Abstract
Neuroimaging studies have identified alterations in frontostriatal circuitry in OCD. Voxel-based morphometry (VBM) allows for the assessment of differences in gray matter density across the whole brain. VBM has not previously been used to examine regional gray matter density in pediatric OCD patients and the siblings of pediatric OCD patients. Volumetric magnetic resonance imaging (MRI) studies were conducted in 10 psychotropic-naïve pediatric patients with OCD, 10 unaffected siblings of pediatric patients with OCD, and 10 healthy controls. VBM analysis was conducted using SPM2. Statistical comparisons were performed with the general linear model, implementing small volume random field corrections for a priori regions of interest (anterior cingulate cortex or ACC, striatum and thalamus). VBM analysis revealed significantly lower gray matter density in OCD patients compared to healthy in the left ACC and bilateral medial superior frontal gyrus (SFG). Furthermore, a small volume correction was used to identify a significantly greater gray matter density in the right putamen in OCD patients as compared to unaffected siblings of OCD patients. These findings in patients, siblings, and healthy controls, although preliminary, suggest the presence of gray matter structural differences between affected subjects and healthy controls as well as between affected subjects and individuals at risk for OCD.
Keywords: Obsessive-compulsive disorder (OCD), pediatric, high-risk, neuroimaging, siblings, voxel-based morphometry (VBM)
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating psychiatric disorder characterized by intrusive thoughts and/or ritualistic behaviors [1]. Genetic epidemiological studies have revealed that OCD has a significant familial aggregation [19]. The aggregate risk in first-degree relative of probands with OCD has been estimated at approximately 8–23% [17, 19, 30]. As relatives of patients are at a significantly higher risk of developing OCD symptoms than the general population, young relatives at risk represent a valuable group to examine potential neurobiological precursors of the disorder.
Structural gray matter abnormalities involving the prefrontal cortex (PFC) and basal ganglia circuits have been described in both adults and children with OCD [34, 33, 36, 41]. However, contradictory reports do exist [5]. This may, in part, be accounted for by the heterogeneity of the illness, differences in illness duration, psychoactive medication exposure and the diversity of neuroimaging methods. New structural imaging analysis methods, such as voxel-based morphometry (VBM), allow for evaluation across the whole brain. This may reduce potential confounds and challenges associated with Region of Interest (ROI) hand-tracing methods, including subjective delineation of landmarks as well as the rigorous training required for rater reliability [3, 28, 47].
Few studies have used VBM to assess whole brain regional gray matter concentrations in OCD patients [22, 31, 44, 47]. To our knowledge, there have been no VBM studies of pediatric OCD patients and no prior published neuroimaging studies in relatives of patients with OCD. We employed VBM to examine structural brain alterations in pediatric OCD patients, as well as in the high-risk siblings of pediatric OCD patients. Based upon prior investigations [34, 33, 12, 22, 41, 44], we predicted regionally specific alterations in frontostriatal-thalamic circuitry in pediatric OCD patients, specifically the anterior cingulate, striatum and thalamus. Given the direction of structural differences involving these regions in prior studies [12, 33, 34, 44], we predicted larger gray matter densities in cingulate and thalamus and smaller gray matter density in striatum in both patients and siblings as compared to healthy controls.
