Abstract
Background
The melanocortin (MC) system is composed of peptides that are cleaved from the polypeptide precursor proopiomelanocortin (POMC). Recent pharmacologic and genetic evidence suggests that MC receptor (MCR) signaling modulates neurobiologic responses to ethanol and ethanol intake. Because ethanol decreases POMC mRNA levels, we determined if exposure to an ethanol-containing diet (ED) would significantly reduce central immunoreactivity of the MC peptide α-MSH in rats. We also determined if ethanol exposure would alter the immunoreactivity of agouti-related protein (AgRP), an endogenous MCR antagonist.
Methods
Male Sprague–Dawley rats were given 18 days of access to normal rodent chow or a control diet (CD), or short-term (4 days) or long-term (18 days) access to an ED. At the end of the study, rats were perfused with 4% paraformaldehyde and their brains were sectioned into two sets for processing with α-MSH or AgRP immunohistochemistry.
Results
Rats exposed to an ED showed significant reductions of central α-MSH immunoreactivity relative to rats exposed to a control diet (CD) or normal rodent chow. Ethanol-induced reductions of α-MSH immunoreactivity were site-specific and were noted in regions of the hypothalamus and extended amygdala, as well as the paraventricular nucleus of the thalamus. Because there were no differences in body weights or caloric intake between the CD and ED groups, reductions of α-MSH immunoreactivity in ED-treated rats are best explained by ethanol exposure rather than altered energy balance. No significant ethanol-induced alterations in hypothalamic AgRP immunoreactivity were detected.
Conclusions
The present study shows that ethanol site specifically reduces α-MSH immunoreactivity in rat brain. These observations, in tandem with recent pharmacologic and genetic studies, suggest that the endogenous MC system modulates neurobiologic responses to ethanol. Thus, compounds which target MCRs may prove to have therapeutic value in the treatment of excessive ethanol consumption and/or the symptoms associated with ethanol withdrawal.
Keywords: AgRP, α-MSH, Ethanol Consumption, Rats, Melanocortin, POMC
The melanocortin mc system is composed of peptides that are cleaved from the polypeptide precursor proopiomelanocortin (POMC). Central MC peptides are produced by neurons within the hypothalamic arcuate nucleus and the medulla (Dores et al., 1986; Jacobowitz and O’Donohue, 1978; O’Donohue and Dorsa, 1982), and include adrenocorticotropic hormone (ACTH), α-melanocyte stimulating hormone (α-MSH), β-MSH, and γ-MSH (Hadley and Haskell-Luevano, 1999). Because of lack of a critical dibasic site, β-MSH is not processed in rodent brain (Pritchard et al., 2002). Agouti related-protein (AgRP), a neuropeptide produced in the hypothalamus and co-secreted with neuropeptide Y (NPY) in the same synaptic complexes as α-MSH, functions as a natural MC receptor (MCR) antagonist (Shutter et al., 1997).
There are several observations, which suggest that the MC system is a prime candidate for regulating neurobiologic responses to drugs of abuse and drug self-administration. For example, α-MSH administered into the ventral tegmental area (VTA) increases dopamine and DOPAC levels in the nucleus accumbens (NAc; Lindblom et al., 2001), and chronic central infusion of the non-selective MCR agonist, melanotan II (MTII), increases dopamine D1 receptor binding in the NAc and dopamine D2 receptor binding in the VTA (Lindblom et al., 2002a). Thus, α-MSH and MTII alter dopamine signaling in these regions. Chronic treatment of a high dose of morphine decreases MC-4 receptor (MC4R) mRNA in the NAc, the periaqueductal gray, and neostriatum (Alvaro et al., 1996), brain regions that modulate drug reward, opiate tolerance, and psychomotor stimulation, respectively (Kalivas and Stewart, 1991; Koob and Bloom, 1988; Wise and Bozarth, 1987). On the other hand, chronic treatment with low doses of morphine or cocaine increases MC4R receptor mRNA in the striatum and NAc (Hsu et al., 2005). Consistent with a role in drug self-administration, central infusion of an MCR agonist decreases the acquisition of heroin self-administration in rats (van Ree et al., 1981).
Importantly, there is also accumulating evidence that MC neuropeptides modulate neurobiologic responses to ethanol. First, α-MSH is expressed in brain regions implicated in ethanol’s effects, including the striatum, NAc, VTA, amygdala, hippocampus, and hypothalamus (Bloch et al., 1979; Dube et al., 1978; Jacobowitz and O’Donohue, 1978; O’Donohue and Jacobowitz, 1980; O’Donohue et al., 1979; Yamazoe et al., 1984). Second, rats selectively bred for high ethanol drinking (AA (Alko, Alcohol)) have low levels of MC-3 receptor (MC3R) in the shell of the NAc, but have high levels ofMC3R andMC4R in various regions of the hypothalamus, when compared with low ethanol drinking rats (Lindblom et al., 2002b). Third, central infusion of MTII significantly reduced voluntary ethanol drinking in AA rats with an established history of ethanol intake (Ploj et al., 2002). Similarly, MTII-induced reduction of ethanol consumption was shown to be receptor-mediated and not associated with alterations of ethanol metabolism in C57BL/6J mice (Navarro et al., 2003). More recently, ventricular infusion of a selective MC4R agonist significantly reduced ethanol drinking, while ventricular infusion of the non-selective MCR antagonist AgRP-(83–132) significantly increased ethanol drinking, by C57BL/6J mice (Navarro et al., 2005).
In light of the above observations, and the fact that ethanol has direct effects on central POMC mRNA activity (Rasmussen et al., 1998, 2002; Scanlon et al., 1992a; Zhou et al., 2000), an important question is whether ethanol exposure influences central MC neuropeptide content and in which brain regions. To this end, the present study determined if short-term (4 day) and/or long-term (18 day) exposure to an ethanol-containing diet would alter the immunoreactivity of α-MSH in rat brain. Because AgRP is an endogenous MCR antagonist (Shutter et al., 1997), and central infusion of AgRP increases ethanol drinking (Navarro et al., 2005), the immunoreactivity of AgRP in rats following chronic exposure to ethanol was also assessed. Here we show that exposure to an ethanol diet for 4 or 18 days significantly reduced α-MSH immunoreactivity in specific regions of the hypothalamus, thalamus, and extended amygdala while having no effect on the immunoreactivity of AgRP in the hypothalamus.
MATERIALS AND METHODS
Subjects
Male Sprague–Dawley rats (Charles River, Raleigh, NC, USA) were obtained at 160–180 g and were maintained at 22°C with a 12:12 light/dark cycle. All rats were individually housed in plastic rat cages with free access to water and standard rodent chow (Teklad, Madison, WI) in the vivarium facilities of the Department of Psychology (University of North Carolina). All procedures used in the present study were in compliance with the National Institute of Health guidelines, and all procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC).
Ethanol and Control Diets
The diet was a lactalbumin/dextrose-based, nutritionally complete diet (Dyets, Inc., Bethlehem, PA). Dextrose calories in the control diet (CD) were equated with ethanol calories in the ethanol diet (ED). Rats were habituated to drinking CD in the absence of rodent chow for 2 days (with the exception of the chow control group described below). During the study, all rats with ED were first habituated with 2 days access to a 4.5% (w/v) ED, followed by access to a 7% (w/v) ED for an additional 2 or 16 days. A modified pair-feeding design was used. To equate the caloric intake between groups, the rats maintained on the CD were given a volume of diet equivalent to the average volume consumed the previous day by the rats maintained on the ED. This diet has been used successful to study withdrawal-induced anxiety-like behavior in rats (Breese et al., 2004; Knapp et al., 2004; Overstreet et al., 2002, 2004). Rodent chow was removed from each rat cage during diet access and rats had access to a second bottle containing tap water at all times.
