumor cells, and mutations in the genes involved in cell cycle control are frequent in human cancers, suggesting that the inactivation of their pathways may be necessary for tumor development (2). Aurora-A (also known as STK15, BTAK, AIKI, and AURKA) is a serine/threonine kinase and plays a pivotal role in proper mitotic entry and the G2-M checkpoint. The overexpression of Aurora-A induces abnormal G2-M transition in mammalian cells and may lead to centrosome amplification and chromosome instability, which results in the development and progression of malignant tumors (3, 4). A recent study showed that Aurora-A is overexpressed in pancreatic tumors and carcinoma cell lines, suggesting that its overexpression plays a role in pancreatic carcinogenesis (5). A common T-A polymorphism (T91A) has been identified at codon 31 of the Aurora-A gene, resulting in a phenylalanine to isoleucine substitution. The A allele has been reported to be preferentially amplified and associated with the degree of aneuploidy in human tumors (6).
p16 (also known as CDKN2, MTS-1, and INK4a) plays a pivotal role in the regulation of the G1-S cell cycle checkpoint. It blocks cell cycle progression by binding CDK4/6 and inhibiting the action of D-type cyclins (7). This leads to accumulation of hypophosphorylated Rb and growth arrest in G1 (8). Germ line mutations in the p16 gene are known to predispose individuals to pancreatic cancer and melanoma (9). Two adjacent polymorphisms in the p16 gene (C540G and C580T) located in the 3′untranslated region of exon 3 are associated with a significantly shorter progression time from primary to metastatic melanoma (10).
Given the major roles that Aurora-A and p16 play in cell cycle checkpoint regulation, we hypothesized that polymorphisms in the two genes may interact or work together to influence the finely tuned mechanisms of cell cycle regulation. This might influence age at diagnosis of pancreatic cancer as well as the dissemination and metastasis of the disease. In this study, on a consecutive series of 148 Caucasian pancreatic cancer patients, we obtained evidence that the Aurora-A T91A polymorphism is associated with an earlier age at diagnosis with pancreatic cancer, and that Aurora-A and p16 C580T polymorphisms synergistically contribute to an earlier age at diagnosis of pancreatic cancer.
Materials and Methods
Study subjects
The study included 148 consecutively registered Caucasian patients with adenocarcinoma of the pancreas evaluated at the University of Texas M. D. Anderson Cancer Center in Houston, Texas, from February 1999 to August 2004. At recruitment, each participant gave written informed consent. The data that we obtained from participants included their age at diagnosis and gender. The presence (M1) or absence (M0) of detectable metastases at diagnosis was determined according to the American Joint Committee on Cancer tumor-node-metastasis classification for pancreatic cancer (11). Each study subject contributed blood from which DNA was extracted with an AUTOPURE LS Automated DNA Purification Instrument (Gentra Systems, Inc.) according to the manufacturer’s instructions. The study was approved by the Institutional Review Board of M. D. Anderson Cancer Center.
Polymorphism analysis
Genotypes of Aurora-A T91A (dbSNP: rs2273535), p16 C540G (dbSNP: rs11515), and p16 C580T (dbSNP: rs3088440) were analyzed by pyrosequencing as directed by the manufacturer (Biotage, Inc.). A PCR was done on 5 ng DNA in a 50-μL reaction mixture containing 50 mmol/L KCl; 10 mmol/L Tris-HCl (pH, 8.3); 2.0 mmol/L MgCl2; 0.125 mmol/L dATP, dCTP, dGTP, and dTTP; 1.5 units AmpliTaq Gold DNA polymerase (Applied Biosystems); and 10 pmol of each primer (Sigma/Genosys). The PCR reaction mixture was initially incubated at 95°C for 6 min, followed by 45 cycles at 95°C for 15 s, 64°C for 30 s for Aurora-A, and 67°C for 30 s for p16, followed by 72°C for 15 s, and then an extension of 72°C for 5 min. The PCR primers used were 5′-CCATTCTAGGCTACAGCTCCA-3′ (forward) and 5′-ATTCTGAACCGGCTTGTGAC-3′ (reverse) for Aurora-A, 5′-GTGCCACACATCTTTGACCTCAG-3′ (forward) and 5′-TACGAAA-GCGGGGTGGGT-3′ (reverse) for p16 (C540G and C580T). The reverse primer was biotinylated for Aurora-A, and the forward primer was biotinylated for p16 to allow subsequent immobilization on a magnetic bead. The sequencing primers were 5′-TCTCGTGACTCAGCAA-3′ (Sigma Genosys) for Aurora-A, 5′-GACTGATGATCTAAGTTTCC-3′ for p16 C540G, and 5′-TGTGGCGGGGGCAGT-3′ for p16 C580T. Sixteen samples for each polymorphism were randomly selected and repeated with 100% concordance, and the genotypes were read independently by two different persons.