Methods
Subjects consisted of the following three age and sex matched groups: 1) Ten psychotropic naïve pediatric outpatients with OCD (mean ± SD age, 13.26±2.46; range: 8 to 16 years); 2) Ten unaffected siblings of pediatric OCD patients (mean ± SD age, 13.11±2.99; range: 8 to 17 years); and 3) Ten healthy comparison subjects (mean ± SD age, 12.97±2.68; range: 9 to 17 years) (Table 1). All subjects were right handed. This group of subjects did not overlap with the group of subjects used in our previous reports [34, 33, 12, 41]. All subjects were recruited through the child psychiatry outpatient clinic at Wayne State University School of Medicine in Detroit, MI. The patients’ diagnoses were made by using DSM-IV criteria [1] using the Kiddie Schedule for Affective Disorders and Schizophrenia of School – Age Children – Present and Lifetime Version (K-SADS-PL) [21]. All subjects and their parents were interviewed by a board certified child and adolescent psychiatrist (DRR). Exclusion criteria for patients and healthy comparison subjects are described in previous reports [12, 41]. Briefly, this included lifetime history of unipolar or bipolar disorder, psychosis, eating disorders, substance abuse or dependence, Sydenham’s chorea, Tourette’s disorder, other tic-related conditions, conduct disorder, significantly debilitating medical or neurological conditions, pervasive developmental disorder, mental retardation, or learning disorder. There was no history of psychiatric illness in the healthy comparison subjects or in any of their first-degree relatives. Siblings of OCD patients had no lifetime history of DSM-IV Axis diagnoses or prior exposure to psychotropic medication. Legal guardians provided written informed consent, and all subjects provided written assent prior to all studies being initiated.
Table 1.
Subject Characteristics
| Clinical and Demographic characteristics | OCD Patients (N=10; 6 male, 4 female) | Siblings (N=10; 6 male, 4 female) | Healthy Comparison (N=10; 6 male, 4 female) | ANOVA | |
|---|---|---|---|---|---|
| Mean/SD | Mean/SD | Mean/SD | F | p | |
| Age (years) | 12.9/2.7 | 13.1/3.0 | 13.4/2.6 | 0.084 | ns |
| Children’s Yale-Brown Obsessive-Compulsive Scale Score | 26.5/5.4 | 1.9/3.1 | 2.1/2.6 | 123.26 | <0.001a,b |
| Obsessive Subscale Score | 13.7/2.9 | 0.3/0.7 | 1.3/2.2 | 123.01 | <0.001a,b |
| Compulsive Subscale Score | 12.7/3.1 | 0.7/0.9 | 0.8/1.3 | 122.17 | <0.001a,b |
| Hamilton Anxiety Rating Scale Score | 10.1/7.3 | 3.2/3.8 | 3.3/3.1 | 7.73 | 0.007 a, 0.006b |
| Hamilton Depression Rating Scale Score | 10.0/7.3 | 1.0/1.6 | 2.3/2.6 | 11.41 | 0.002 a, >0.001 b |
Patients vs. Controls and
Patients vs. Siblings
OCD symptom severity was assessed with the Children’s Yale Brown Obsessive Compulsive Scale [37]. The 17-item Hamilton Depression Rating Scale [16] was used to measure severity of depression, and the Hamilton Anxiety Rating Scale [15] was used to measure severity of anxiety.
MRI examinations were conducted at the Children’s Hospital of Michigan Imaging Center by using image acquisition methods described previously [19]. Images were acquired in the coronal plane using a three-dimensional spoiled gradient echo pulse sequence with a 40 degree flip angle, 25-msec repetition time, and 5-msec echo time on a 1.5-T whole body superconducting imaging system (Horizon 5.7, General Electric, Milwaukee). This produced 124 contiguous coronal slices (thickness=1.5mm) through the whole head with nominal in-plane resolution of 0.94x0.94 mm in a 256x256 matrix. (TR = 25 ms, TE = 5 ms, rotation angle = 40, FOV = 180 mm in the phase encoding direction and 240 mm in the read direction, slice thickness = 1.5 mm, NEX = 1, matrix = 256x192, and scan time = 7 minutes and 44 seconds). Axial proton density and T2-weighted images were obtained to exclude visually detectable abnormalities on MRI scans.
Images were preprocessed as described in Wilke et al [45, 46]. After an initial step wherein images were examined for artifacts and moderate to severe head movement and low quality scans were eliminated, the remaining high quality images were resliced using MEDx imaging software [27] to produce isotropic 1.0x1.0x1.0mm voxels with an axial image orientation. Thereafter, image signal intensity inhomogeneities were corrected using a script in the Freesurfer software package in order to establish standardized signal intensity baselines for all images [38]. Finally, using SPM2 software (http://www.fil.ion.ucl.ac.uk/spm) running in Matlab 7 (MathWorks, Natick, MA), a trained evaluator (PCE), blinded to subject diagnosis, realigned the scan origin along the anterior/posterior commissure line to correct for variations in head positioning during the scanning procedure in order to attain the best alignment with our template.