Following habituation, rats were distributed to 4 groups matched on body weight (n = 10/group) so that each group had approximately the same average weight at the beginning of the study. To control for potential effects of diet on the immunoreactivity of α-MSH or AgRP, one group of rats was maintained on normal rodent chow (Chow) for the entire study. A second control group received CD in place of rodent chow for the duration of the study. A third group was given CD for 14 days, the 4.5% ED for 2 days, and the 7% ED for 2 days (group ED4). A fourth group received the 4.5% ED for 2 days, followed by the 7% ED for 16 days (group ED18). Rats that experienced a similar protocol (15 days of access to at 7% ED) achieved blood ethanol concentrations ranging from 100 mg/dl after the first day of access to 200 mg/dl during the 15th day of access (Overstreet et al., 2002). Throughout the study, diet intake and body weight measures were recorded daily.
Perfusions, Brain Preparation, and Immunohistochemistry (IHC)
Immunohistochemistry procedures were based on those routinely used in our laboratory (Hayes et al., 2005; Knapp et al., 1998; Thiele et al., 1996, 1997, 1998a,b, 2000). Immediately after 18 days of access to diet, rats were injected with pentobarbital (100 mg/kg) and were then perfused within 10 minutes transcardially with 0.1 mM phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde in phosphate buffer. Rats were perfused in pairs, and the order that rats were perfused was counterbalanced by diet condition. Rats had access to diet up to the time of perfusions to avoid ethanol withdrawal in the ED groups. All perfusions were completed within a 5-hour window of time. The brains were collected and post-fixed in paraformaldehyde for 24 hours at 4°C, at which point they were transferred to PBS. Rat brains were cut using a vibratome into 40 μm sections that were then stored in PBS until the IHC assay. Sections were evenly divided into two sets (every-other section) for processing with α-MSH or AgRP antibodies. After rinsing in fresh PBS 4 times (10 minutes each), tissue sections were blocked in 10% rabbit serum (for α-MSH) or 10% goat serum (for AgRP) and 0.1% triton-X-100 in PBS for 1 hour. Sections were then transferred to fresh PBS containing primary sheep anti-α-MSH (Millipore, Billerica, MA; 1:10,000) or primary rabbit anti-AgRP (Phoenix Pharmaceuticals, Inc., Burlingame, CA; 1:4,000) for 3 days at 4°C. As a control to determine if staining required the presence of the primary antibodies, some sections were run through the assay without primary antibody (α-MSH or AgRP). In each assay described below, tissue processed without the primary antibody failed to show staining that was evident in tissue processed with primary antibody. After the 3 days of incubation, the sections were rinsed 4 times and then processed with Vectastain Elite kits (Vector Labs) as per the manufacturer’s instructions for standard ABC/HRP/diaminobenzidine-based immunohistochemistry. The sections processed for α-MSH or AgRP were visualized by reacting the sections with a 3,3′-diamino-benzidine tetrahydrochloride (DAB, Polysciences, Inc., Warrington, PA) reaction solution containing 0.05% DAB, 0.005% cobalt, 0.007% nickel ammonium sulfate, and 0.006% hydrogen peroxide. All sections were mounted on glass slides, air-dried overnight, and cover slipped for viewing.
Digital images of α-MSH and AgRP immunohistochemistry were obtained on a Nikon E400 microscope equipped with a Nikon Digital Sight DS-U1 digital camera run with Nikon-provided software. For analysis, great care was taken to match sections through the same region of brain and at the same level using anatomic landmarks with the aid of a rat stereotaxic atlas (Paxinos and Watson, 1986). For cell counting, all visible cell bodies stained within the defined brain region were counted manually by an experimenter blinded to group condition. Data from each brain region in an animal were calculated by taking the average counts from 2 brain slices. Data from each slice were calculated by taking the average counts from the left and right sides of the brain at the specific brain region of interest. For non-cell body localization of the α-MSH or AgRP in a given brain region, densitometric procedures were used to assess protein levels. Flat-field corrected digital pictures (8-bit grayscale) were taken using the Digital Sight DS-U1 camera and density of staining was analyzed using Image J software (Image J, National Institute of Health, Bethesda, MD) by calculating the percent of the total area examined that showed signal (cell bodies and processes) relative to a subthreshold background. The size of the areas that were analyzed was the same between animals and groups. The subthreshold level for the images was set in such a way that any area without an experimenter defined level of staining was given a value of zero. Anatomically matched pictures of the left and right sides of the brain were used to produce an average density for each brain region from each slice. In all cases, quantification of immunohistochemistry data was conducted by an experimenter that was blinded to group identity.
Data Analyses
All data in this report are presented as mean ± SEM differences between groups were analyzed using one-way analyses of variance (ANOVA) procedures. Because we expected that ethanol-induced alterations of α-MSH would be site specific, separate ANOVAs were performed for each brain region. When significant differences are found, post hoc analyses were conducted using the Tukey’s HSD test. For one set of analyses, t-tests were used for planned comparisons in accordance with a priori hypotheses. In all cases, p < 0.05 (two-tailed) was used as the level of statistical significance.
RESULTS
Body Weights
Because both α-MSH and AgRP have been implicated in the modulation of food intake and body weight (Sainsbury et al., 2002), it was important to determine if there were body weight differences between the different treatment groups over the course of the experiment. A one-way ANOVA performed on baseline body weight data collected the day before the initiation of diet exposure failed to achieve statistical significance (F3, 36 = 0.009; p > 0.05), a result verifying the similar body weights between the Chow (286.6 ± 6.5 g), CD (287.7 ± 5.7 g), ED4 (287.7 ± 4.4 g), and ED18 (287.5 ± 4.8 g) groups. A one-way ANOVA performed on body weight data collected at the end of the study was significant (F3, 36 = 16.19; p < 0.001). In this case, Tukey’s HSD post-hoc tests revealed that while the Chow group (396.5 ± 9.4 g) weighed significantly more than the CD (344.8 ± 5.0 g), ED4 (339.9 ± 6.1 g), and ED18 (326.8 ± 9.1 g) groups, none of the diet treated groups differed significantly from one another. Furthermore, as the pair-feeding procedure equated the volume of diet consumption between CD and ED groups, and because the CD and ED were calorically equated, there were no differences in caloric intake between groups given access to liquid diet. These observations reinforce the conclusion the differences between CD- and ED-treated groups below are best explained by the presence or absence of ethanol exposure rather than group differences in caloric intake or body weight.