Statistical analysis
We tested the association between age at diagnosis and genotype by comparing Kaplan-Meier curves according to genotype. The log-rank test was used to evaluate the homogeneity of the Kaplan-Meier curves by genotype. The Cox proportional hazard regression model was then used to estimate the association between time to diagnosis for pancreatic cancer and the polymorphic genotypes, adjusting for gender. We tested for Hardy-Weinberg equilibrium by using an exact test based on genotypic frequencies (12). We used the LDA software to calculate the linkage disequilibrium index (13). Haplotype associations with the genotypes and age at diagnosis of pancreatic cancer were calculated with the haplotype procedure in the SAS/Genetics module (SAS, version 9.1). The χ2 test was used to determine the difference in the distribution of genotypes between patients with metastatic and nonmetastatic pancreatic cancer. The association between the polymorphic genotypes and the risk of metastatic pancreatic cancer was estimated by odds ratios (OR) and 95% confidence intervals (95% CI), which were calculated by unconditional logistic regression models. The ORs were adjusted for age and gender. We tested the null hypotheses of multiplicative gene-gene interaction by including main-effect variables and their product terms in the Cox regression model. A more-than-multiplicative interaction is suggested by the hazard ratio (HR). HR11 > HR10 × HR01, in which HR11 is the HR when both factors were present, HR10 is the HR when only factor 1 was present, HR01 is the HR when only factor 2 was present. We also tested for a more-than-additive gene-gene interaction by a bootstrapping test. A more-than-additive interaction is indicated if HR11 > HR10 + HR01 − 1. All statistical analyses were done using the Stata 8.0 (Stata Corporation).
Results
Subject characteristics
Of the 148 Caucasian patients in our study, 82 (55.4%) were men, and 66 (44.6%) were women. At the time of diagnosis, metastases were detected (M1) in 39 (26.4%) patients, whereas detectable metastases were absent (M0) in 109 (73.6%) patients. We genotyped the Aurora-A and p16 polymorphisms in 148 pancreatic cancer patients by pyrosequencing. The percentages in subjects were, for Aurora-A, 68.2% (101) TT, 27.1% (40) TA, and 4.7% (7) AA; for p16 C540G, 72.3% (107) CC, 25.7% (38) CG, and 2.0% (3) GG; for p16 C580T, 81.1% (120) CC, 16.9% (25) CT, and 2.0% (3) TT. The genotypic frequencies for Aurora-A and p16 were consistent with the Hardy-Weinberg equilibrium (for Aurora-A, χ2 = 1.307, P = 0.253; for p16 C540G, χ2 = 0.031, P = 0.861; for p16 C580T, χ2 = 1.457, P = 0.228). The allelic frequencies for Aurora-A are T, 81.8%, A, 18.2%; for p16 C540G, C, 85.1% and G, 14.9%; and for p16 C580T, C, 89.5%, T, 10.5%.