Image analysis and processing were performed following the optimized voxel based morphometry procedure [14]. T1 -weighted images from subjects were prepared for VBM analyses using a fully automated algorithm script in Matlab. Raw images from scans of all subjects were normalized to the standard SPM MNI T1 template using an affine-only procedure and then averaged to create a study specific whole brain template. Normalized images were then segmented into their gray matter, white matter, and cerebrospinal fluid components with an automated algorithm [3]. Finally, the whole brain template and all segmented tissue templates were smoothed with an 8-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel.
Between-group statistical comparisons of mean gray matter density were performed with the general linear model, based upon Gaussian field theory. Unmodulated gray matter images were analyzed, global brain density measurements of all subjects were generated and compared using independent samples t-tests. Each group comparison generated 2 t-statistic maps (SPM {t}) corresponding to 2 opposite contrasts: density decrease and increase, displayed at a threshold of p<0.001, uncorrected. Four small volume corrections were applied: (1) prefrontal (x = 0, y = 52, z = 10, 30 x 50 x 30 mm), (2) thalamus (x = 0, y = −11, z = 6, 30 x 30 x 30 mm), (3) right striatum (x = 17, y = 10, z = 5, 30x50x30 mm) and left striatum (x = −17, y = 10, z = 5, 30 x 50 x 30 mm). Within the small volume correction voxels, we set significance at p (FDR-corrected) < 0.05 and z –score > 3.50. The observed Montreal Neurological Institute (MNI) coordinates were converted to Talairach coordinates to classify the locations of significance [40]. For additional gray matter localization, coordinates were then entered into the Talairach Daemon [24].
All clinical and demographic data were uploaded to SPSS for Windows 11.0 (Chicago, SPSS, 2001) computer statistic package software. For comparison of groups, the One-Way Analysis of Variance (ANOVA) procedure was used. For significant differences between means, Bonferroni pairwise multiple comparisons were carried out at an alpha level of 0.05.
Results
The patients, siblings, and comparison subjects did not differ significantly in distributions of age, or sex (see table 1). All patients had significant symptom severity as measured by their Children’s Yale Brown Obsessive Compulsive Scale (CYBOCS) (total 26.5), Hamilton Anxiety Rating Scale (10.1), and Hamilton Depression Rating Scale (10.0) scores. Using the same instruments, siblings and controls did not have significant symptom severity. There were significant differences in all clinical assessment scores between patients and both siblings and healthy controls.
The significant differences and trends towards significant differences in regional gray matter density between subjects are summarized in Table 2. VBM analysis revealed significantly lower gray matter density in the left ACC and bilateral medial SFG in OCD patients as compared to healthy controls. Furthermore, a small volume correction identified significantly greater gray matter density in the right putamen in OCD patients as compared to unaffected siblings of OCD patients (see figure 1). No differences were noted in the thalamus or left striatum selections.
Table 2.
Regional Changes in Gray Matter Density Between OCD Patients and Healthy Control Subjects*
| Coordinates | Statistic | ||||
|---|---|---|---|---|---|
| OCD > Siblings | X | Y | Z | Z Score | p (FDR-corr)1 |
| Right Putamen | 31 | 4 | 0 | 3.99 | 0.04 |
| Right Putamen | 32 | 0 | 3 | 3.85 | 0.04 |
| OCD < Control | X | Y | Z | Z Score | p (FDR-corr)2 |
| Left Anterior Cingulate Gyrus | −8 | 46 | 3 | 4.43 | 0.01 |
| Right Medial Superior Frontal Gyrus | 12 | 50 | 15 | 3.51 | 0.01 |
| Left Medial Superior Frontal Gyrus | −4 | 63 | 12 | 3.91 | 0.01 |
OCD > Controls and OCD < Siblings did not demonstrate any significant differences.