Immunoreactivity of α-MSH in Regions of the Hypothalamus After Ethanol Exposure
Arcuate Nucleus of the Hypothalamus (Arc)
Data representing the average immunoreactivity of α-MSH in the Arc are presented in Fig. 1, and represented photomicrographs of α-MSH immunoreactivity in the Arc of groups CD and ED18 are depicted in Fig. 2A and 2C (to conserve space, pictures of sections from the Chow and ED4 groups are not presented in photomicrograph figures). The Arc was the only brain region in which α-MSH was expressed in cell bodies rather than cellular processes. Thus, to verify that cell counting and densitometric procedures yielded similar results, we quantified and analyzed α-MSH immunoreactivity in this region using both procedures. Average cell counts of α-MSH-positive cells in the Arc are presented in Fig. 1A. A one-way ANOVA performed on these data was significant (F3, 36 = 36.28; p < 0.001). Tukey’s HSD post hoc tests revealed that both groups ED4 and ED18 showed significantly lower immunoreactivity of α-MSH relative to the control groups (Chow and CD). Average densities (% area) of α-MSH immunoreactivity in the Arc are presented in Fig. 1B. Similar to the cell counting data, a one-way ANOVA performed on density data was significant (F3, 36 = 44.81; p < 0.001), and Tukey’s HSD tests showed that groups ED4 and ED18 showed significantly lower immunoreactivity of α-MSH relative to groups Chow and CD.
Fig. 1.
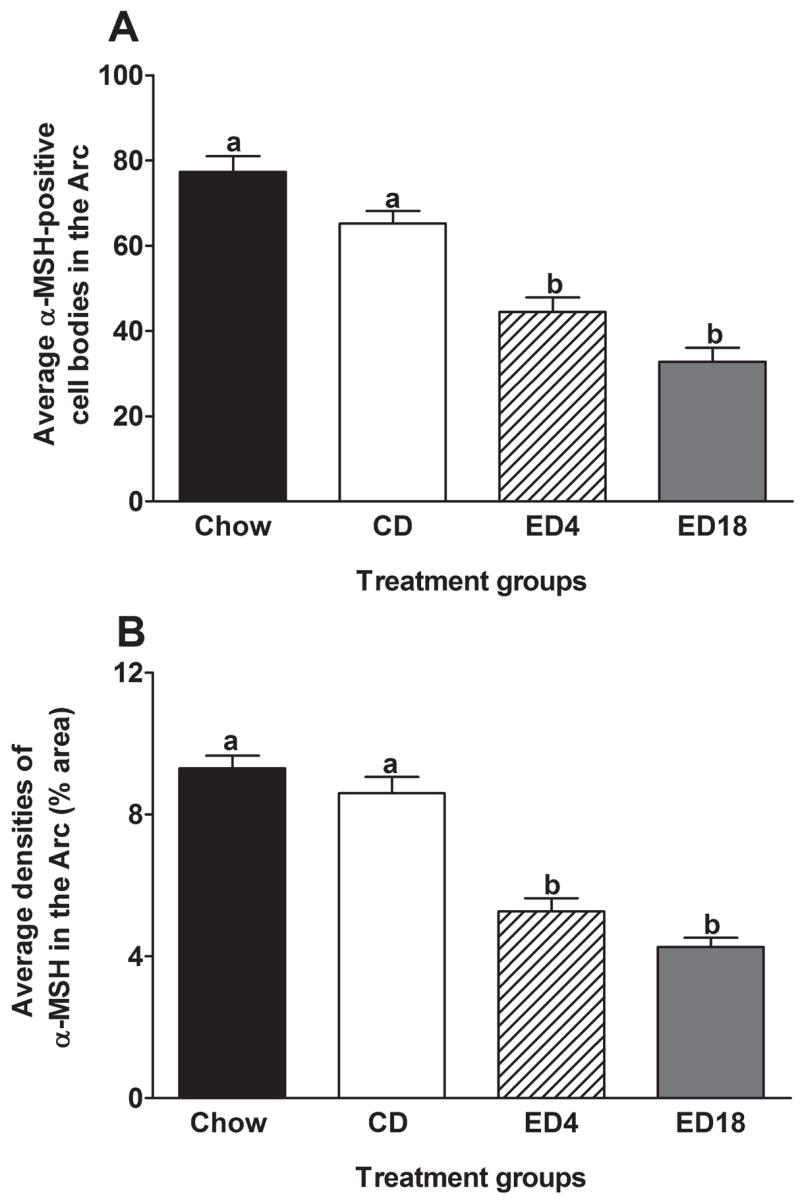
Quantification of α-melanocyte-stimulating hormone (MSH) immunoreactivity in the arcuate nucleus of the hypothalamus (Arc). Quantification was done by counting α-MSH-positive cell bodies (A) or by measuring the density of α-MSH staining (B) using Image J software, which calculated the percent of the total area examined (% area) that showed signal (cell bodies and processes) relative to a subthreshold background. Groups were given 18-days of access to normal rodent chow (Chow) or an ethanol-free control diet (CD), or an ethanol diet for 4 (ED4) or 18 (ED18) days. Values are represented as mean ± SEM. There are statistical differences between groups that do not share overlapping lettering (a or b; p < 0.05).
Fig. 2.
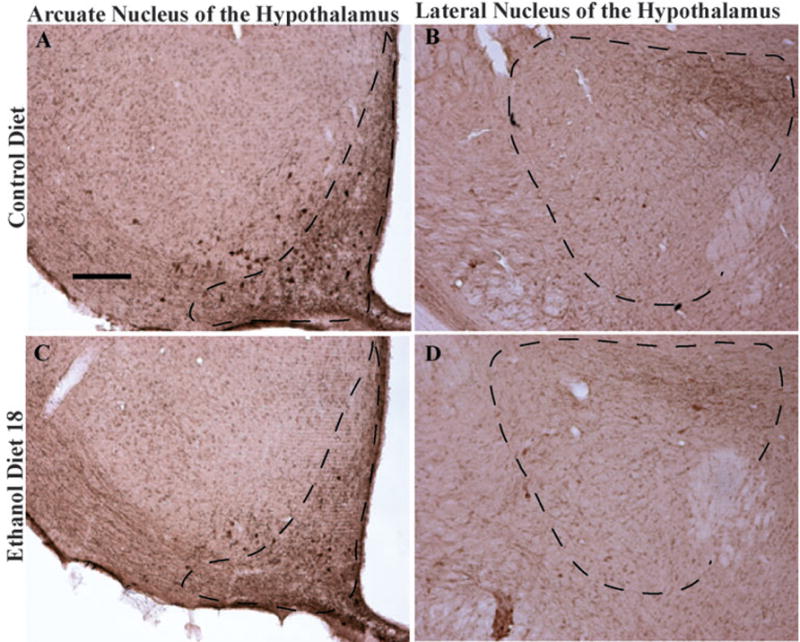
Representative photomicrographs of 40 μm coronal sections showing α-melanocyte-stimulating hormone (MSH) immunoreactivity through the arcuate nucleus of the hypothalamus (A and C) and the lateral nucleus of the hypothalamus (B and D) of rats given 18 days of exposure to the control diet or the ethanol diet (Ethanol Diet 18). Dashed line depicts the region that was selected for quantification. Images were photographed and quantified at a magnification of 10×. Scale bar = 200 μm. α-MSH immunoreactivity in the arcuate nucleus appears in cell bodies, while staining in the lateral hypothalamus is located primarily in cellular processes.