Aurora-A genotype, age at diagnosis, and cancer risk
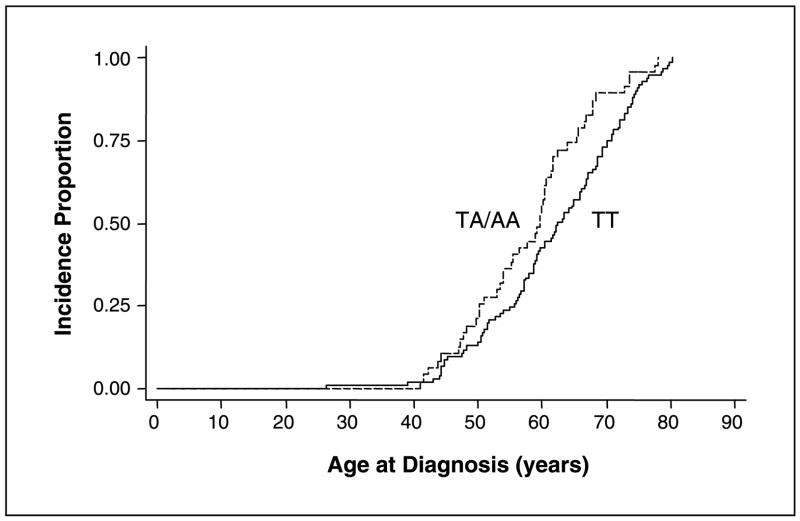
Because the number of subjects with the Aurora-A homozygous polymorphic genotype was too low to provide meaningful results, we combined the heterozygous and homozygous polymorphic genotypes for the analysis. Kaplan-Meier estimates showed that the median age at diagnosis in patients with the Aurora-A polymorphic genotype was 59.6 years, which was 2.8 years earlier than those with the Aurora-A wild-type genotype (Fig. 1). The median age at diagnosis for the different genotypes was significantly different (log-rank test, P = 0.015). Using the subjects with the Aurora-A wild-type genotype as a reference in the Cox proportional hazards regression model, we found a HR of 1.55 (95% CI, 1.09–2.20; Table 1). This result indicated that the subjects with the polymorphic genotypes had a significantly greater probability of being diagnosed with pancreatic cancer during any interval than those with the wild-type genotype.
Fig. 1.
Kaplan-Meier curves showing cumulative risk for diagnosis of pancreatic cancer by patient age for the TA/AA andTTgenotypes of the Aurora-A polymorphism.
Table 1.
Genotypes of Aurora-A and p16 and their association with risk for early diagnosis of pancreatic cancer
| Genotype | n (%), N = 148 | Median age at diagnosis (y) | P* | HR (95% CI) |
|---|---|---|---|---|
| Aurora-A (T→A) | ||||
| TT | 101 (68.2) | 62.4 | 0.015 | 1.00 (reference) |
| TA/AA | 47 (31.8) | 59.6 | 1.55 (1.09–2.20) | |
| p16 C540G (C→G) | ||||
| CC | 107 (72.3) | 60.4 | 0.252 | 1.00 (reference) |
| CG/GG | 41 (27.7) | 61.4 | 0.80 (0.56–1.17) | |
| p16 C580T (C→T) | ||||
| CC | 120 (81.1) | 62.1 | 0.050 | 1.00 (reference) |
| CT/TT | 28 (18.9) | 58.9 | 1.52 (1.00–2.30) |
Log-rank test for homogeneity between genotypes.
p16 genotype, haplotype, age at diagnosis, and cancer risk
Because of low frequency of homozygous polymorphic carriers, we combined the heterozygous and homozygous polymorphic genotypes for the analysis. The median ages of diagnosis for the different genotypes were determined from Kaplan-Meier plots and are shown in Table 1. There was no significant difference in age at diagnosis between the polymorphic genotype and wild-type genotype for C540G (log-rank test, P = 0.252) and a borderline significant difference between polymorphic genotypes and wild-type genotypes for C580T (log-rank test, P = 0.050). Compared with patients carrying wild-type genotype, patients with polymorphic genotypes of C540G did not show a significant increase in the HR (0.80; 95% CI, 0.56–1.17), and C580T showed a borderline significant increase in the HR (1.52; 95% CI, 1.00–2.30) in age-associated risk for the diagnosis of pancreatic cancer. Significant linkage disequilibrium (LD) was found between the two adjacent polymorphisms (D′ = 1, P = 0.025). Using the expectation-maximization (EM) algorithm to estimate the frequencies of the haplotypes, there were three of four possible haplotypes derived from the known genotypes (540C–580C, 74.7%; 540G–580C, 14.8%; and 540C–580T, 10.5%). The frequency of 540G–580T haplotype was zero. Therefore, only six diplotypes were determined in this study population. Most common diplotypes were 540C–580C/540C–580C (83, 56.1%), 540C–580C/540G–580C (34, 23.0%), and 540C–580C/540C–580T (21, 14.2%). Although 3 (2.0%) were 540G–580C/540G–580C, 4 (2.7%) were 540G–580C/540C–580T, and 3 (2.0%) were 540C–580T/540C–580T. No difference was observed in age-associated risk for the diagnosis of pancreatic cancer among diplotypes (data not shown).