Based on small volume correction and
main comparison.
Figure 1.
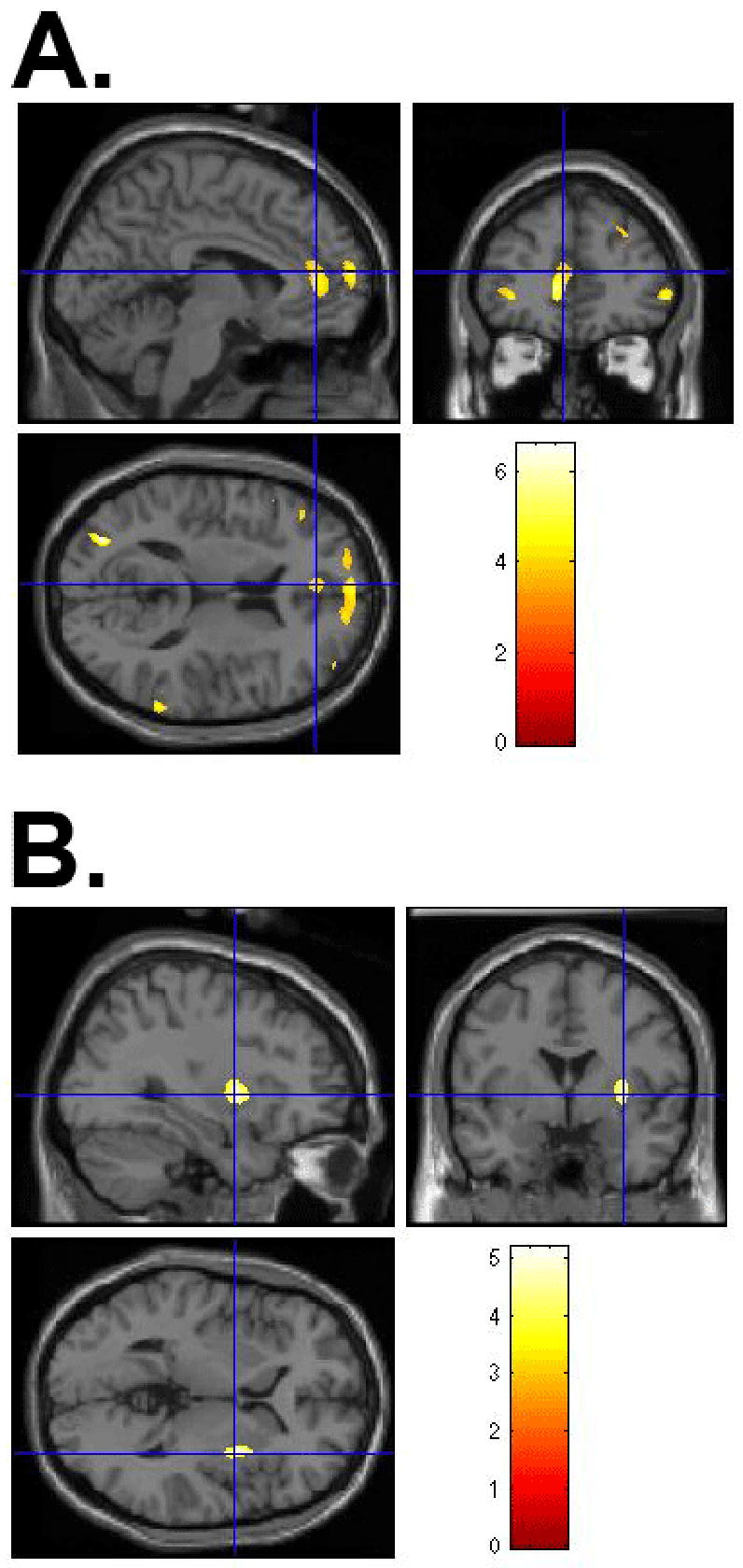
(A) VBM-measured significantly lower gray matter density in the left ACC and bilateral medial SFG in pediatric OCD patients compared to healthy controls and (B) VBM-measured significantly greater gray matter density in the right putamen in pediatric OCD patients compared to unaffected siblings. (Non-radiologic convention: Right=Right/Left=Left; color map reflects z-score)
Discussion
This is the first study, to our knowledge, using VBM to examine brain morphology in pediatric OCD patients as well as in unaffected siblings of OCD patients. Based upon prior neuroimaging studies implicating aberrant frontostriatal circuits in both adult and pediatric OCD patients [34, 33, 36; 12, 20, 41], we predicted abnormalities in the anterior cingulate, striatum and thalamus. Consistent with one of these predictions, alterations in the left ACC were observed as well as an, unexpected alteration in the bilateral SFG in OCD patients compared to healthy controls. Furthermore, we found increased gray matter density in the right putamen of OCD patients compared to their unaffected siblings.