Lateral (LH), Dorsomedial (DMH), and Paraventricular (PVN) Nuclei of the Hypothalamus
Data representing the average immunoreactivity of α-MSH in the LH, DMH, and PVN are presented in Fig. 3, and representative photomicrographs of α-MSH immunoreactivity in the LH of groups CD and ED18 are depicted in Fig. 2B and 2D. A one-way ANOVA performed on average densities of α-MSH immunoreactivity in the LH was significant (F3, 36 = 5.77; p < 0.05). Tukey’s HSD tests revealed that while group ED18 had significantly lower α-MSH immunoreactivity relative to both control groups (Chow and CD), group ED4 did not differ significantly from the control groups (Fig. 3A). One-way ANOVAs performed on average densities of α-MSH immunoreactivity in the DMH (F3, 36 = 0.38; p > 0.05) and PVN (F3, 36 = 0.23; p > 0.05) both failed to achieve statistical significance (see Fig. 3B and C, respectively).
Fig. 3.
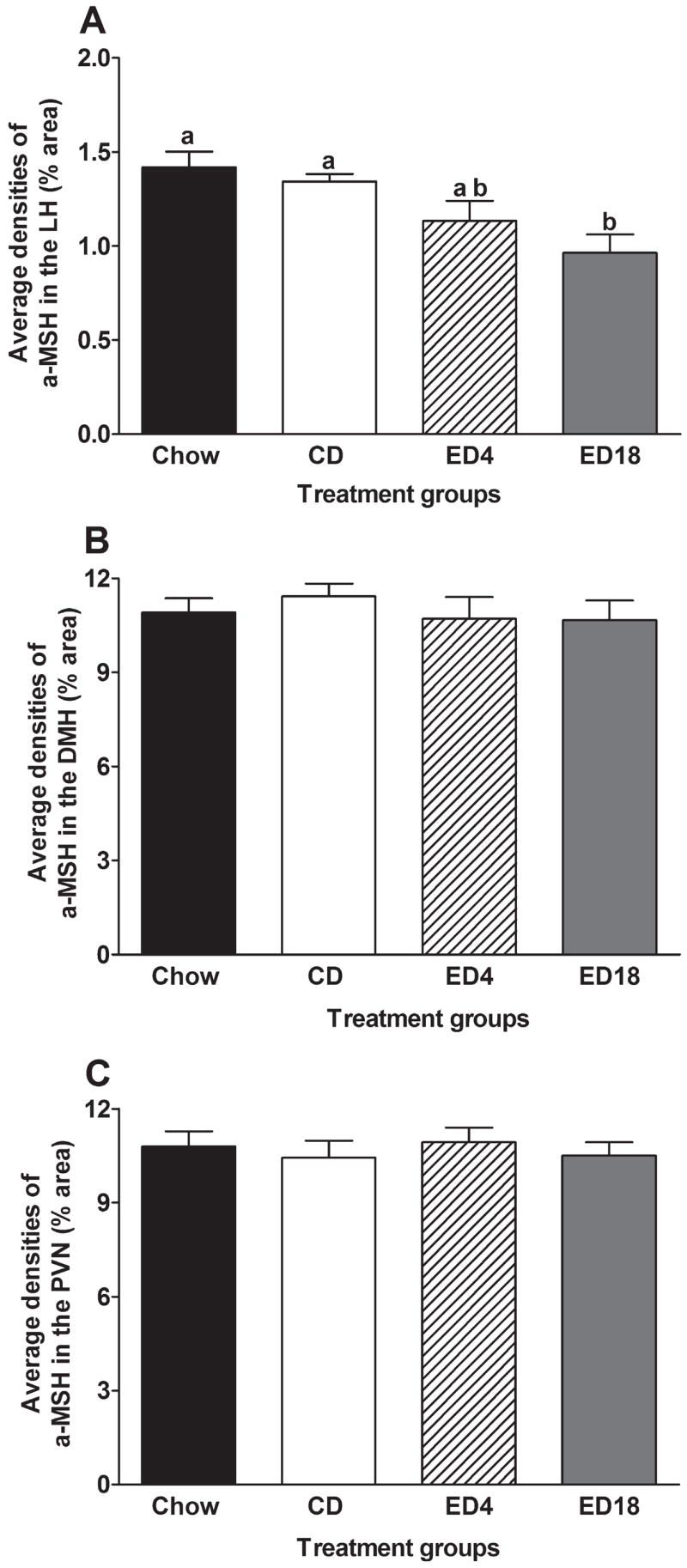
Quantification of α-melanocyte-stimulating hormone immunoreactivity (% area) in the lateral nucleus of the hypothalamus (LH; A), the dorsomedial nucleus of the hypothalamus (DMH; B), and the paraventricular nucleus of the hypothalamus (PVN; C). Groups were given 18 days of access to normal rodent chow (Chow) or an ethanol-free control diet (CD), or an ethanol diet for 4 (ED4) or 18 (ED18) days. Values are represented as mean ± SEM. There are statistical differences between groups that do not share overlapping lettering (a or b; p < 0.05).
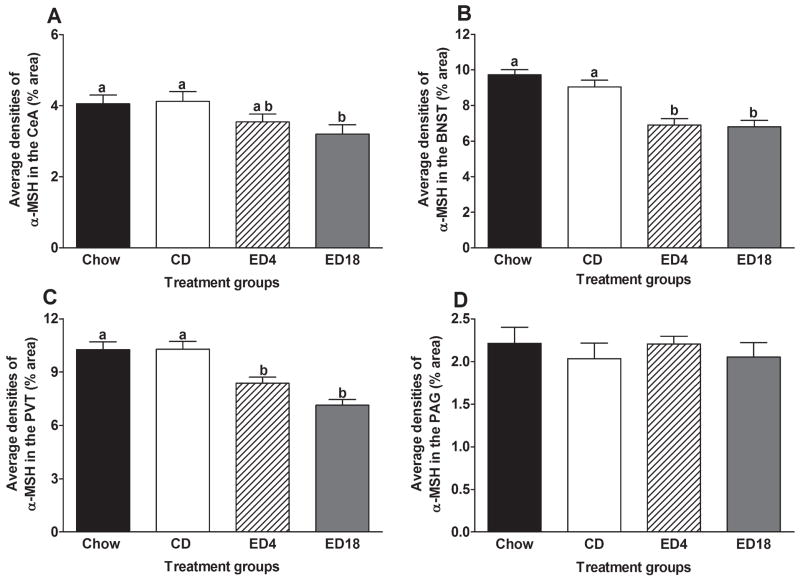
Immunoreactivity of α-MSH in the Extended Amygdala, Thalamus, and Periaqueductal Gray After Ethanol Exposure
Central Nucleus of the Amygdala (CeA) and Bed Nucleus of the Stria Terminalis (BNST)
Data representing the average immunoreactivity of α-MSH in the extended amygdala regions CeA and BNST are presented in Fig. 4A and B, respectively, and representative photomicrographs from groups CD and ED18 in these regions are presented in Fig. 5. A one-way ANOVA performed on the average densities of α-MSH immunoreactivity in the CeA was significant (F3, 36 = 3.05; p < 0.05). Although Tukey’s HSD tests did not reveal significant group differences, planned t-test comparisons indicated that while group ED18 showed significantly lower levels of α-MSH immunoreactivity relative to the control groups (Chow and CD), group ED4 did not differ significantly from the control groups. A one-way ANOVA performed on average densities of α-MSH immunoreactivity in the BNST was significant (F3, 36 = 17.96; p < 0.001), and Tukey’s HSD tests revealed that both of the ethanol diet groups (ED4 and ED18) showed significantly lower levels of α-MSH immunoreactivity relative to the control groups (Chow and CD).
Fig. 4.