Gene-gene interaction and pancreatic cancer risk
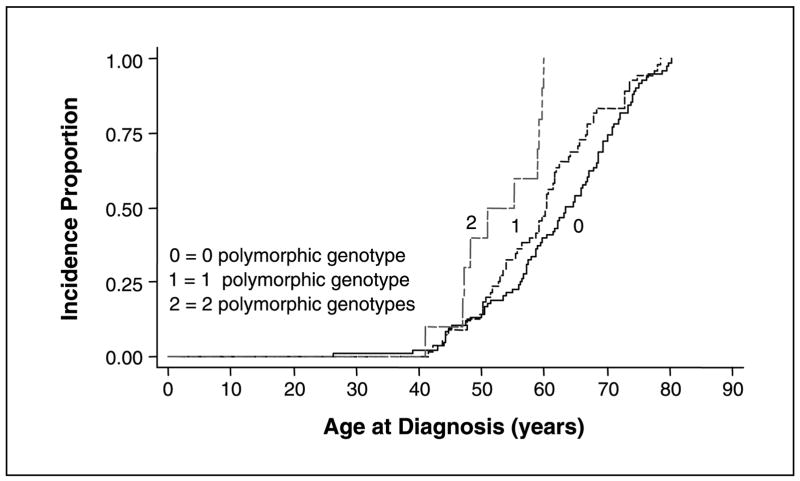
We evaluated the effect that carriage of multiple polymorphic alleles had on time to diagnosis of cancer. Interestingly, we found that patients with polymorphic genotypes of both Aurora-A and p16 C580T were diagnosed with pancreatic cancer 12.6 years earlier than those with both wild-type genotypes. The median ages at diagnosis for patients with zero, one, or two polymorphic genotypes were 63.4, 60.3, and 50.8 years, respectively. Kaplan-Meier plots (Fig. 2) showed a significant difference in age at diagnosis among the different genotypes (log-rank test, P = 0.0002). To determine if the earlier median age at diagnosis among the patients with two polymorphic genotypes might be due to familial pancreatic cancer, we assessed family history. None of the patients had a history of pancreatic cancer and/or melanoma. Although seven patients were homozygous for the rare allele of the Aurora-A gene and three patients were homozygous for the rare allele of the p16 gene, none of the subjects were homozygous for the rare allele for both p16 and Aurora-A. The expected frequency of homozygosity for the rare allele of the Aurora-A gene is 3.3% (we observed 4.7%) and, for p16 gene, 1.1% (we observed 2%). The probability of homozygosity for the rare allele for both p16 and Aurora-A is very low (P = 0.0004). Therefore, it is not surprising that we did not observe this genotype in our subjects.
Fig. 2.
Kaplan-Meier curves showing cumulative risk for diagnosis of pancreatic cancer by patient age for the combined effect of Aurora-A and p16 C580T polymorphisms. The patients have either zero (both genes are wild type), one (only one of two genes is polymorphic), or two (both genes are polymorphic) polymorphic genotypes.
We also determined whether the Aurora-A and p16 (C580T) polymorphisms had a joint effect on the age-associated risk of pancreatic cancer. We found that patients with a polymorphic genotype for only one of the two genes had a slightly higher nonsignificant age-associated risk for diagnosis of pancreatic cancer than patients with wild-type genotypes for both genes (HR, 1.29; 95% CI, 0.77–2.15 for p16; HR, 1.41; 95% CI, 0.95–2.10 for Aurora A; Table 2). Interestingly, the HR increased to 3.88 (95% CI, 1.94–7.76) for patients carrying polymorphic genotypes for both genes (log-rank test, P = 0.0006), and these results were confirmed using bootstrapping, which showed a 95% CI of 2.2–6.8. These results indicate a more-than-multiplicative joint effect between the Aurora-A polymorphic genotypes and p16 (C580T) polymorphic genotypes on the age-associated risk for diagnosis of pancreatic cancer. For example, the product of the separate effects is 1.29 × 1.41 = 1.82 (i.e., a multiplicative effect), which is much smaller than the observed combined effect of 3.88 (Table 2). However, the test for multiplicative gene-gene interaction was not quite significant (P = 0.091). A test of an additive model done by bootstrapping showed significant departure from an additive model (P = 0.029), further supporting multiplicative or synergistic effect when both risk genotypes were present.