Our finding of reduced left ACC gray matter density in OCD patients compared to healthy controls is intriguing. Alterations in the ACC may reflect previously described abnormalities of the direct-indirect basal ganglia pathway in the orbitofrontal-subcortical and limbic circuits [32, 29, 6, 36, 7]. It has been suggested that abnormal ACC activity may reflect a dysfunctional action-monitoring system in patients with OCD [43]. Developmental studies of the ACC in children have demonstrated correlations between its size and ability to regulate inhibitory processes [8]. Indeed, the presence of alterations in ACC in pediatric OCD patients may support and reflect prior findings of inhibitory control abnormalities present early in the course of illness [35]. In a previous study, we have reported larger total ACC gray matter volumes in pediatric OCD [33]. However, our current study, using VBM, is using a whole brain approach, which may only reveal partial and not total gray matter differences between subjects. Our findings here may differ from our prior study in that we are reporting gray matter density, the probability of finding gray matter in a given voxel, rather than gray matter volume. It is also possible that these conflicting results may relate to differences between subject samples between these studies as well as the heterogeneity of OCD [26]. Concurrent with our findings, a recent VBM study of adults with OCD reported reduced ACC gray matter density [44].
The SFG has also been identified as a potential area of interest in the pathophysiology of obsessive-compulsive disorder. Abnormal functionality of the SFG has been previously implicated in several studies in adults using positron emission tomography (PET) [25] and single photon emission computed tomography (SPECT) [23, 9], though the results of these studies are conflicting. Lacerda et al [23] found significant increases in regional cerebral blood flow (rCBF) in the right SFG in OCD patients versus healthy control subjects, while Lucey et al [25], found significant bilateral decreases in resting rCBF in a population of OCD patients versus healthy controls. Castillo et al [9], found no significant differences in average ratios of rCBF in a population of pediatric OCD patients before and after treatment with clomipramine. Adler et al [2] found significant increases in neural activation of several frontal cortical regions, including the SFG, during symptom provocation using functional magnetic resonance imaging (fMRI). Another study, using electroencephalography (EEG), found reduced activity in the SFG in a sample of OCD patients versus controls during the NoGo portion (i.e. inhibition response) of a Go-NoGo task [18]. Despite evidence indicating functional SFG abnormalities in OCD, there has been only one volumetric analysis of the SFG [42], which reported no significant differences in combined gray and white matter in adult OCD subjects compared to healthy controls.
Our finding of greater gray matter density in the right putamen of OCD patients as compared to their unaffected siblings is especially interesting. The putamen has been implicated in the pathophysiology of OCD; indeed, a recent VBM analysis of adult OCD patients reported increased gray matter volumes bilaterally in the ventral putamen [31]. Previously, we found smaller putamen volumes in OCD patients as compared to healthy controls [34]. In our current study, no difference was noted between siblings and healthy controls nor OCD patients and healthy controls with regard to putamen gray matter density.