Quantification of α-melanocyte-stimulating hormone immunoreactivity (% area) in the central nucleus of the amygdala (CeA; A), the bed nucleus of the stria terminalis (BNST; B), the paraventricular nucleus of the thalamus (PVT; C), and the periaqueductal gray (PAG; D). Groups were given 18 days of access to normal rodent chow (Chow) or an ethanol-free control diet (CD), or an ethanol diet for 4 (ED4) or 18 (ED18) days. Values are represented as mean ± SEM. There are statistical differences between groups that do not share overlapping lettering (a or b; p < 0.05).
Fig. 5.
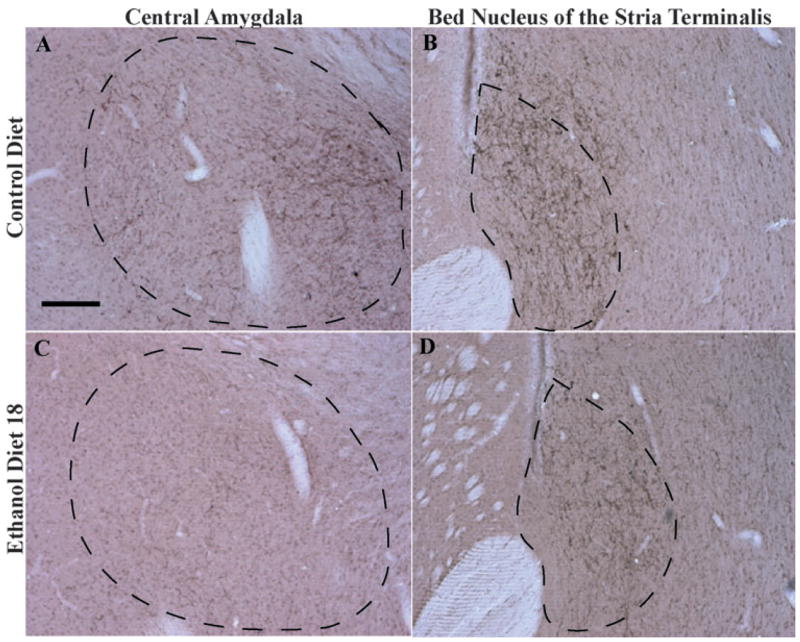
Representative photomicrographs of 40 μm coronal sections showing α-melanocyte-stimulating hormone (MSH) immunoreactivity through the central nucleus of the amygdala (A and C) and the bed nucleus of the stria terminalis (B and D) of rats given 18 days of exposure to the control diet or the ethanol diet (Ethanol Diet 18). Dashed line depicts the region that was selected for quantification. Images were photographed and quantified at a magnification of 10×. Scale bar = 200 μm. α-MSH immunoreactivity in these regions is located primarily in cellular processes.
Paraventricular Nucleus of the Thalamus (PVT) and Periaqueductal Gray (PAG)
Data representing the average immunoreactivity of α-MSH in the PVT and PAGare presented in Fig. 4C and 4D, respectively, and representative photomicrographs from groups CD and ED18 in the PVT are presented in Fig. 6. A one-way ANOVA performed on the average densities of α-MSH immunoreactivity in the PVT was significant (F3, 36 = 16.14; p < 0.001) and Tukey’s HSD tests showed that both groups ED4 and ED18 had significantly lower α-MSH immunoreactivity relative to the control groups (Chow and CD). A one-way ANOVA performed on the average densities of α-MSH immunoreactivity in the PAG failed to reach statistical significance (F3, 36 = 0.32; p > 0.05).
Fig. 6.
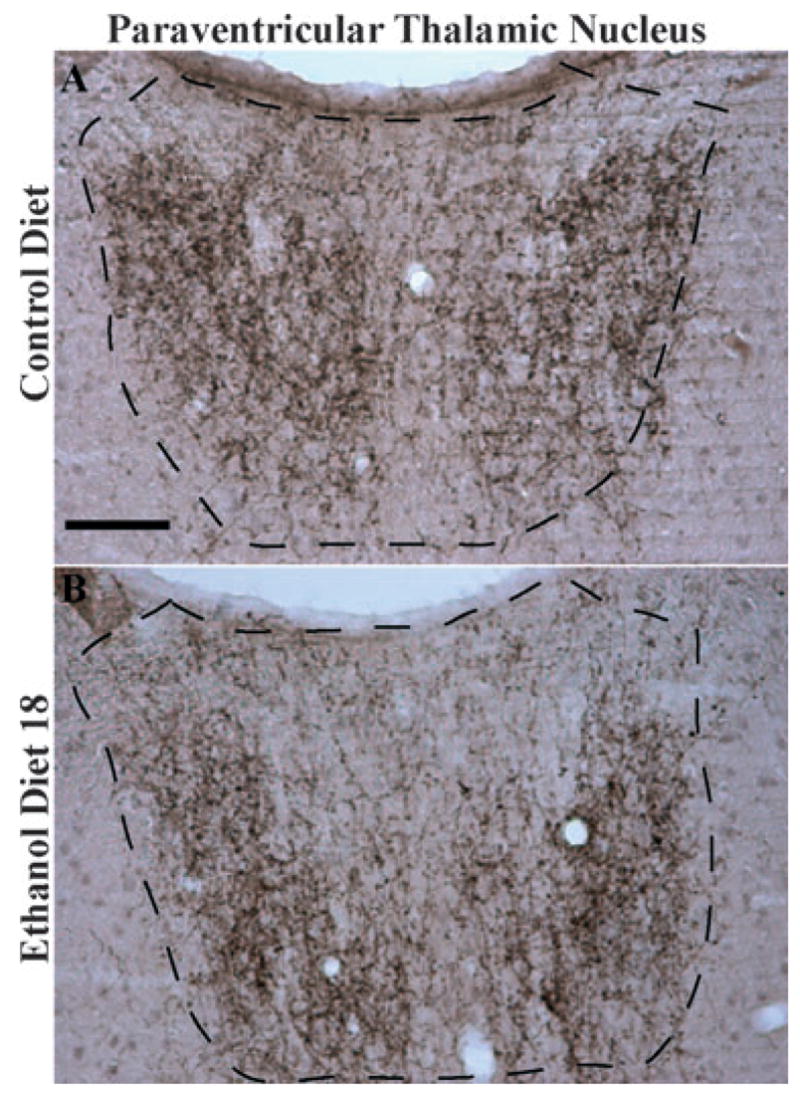
Representative photomicrographs of 40 μm coronal sections showing α-melanocyte-stimulating hormone (MSH) immunoreactivity through the paraventricular nucleus of the thalamus of rats given 18 days of exposure to the control diet (A) or the ethanol diet (B, Ethanol Diet 18). Dashed line depicts the region that was selected for quantification. Images were photographed and quantified at a magnification of 10×. Scale bar = 200 μm. α-MSH immunoreactivity in this region is located primarily in cellular processes.
Immunoreactivity of AgRP in the Arc After Ethanol Exposure
Data representing the average densities (% of area) of AgRP immunoreactivity in the Arc are presented in Fig. 7, and representative photomicrographs from groups CD and ED18 in this region are depicted in Fig. 8. A one-way ANOVA performed on these data failed to achieve statistical significance (F3, 36 = 1.20; p > 0.05).
Fig. 7.