Table 2.
Joint effects of polymorphisms of Aurora-A and p16 on risk for diagnosis of pancreatic cancer at an early age
| Genotypes Aurora-A | p16 C580T | n (%), N = 148 | Median age at diagnosis (y) | HR (95% CI) |
|---|---|---|---|---|
| (T→A) | (C→T) | |||
| TT | CC | 83 (56.1) | 63.4 | 1.00 (reference) |
| TT | CT/TT | 18 (12.2) | 59.1 | 1.29 (0.77–2.15) |
| TA/AA | CC | 37 (25.0) | 60.5 | 1.41 (0.95–2.10) |
| TA/AA | CT/TT | 10 (6.7) | 50.8 | 3.88 (1.94–7.76)* |
P < 0.05, departure from additive model.
We also evaluated the effect that carriage of multiple polymorphic alleles of the Aurora-A and p16 (C540G) had. No joint effect on the age-associated risk of pancreatic cancer was observed (data not shown).
The effect of Aurora-A and p16 polymorphisms on cancer status
We also determined the potential effect of the Aurora-A genotype on tumor invasion and metastasis. No significant differences in the Aurora-A genotypic frequencies between subgroup M1 and subgroup M0 were observed (χ2 = 0.419; P = 0.517). Additionally, the polymorphic genotype was not significantly associated with the risk of metastatic disease compared with the wild-type genotype (OR, 1.29; 95% CI, 0.60–2.77) or survival after diagnosis (log-rank test, P = 0.786). Similarly, no significant differences in the p16 genotypic frequencies between subgroup M1 and subgroup M0 were observed (data not shown). Furthermore, no significant association between the polymorphism and disease status was found when patients in stage I were compared with patients in stages II to IV for Aurora-A and p16 (data not shown).
Discussion
In this study, we examined polymorphisms in two cell cycle genes, Aurora-A and p16. We found that Aurora-A T91A and p16 C580T polymorphisms had a synergistic effect on age-associated risk for diagnosis of pancreatic cancer. Patients with one or two polymorphic alleles in both genes had a 3.88-fold increased risk for earlier age at diagnosis of pancreatic cancer compared with wild-type genotypes and were diagnosed with pancreatic cancer 12.6 years earlier. This is the first report, to our knowledge, to show that the Aurora-A and p16 polymorphisms synergistically contribute to an earlier age at diagnosis for any cancer.
When the functional Aurora-A T91A polymorphism was analyzed alone for influence on age at diagnosis of pancreatic cancer, patients with the polymorphic genotypes developed pancreatic cancer earlier than did those with the wild-type genotype and had an approximately 1.55-fold increased age-associated risk. These findings are consistent with those of other studies, which show the A allele to be an adverse genotype (14–16). Ewart-Toland et al. (17) also reported that the A allele increased the risk of multiple cancer types and confirmed that the allele is a low-penetrance cancer susceptibility allele by carrying out a meta-analysis. In contrast, other recent studies reported that the A allele was associated with a significantly reduced risk for lung cancer in Caucasians (18), and no association between the A allele and the risk for breast cancer was observed in English women (19).
No significant association was observed between p16 C540G or p16 C580T genotypes alone and age at diagnosis with pancreatic cancer. Although the two adjacent polymorphisms of p16 were reportedly associated with tumor aggressiveness for melanoma (10), Zheng et al. (20) did not find that they were associated with the risk of developing squamous cell carcinoma of the head and neck.
The mechanism for the synergistic effect between Aurora-A T91A and p16 C580T upon age at diagnosis is unknown. It may be that there are physical interactions between the two genes that are influenced by the polymorphisms or the polymorphisms may influence the physical interactions of Aurora-A and p16 with other proteins or with environmental factors such as tobacco smoke. The overexpression of Aurora-A has been shown to lead to centrosome amplification, chromosomal instability, and transformation in mammalian cells (4). Aurora-A localizes to centrosomes immediately after the centrioles have been duplicated at the end of S phase and becomes phosphorylated and activated in centrosomes late in the G2 phase. The protein then positively regulates the G2-M phase transition of the cell cycle. p16 has also been shown to be important in proper centrosome duplication. Loss of p16 results in generation of supernumerary centrosomes in studies on primary diploid epithelial and fibroblast cultures (21). The synergism between Aurora-A and p16 could be a result of subtle variations caused by polymorphisms in the two genes, which influence their ability to carry out their roles in proper maintenance and duplication of the centrosomes.
Another possible mechanism by which Aurora-A and p16 could have a synergistic effect on age at diagnosis of pancreatic cancer is through their influence on the G1-S checkpoint. p16 is important in the regulation of the G1-S checkpoint as it binds to CDK4 and CDK6, inhibiting the ability of either protein to interact with cyclin D1 and stimulate passage through the G1-S phase transition of the cell cycle. Although Aurora-A is important in mitotic entry and G2-M checkpoint, it may also play a role in the G1-S checkpoint because Aurora-A kinase activity has been shown to modulate p53 stability. p53 is important in several functions, including regulation of the G1-S checkpoint where it positively regulates p21, in response to DNA damage, which then binds to cyclin D1/CDK4 or CDK6 complexes to inhibit their activity and induce cell cycle arrest (22, 23). The synergism might therefore arise as a result of the combined influence that Aurora-A and p16 have on the G1-S cell cycle checkpoints.
Further confirmation of the synergism between the p16 and Aurora-A polymorphisms in additional clinical populations and a better understanding of the mechanism by which this synergism occurs are needed.
Several studies have shown that the overexpression of Aurora-A is more relevant for early events in tumorigenesis than for tumor progression (5, 24–26). Our findings are in agreement with these previous studies because the Aurora-A polymorphism was significantly correlated with the age at diagnosis of pancreatic cancer but not with its progression, as shown by the lack of significant correlation between the polymorphism and the cancer’s metastatic status or stage. Thus, our findings agree with previous studies and further substantiate that Aurora-A is a potential low-penetrance cancer susceptibility gene in humans (6).
Because pancreatic cancer is usually not detected until it reaches an advanced stage, new approaches to earlier diagnosis are important for improving the prognosis of the disease. If confirmed, the present findings, combined with the identification of additional environmental and genetic risk factors, could provide a panel of risk markers, which could help identify those patients who are more likely to develop pancreatic cancer, and this could lead to the earlier detection and treatment, longer survival time, and lower mortality of pancreatic cancer. Future studies will focus on identifying additional risk factors.
Acknowledgments
We thank Haidee Chancoco for DNA extraction, and Christopher Yeager, Angelique Siy, and Monica Domingue for their editorial comments.
Grant support: National Cancer Institute grants P20 CA101936, and U01 CA111302; NIHCancer Center Support grant CA16672.