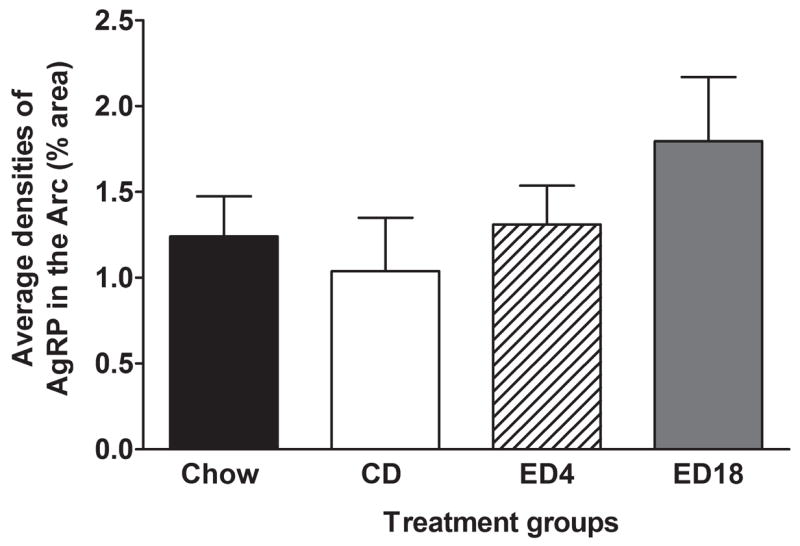
Quantification of AgRP immunoreactivity (% area) in arcuate nucleus of the hypothalamus (Arc). Groups were given 18 days of access to normal rodent chow (Chow) or an ethanol-free control diet (CD), or an ethanol diet for 4 (ED4) or 18 (ED18) days. Values are represented as mean ± SEM.
Fig. 8.
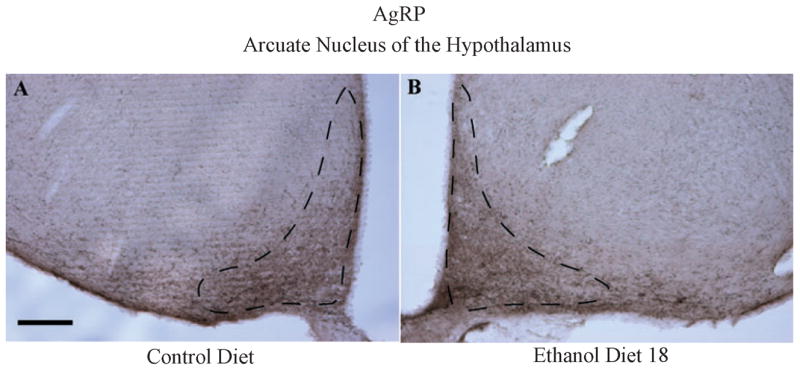
Representative photomicrographs of 40 μm coronal sections showing AgRP immunoreactivity through the arcuate nucleus of the hypothalamus of rats given 18 days of exposure ethanol diet (Ethanol Diet 18; A) or to control diet (B). Dashed line depicts the region that was selected for quantification. Images were photographed and quantified at a magnification of 10×. Scale bar = 200 μm. AgRP immunoreactivity in this region is located primarily in cellular processes.
DISCUSSION
Here we show that Sprague–Dawley rats exposed to an ethanol containing diet exhibit significant reductions of central α-MSH immunoreactivity relative to rats exposed to a control diet or normal rodent chow. Ethanol-induced reductions of α-MSH immunoreactivity were noted in regions of the hypothalamus (the Arc and LH) and extended amygdala (the CeA and BNST) as well as the paraventricular nucleus of the thalamus (PVT). Regions that did not show ethanol-induced alterations of α-MSH immunoreactivity were the DMH, PVN, and PAG. We did not find quantifiable levels of α-MSH immunoreactivity in other brain regions. The observation that ethanol exposure reduced α-MSH in some, but not all, brain regions indicates that the effects of ethanol exposure on α-MSH immunoreactivity are brain-region specific. This observation limits the likelihood that ethanol-induced reductions of α-MSH immunoreactivity were secondary to any global effects of ethanol on brain morphology or cellular toxicity. Importantly, because there were no differences at the end of the study in body weights between the group that received the CD versus the groups that received ED, and because caloric intake between diet groups were matched, reductions of α-MSH immunoreactivity in ED-treated groups are best explained by ethanol exposure, rather than group differences in caloric intake or body weight. As rats that experienced a similar protocol (15-days of access to at 7% ED) achieved blood ethanol concentrations ranging from 100 mg/dl after the first day of access to 200 mg/dl during the 15th day of access (Overstreet et al., 2002), the effects of ethanol exposure on α-MSH immunoreactivity are probably related to the central pharmacologic actions of this drug. These observations extend an earlier finding of ED-induced reduction of α-MSH immunoreactivity in the Arc and substantia nigra (Rainero et al., 1990). On the other hand, neither 4 or 18 days of exposure to ED caused significant alterations of AgRP immunoreactivity in the Arc. Given that we observed AgRP immunoreactivity in only one brain region, and in the absence of other measures (e.g., mRNA levels), it would be premature to conclude that ethanol exposure does not influence AgRP immunoreactivity.
With the exception of the LH and the CeA, which showed reductions of α-MSH immunoreactivity only after 18 days of exposure to the ED, all other regions that were affected by ED had reduced α-MSH immunoreactivity with both short-term (4 days) and long-term (18 days) exposure to ethanol. This observation raises the interesting possibility that the reduction of α-MSH immunoreactivity in the LH and CeA progresses with the development of ethanol dependence over the course of continued ethanol exposure. Consistent with this idea, Sprague–Dawley rats show increased withdrawal-induced anxiety-like behavior after 17-days of access to a 7% ED (Knapp et al., 2004), but not after 5-days of access to a 7% ED (Overstreet et al., 2005). On the other hand, reductions of α-MSH immunoreactivity in regions after only 4-days of ED access may represent neurobiologic responses to ethanol prior to the development of ethanol dependence.
With immunohistochemistry procedures, reduced α-MSH immunoreactivity in response to ethanol exposure could indicate that ethanol inhibits normal α-MSH signaling via reduced production of α-MSH and/or interference of the normal transport of α-MSH to the terminals. Alternatively, ethanol-induced reduction of α-MSH immunoreactivity may reflect an augmentation of α-MSH signaling via potentiated release and/or the inhibition of α-MSH re-uptake into presynaptic terminals. While either option is possible, the observation that exposure to ethanol inhibits POMC mRNA (Rasmussen et al., 2002; Scanlon et al., 1992b; Zhou et al., 2000) leads us to speculate that chronic exposure to ethanol disrupts normal α-MSH synthesis which ultimately depletes α-MSH immunoreactivity in the terminals of brain regions involved with neurobiologic responses to ethanol. A mechanism for the effects of ethanol on POMC mRNA may involve GABA signaling. Ethanol is a sedative drug that enhances GABAergic transmission (Criswell and Breese, 2005; Weiner and Valenzuela, 2006). Because peripheral administration of GABA agonists reduced POMC mRNA in the arcuate nucleus of Sprague–Dawley rats (Garcia de Yebenes and Pelletier, 1994), it is likely that reduction of POMC mRNA (and thus reduced α-MSH immunoreactivity) results from increased GABAergic transmission in the presence of ethanol.