This research was supported, in part, by the Janis Davis Gordon Memorial Postdoctoral Fellowship, Division of Cancer Prevention, University of Texas M. D. Anderson Cancer Center.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 3.Marumoto T, Hirota T, Morisaki T, et al. Roles of aurora-A kinaseinmitotic entryand G2 checkpointinmammalian cells. Genes Cells. 2002;7:1173–82. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Zhu J, Firozi PF, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–7. [PubMed] [Google Scholar]
- 6.Ewart-Toland A, Briassouli P, de Koning JP, et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34:403–12. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- 7.Macaluso M, Montanari M, Cinti C, Giordano A. Modulation of cell cycle components by epigenetic and genetic events. Semin Oncol. 2005;32:452–7. doi: 10.1053/j.seminoncol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFβ, and contact inhibition. Cell. 1999;97:53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- 9.Whelan AJ, Bartsch D, Goodfellow PJ. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N Engl J Med. 1995;333:975–7. doi: 10.1056/NEJM199510123331505. [DOI] [PubMed] [Google Scholar]
- 10.Sauroja I, Smeds J, Vlaykova T, et al. Analysis of G(1)/S checkpoint regulators in metastatic melanoma. Genes Chromosomes Cancer. 2000;28:404–14.1. doi: 10.1002/1098-2264(200008)28:4<404::aid-gcc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al. American Joint Committee on Cancer cancer staging manual. 6. NewYork: Springer; 2002. [Google Scholar]
- 12.Weir BS. Genetic data analysis II: methods for discrete population genetic data. Sunderland, Massachusetts: SinauerAssociates; 1996. [Google Scholar]
- 13.Ding K, Zhou K, He F, Shen Y. LDA—a Java-based linkage disequilibrium analyzer. Bioinformatics. 2003;19:2147–8. doi: 10.1093/bioinformatics/btg276. [DOI] [PubMed] [Google Scholar]
- 14.Miao X, Sun T, Wang Y, Zhang X, Tan W, Lin D. Functional STK15 Phe31Ile polymorphism is associated with the occurrence and advanced disease status of esophageal squamous cell carcinoma. Cancer Res. 2004;64:2680–3. doi: 10.1158/0008-5472.can-04-0651. [DOI] [PubMed] [Google Scholar]
- 15.Egan KM, Newcomb PA, Ambrosone CB, et al. STK15 polymorphism and breast cancer risk in a population-based study. Carcinogenesis. 2004;25:2149–53. doi: 10.1093/carcin/bgh231. [DOI] [PubMed] [Google Scholar]
- 16.Dicioccio RA, Song H, Waterfall C, et al. STK15 polymorphisms and association with risk of invasive ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1589–94. [PubMed] [Google Scholar]
- 17.Ewart-Toland A, Dai Q, Gao YT, et al. Aurora-A/ STK15 T+91A is a general low penetrance cancer susceptibility gene: a meta-analysis of multiple cancer types. Carcinogenesis. 2005;26:1368–73. doi: 10.1093/carcin/bgi085. [DOI] [PubMed] [Google Scholar]
- 18.Gu J, Gong Y, Huang M, Lu C, Spitz MR, Wu X. Polymorphisms of STK15 (Aurora-A) gene and lung cancer risk in Caucasians. Carcinogenesis. 2007;28:350–5. doi: 10.1093/carcin/bgl149. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher O, Johnson N, Palles C, et al. Inconsistent association between the STK15 F31I genetic polymorphism and breast cancer risk. JNatl Cancer Inst. 2006;98:1014–8. doi: 10.1093/jnci/djj268. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Shen H, Sturgis EM, et al. Haplotypes of two variants in p16 (CDKN2/MTS-1/INK4a) exon 3 and risk of squamous cell carcinoma of the head and neck: a case-control study. Cancer Epidemiol Bio-markers Prev. 2002;11:640–5. [PubMed] [Google Scholar]
- 21.McDermott KM, Zhang J, Holst CR, Kozakiewicz BK, Singla V, Tlsty TD. p16(INK4a) prevents centrosome dysfunction and genomic instability in primary cells. PLoS Biol. 2006;4:o350–65. doi: 10.1371/journal.pbio.0040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama H, Sasai K, Kawai H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 23.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 24.Hoque A, Carter J, Xia W, et al. Loss of aurora A/ STK15/BTAK overexpression correlates with transition of in situ to invasive ductal carcinoma of the breast. Cancer Epidemiol Biomarkers Prev. 2003;12:1518–22. [PubMed] [Google Scholar]
- 25.Miyoshi Y, Iwao K, Egawa C, Noguchi S. Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int J Cancer. 2001;92:370–3. doi: 10.1002/ijc.1200. [DOI] [PubMed] [Google Scholar]
- 26.Royce ME, Xia W, Sahin AA, et al. STK15/Aurora-A expression in primary breast tumors is correlated with nuclear grade but not with prognosis. Cancer. 2004;100:12–9. doi: 10.1002/cncr.11879. [DOI] [PubMed] [Google Scholar]