Central MC signaling modulates food intake and body weight (Shimizu et al., 2007). Site-directed injection of a melanocortin receptor agonist into the PVN, DMH, LH, Arc, and CeA have been shown to significantly reduce food intake in rats (Giraudo et al., 1998; Kim et al., 2000), and ventricular infusion of the melanocortin receptor agonist MTII, significantly elevated c-Fos immunoreactivity in the PVN and Arc (Thiele et al., 1998b). While there is clear overlap of brain regions in which melanocortin signaling controls feeding with those regions exhibiting ethanol-induced reductions of α-MSH immunoreactivity, the PVN and DMH are critical sites in which α-MSH signaling modulates feeding, yet no effects of ethanol exposure on α-MSH immunoreactivity in the PVN or DMH were noted in the present report. This observation, and the fact that ethanol-induced reductions of α-MSH immunoreactivity were not attributable to calories or body weight change, suggests that the α-MSH pathways that modulate feeding/body weight and neurobiologic responses to ethanol are not identical.
It is of interest to consider the possible role(s) that α-MSH signaling plays in the modulation of neurobiologic responses to ethanol. One possibility is that α-MSH signaling is part of a mechanism that prevents excessive ethanol drinking. While we did not assess the effects of ED exposure on voluntary ethanol drinking in the present study, there is abundant evidence that chronic exposure to an ethanol-containing diet or ethanol vapor augments voluntary ethanol drinking during periods of withdrawal (Becker and Lopez, 2004; Finn et al., 2007; Schulteis et al., 1996; Valdez et al., 2002). Thus, down-regulation of α-MSH signaling following chronic exposure to ethanol may leave rats vulnerable to excessive ethanol intake. Consistent with this hypothesis are the observations that MCR agonists attenuate ethanol drinking in rats and mice (Navarro et al., 2003, 2005; Ploj et al., 2002), while blockade of the MCR augments ethanol drinking in mice (Navarro et al., 2005). As pretreatment with MTII blocked ethanol-induced decreases of Met-enkephalin-Arg6Phe7 (MEAP) immunoreactivity in the ventral tegmental area of rats, it is possible that MCR agonism reduces ethanol intake through actions on the opioid system (Ploj et al., 2002). More recently we found that site-directed infusion of a selective MC4R antagonist into the NAc increased ethanol drinking in Sprague–Dawley rats (Carvajal et al., 2007), a finding that suggests that endogenous α-MSH may negatively modulate the rewarding properties of ethanol via MC4R signaling in the NAc. It should be noted, however, that there is also evidence that MCR signaling enhances the rewarding properties of cocaine and amphetamine (Cabeza de Vaca et al., 2002; Hsu et al., 2005). Regardless, while the mechanisms by which MCR signaling modulates the consumption of ethanol and other drugs of abuse are not completely understood, such mechanisms may involve an interaction of α-MSH with the opioid system as mentioned above (Ploj et al., 2002), and/or by α-MSH actions within the mesolimbic dopaminergic pathway (Lindblom et al., 2001, 2002a).
Another possibility is the reduced α-MSH immunoreactivity following ethanol exposure contributes to the anxiolytic effects of ethanol. In fact, there is a growing body of literature showing that MCR agonists induce, while MCR antagonists inhibit, anxiety-like behaviors in rodents (Chaki and Okuyama, 2005; Chaki et al., 2003, 2005; Kokare et al., 2005; Nozawa et al., 2007; Shimazaki and Chaki, 2005). Consistent with this idea is a recent report showing that the anxiolytic effect of an intraperitoneal ethanol injection was suppressed by central infusion of α-MSH but enhanced by central infusion of a MC4R antagonist or antiserum against α-MSH (Kokare et al., 2006). Thus, ethanol- induced reduction of α-MSH immunoreactivity may be part of the mechanism by which ethanol induces anxiolytic effects. If in fact ethanol-induced reduction of α-MSH immunoreactivity contributes to the anxiolytic effects of ethanol, one would predict that acute ethanol exposure would induce rapid reductions of α-MSH immunoreactivity in critical brain regions as the anxiolytic effects of ethanol are immediate. Furthermore, α-MSH immunoreactivity would be expected to return to normal levels soon after ethanol is eliminated from the blood. These are important questions that will be the focus of future research. Taken together, MCR signaling may modulate any number of neurobiologic responses to ethanol, including ethanol ingestion, the rewarding properties of ethanol, and/or ethanol’s anxiolytic properties. It will be important to determine the specific brain regions in which α-MSH modulates these different neurobiologic responses.
In conclusion, here we show that chronic exposure to an ethanol-containing diet leads to significant reductions of α-MSH within specific brain regions. Reductions of α-MSH are not related to group differences in body weight or caloric intake. Future research is needed to determine the precise mechanism by which ethanol modulates central α-MSH immunoreactivity. The present observations, in tandem with recent genetic and pharmacologic studies, strongly suggest that the endogenous MC system modulates neurobiologic responses to ethanol. Thus, compounds which target MCRs may prove to have therapeutic value in the treatment of excessive ethanol consumption and/or the symptoms associated with ethanol withdrawal.
Acknowledgments
This work was supported by NIH grants AA013573, AA015148, AA011605, AA14949, the Department of Defense grant W81XWH-06-1-0158, and grant FPD/I from the Spanish Government.
References
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bloch B, Bugnon C, Fellmann D, Lenys D, Gouget A. Neurons of the rat hypothalamus reactive with antisera against endorphins, ACTH, MSH and beta-LPH. Cell Tissue Res. 1979;204:1–15. doi: 10.1007/BF00235160. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kim GY, Carr KD. The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine in ad-libitum-fed and food-restricted rats. Psychopharmacology. 2002;161:77–85. doi: 10.1007/s00213-002-0998-1. [DOI] [PubMed] [Google Scholar]
- Carvajal F, Cubero I, Navarro M, Sanchez-Amate MC, Thiele TE. A melanocortin (MC)-4 receptor antagonist increases ethanol, but not food, intake when infused into the nucleus accumbens and hypothalamus. Alcohol Clin Exp Res. 2007;31:119A. [Google Scholar]
- Chaki S, Hirota S, Funakoshi T, Suzuki Y, Suetake S, Okubo T, Ishii T, Nakazato A, Okuyama S. Anxiolytic-like and antidepressant-like activities of MCL0129 (1-[(S)-2-(4-fluorophenyl)-2-(4-isopropylpiperadin-1-yl)ethyl]-4-[4-(2-met hoxynaphthalen-1-yl)butyl]piperazine), a novel and potent nonpeptide antagonist of the melanocortin-4 receptor. J Pharmacol Exp Ther. 2003;304:818–826. doi: 10.1124/jpet.102.044826. [DOI] [PubMed] [Google Scholar]
- Chaki S, Okuyama S. Involvement of melanocortin-4 receptor in anxiety and depression. Peptides. 2005;26:1952–1964. doi: 10.1016/j.peptides.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Chaki S, Oshida Y, Ogawa S, Funakoshi T, Shimazaki T, Okubo T, Nakazato A, Okuyama S. MCL0042: a nonpeptidic MC4 receptor antagonist and serotonin reuptake inhibitor with anxiolytic- and antidepressant-like activity. Pharmacol Biochem Behav. 2005;82:621–626. doi: 10.1016/j.pbb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Dores RM, Jain M, Akil H. Characterization of the forms of beta-endorphin and alpha-MSH in the caudal medulla of the rat and guinea pig. Brain Res. 1986;377:251–260. doi: 10.1016/0006-8993(86)90866-8. [DOI] [PubMed] [Google Scholar]
- Dube D, Lissitzky JC, Leclerc R, Pelletier G. Localization of alpha-melanocyte-stimulating hormone in rat brain and pituitary. Endocrinology. 1978;102:1283–1291. doi: 10.1210/endo-102-4-1283. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist d-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Garcia de Yebenes E, Pelletier G. Negative regulation of proopiomelanocortin gene expression by GABAA receptor activation in the rat arcuate nucleus. Peptides. 1994;15:615–618. doi: 10.1016/0196-9781(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann NY Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- Hayes DM, Knapp DJ, Breese GR, Thiele TE. Comparison of basal NPY and CRF levels between the high ethanol drinking C57BL/6J and low ethanol drinking DBA/2J inbred mouse strains. Alcohol Clin Exp Res. 2005;29:721–729. doi: 10.1097/01.ALC.0000164375.16838.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz DM, O’Donohue TL. Alpha-melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. PNAS. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR, Smith DM, Ghatei MA, Bloom SR. Hypothalamic localization of the feeding effect of agoutirelated peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49:177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Foslike proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokare DM, Chopde CT, Subhedar NK. Participation of alpha-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology. 2006;51:536–545. doi: 10.1016/j.neuropharm.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Chopde CT, Subhedar N. Interaction between neuropeptide Y and alpha-melanocyte stimulating hormone in amygdala regulates anxiety in rats. Brain Res. 2005;1043:107–114. doi: 10.1016/j.brainres.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Kask A, Hagg E, Harmark L, Bergstrom L, Wikberg J. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacol Res. 2002a;45:119–124. doi: 10.1006/phrs.2001.0913. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergstrom L, Wikberg JE. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. NeuroReport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Wikberg JES, Bergstrom L. Alcohol-preferring AA rats show a derangement in their central melanocortin signalling system. Pharm Biochem Behav. 2002b;72:491–496. doi: 10.1016/s0091-3057(02)00719-0. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE. Effects of melanocortin receptor activation and blockade on ethanol intake: a possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res. 2005;29:949–957. doi: 10.1097/01.ALC.0000167740.19702.8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83-132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Nozawa D, Okubo T, Ishii T, Takamori K, Chaki S, Okuyama S, Nakazato A. Novel piperazines: potent melanocortin-4 receptor antagonists with anxiolytic-like activity. BioorgMed Chem. 2007;15:2375–2385. doi: 10.1016/j.bmc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- O’Donohue TL, Dorsa DM. The opiomelanotropinergic neuronal and endocrine systems. Peptides. 1982;3:353–395. doi: 10.1016/0196-9781(82)90098-5. [DOI] [PubMed] [Google Scholar]
- O’Donohue TL, Jacobowitz DM. Studies of alpha-MSH-containing nerves in the brain. Prog Biochem Pharmacol. 1980;16:69–83. [PubMed] [Google Scholar]
- O’Donohue TL, Miller RL, Jacobowitz DM. Identification, characterization and stereotaxic mapping of intraneuronal alpha-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Res. 1979;176:101–123. doi: 10.1016/0006-8993(79)90873-4. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Pharmacological modulation of repeated ethanol withdrawal-induced anxiety-like behavior differs in alcohol- preferring P and Sprague–Dawley rats. Pharmacol Biochem Behav. 2005;81:122–130. doi: 10.1016/j.pbb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Ploj K, Roman E, Kask A, Hyytia P, Schioth HB, Wikberg J, Nylander I. Effects of melanocortin receptor ligands on ethanol intake and opioid levels in alcohol-preferring AA rats. Brain Res Bull. 2002;59:97–104. doi: 10.1016/s0361-9230(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- Rainero I, De Gennaro T, Visentin G, Brunetti E, Cerrato P, Torre E, Portaleone P, Pinessi L. Effects of chronic ethanol treatment on alpha-MSH concentrations in rat brain and pituitary. Neuropeptides. 1990;15:139–141. doi: 10.1016/0143-4179(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR. Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol Clin Exp Res. 2002;26:535–546. [PubMed] [Google Scholar]
- Rasmussen DD, Bryant CA, Boldt BM, Colasurdo EA, Levin N, Wilkinson CW. Acute alcohol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res. 1998;22:789–801. [PubMed] [Google Scholar]
- van Ree JM, Bohus B, Csontos KM, Gispen WH, Greven HM, Nijkamp FP, Opmeer FA, de Rotte GA, van Wimersma Greidanus TB, Witter A, de Wied D. Behavioral profile of gamma-MSH: relationship with ACTH and beta-endorphin action. Life Sci. 1981;28:2875–2878. doi: 10.1016/0024-3205(81)90104-1. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Cooney GJ, Herzog H. Hypothalamic regulation of energy homeostasis. Best Pract Res Clin EndocrinolMetab. 2002;16:623–637. doi: 10.1053/beem.2002.0230. [DOI] [PubMed] [Google Scholar]
- Scanlon MN, Lazar-Wesley E, Csikos T, Kunos G. Rat hypothalamic proopiomelanocortin messenger RNA is unaffected by adrenalectomy. Biochem Biophys Res Commun. 1992a;186:418–425. doi: 10.1016/s0006-291x(05)80824-1. [DOI] [PubMed] [Google Scholar]
- Scanlon MN, Lazar-Wesley E, Grant KA, Kunos G. Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcohol Clin Exp Res. 1992b;16:1147–1151. doi: 10.1111/j.1530-0277.1992.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytia P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcohol Clin Exp Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Chaki S. Anxiolytic-like effect of a selective and non-peptidergic melanocortin 4 receptor antagonist, MCL0129, in a social interaction test. Pharmacol Biochem Behav. 2005;80:395–400. doi: 10.1016/j.pbb.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Inoue K, Mori M. The leptin-dependent and -independent melanocortin signaling system: regulation of feeding and energy expenditure. J Endocrinol. 2007;193:1–9. doi: 10.1677/JOE-06-0144. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Cubero I, van Dijk G, Mediavilla C, Bernstein IL. Ethanolinduced c-Fos expression in catecholamine- and neuropeptide Y-producing neurons in rat brainstem. Alcohol Clin Exp Res. 2000;24:802–809. [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. c-Fos induction in rat brainstem in response to ethanol- and lithium chloride-induced conditioned taste aversions. Alcohol Clin Exp Res. 1996;20:1023–1028. doi: 10.1111/j.1530-0277.1996.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. Learned tolerance to ethanolinduced c-Fos expression in rats. Behav Neurosci. 1998a;112:193–198. doi: 10.1037//0735-7044.112.1.193. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Bernstein IL. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res. 1997;756:278–282. doi: 10.1016/s0006-8993(97)00228-x. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol. 1998b;274:R248– 254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yamazoe M, Shiosaka S, Yagura A, Kawai Y, Shibasaki T, Ling N, Tohyama M. The distribution of alpha-melanocyte stimulating hormone (alpha-MSH) in the central nervous system of the rat: an immunohistochemical study. II. Lower brain stem. Peptides. 1984;5:721–727. doi: 10.1016/0196-9781(84)90013-5. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily “binge” intragastric alcohol administration. Alcohol Clin Exp Res. 2000;24:1575–1582. [PubMed] [Google Scholar]