These neuroimaging findings may reflect potential brain maturational deviations in both affected and unaffected populations, although due to the cross-sectional approach of these analyses, we are unable to draw any conclusions regarding age-related changes in our subjects. Prior neuroimaging studies have reported significant age-related changes in gray matter during childhood and adolescence [11, 39]. It has been postulated that smaller gray matter density may reflect increased synaptic pruning during adolescence and early adulthood [13]. Prior studies of pediatric OCD patients suggest that a developmentally mediated network dysplasia, secondary to abnormal peri-adolescent pruning mechanisms may contribute to the development of OCD in childhood [33]. Different gray matter patterns in affected patients and unaffected at-risk subjects may reflect a failure of normal pruning or defective neural proliferation. Finally, low GM density in subjects with OCD may reflect white matter hypertrophy expanding into the cortex and lowering the probability of finding GM in a particular cortical or nuclear voxel.
The absence of predicted alterations [12] in the thalamus may be a result of the small sample size. Furthermore, as OCD is a clinically heterogenous disorder, and studies have reported specific neural correlates of OCD symptom dimensions, structural and functional abnormalities may be missed in studies that do not examine groups of patients differentiated by specific symptom dimensions [26].
There are several limitations to the preliminary findings, especially regarding the exploratory analysis of this novel high-risk sibling population. The sample size is small and, despite small volume corrections for multiple comparisons, future assessment of a larger cohort of patients and siblings will be important to reduce the potential for both Type I and Type II errors. Comorbid disorders in OCD patients are another limitation. Furthermore, although the sibling subjects are at greater risk for the development of OCD than healthy controls, we are unable to verify that they will indeed develop the disorder. Therefore, it is premature to conclude that differences between unaffected high-risk subjects and affected siblings represent markers for risk of developing OCD. Nonetheless, we believe that the uniqueness of our sample, e.g., psychotropic naïve pediatric OCD patients and unaffected siblings, may contribute to a broader understanding of neurodevelopmental factors involved in the etiopathogenesis of OCD. Clearly, future reports of longitudinal analyses of these populations will be informative.
Acknowledgments
This work was supported in part by the State of Michigan Joe F. Young Sr Psychiatric Research and Training Program, the Miriam L. Hamburger Endowed Chair of Child Psychiatry at Children’s Hospital of Michigan and Wayne State University, Detroit, MI, and grants from the National Institute of Mental Health (R01MH59299, R01MH65122, K24MH02037) and the Mental Illness Research Association (MIRA). The research was also supported by Grant Number 1 KL2 RR024154-01 from the National Center for Research Resources (NCRR) to Dr. Gilbert.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2004. [Google Scholar]
- 2.Adler CM, McDonough RP, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatry Res. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Map. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 5.Aylward EH, Harris GJ, Hoeghn-Saric R, Barta PE, Machlin SR, Pearlson GD. Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Arch Gen Psychiatry. 1996;53:577–584. doi: 10.1001/archpsyc.1996.01830070021006. [DOI] [PubMed] [Google Scholar]
- 6.Baxter LR, Schwartz JM, Guze BH, Bergman K, Szuba MP. PET imaging in obsessive compulsive disorder with and without depression. J Clin Psychiatry. 1990;51:61–69. [PubMed] [Google Scholar]
- 7.Bosatto GF, Zamignani DR, Buchpiquel CA. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computer tomography (SPECT) Psychiatry Res: Neuorimagin. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 8.Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- 9.Castillo AR, Buchpiguel CA, de Araujo LA, Castillo JC, Asbahr FR, Maia AK, de Oliveira Latorre MR. Brain SPECT imaging in children and adolescents with obsessive-compulsive disorder. J Neural Transm. 2005;112:1115–1129. doi: 10.1007/s00702-004-0240-x. [DOI] [PubMed] [Google Scholar]
- 10.Flament MF, Whitaker A, Rapoport JL. Obsessive compulsive disorder in adolescence: An epidemiological study. J Am Acad Child Adolesc Psychiatry. 1998;27:764–771. doi: 10.1097/00004583-198811000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Giedd JN. Brain developmental during childhood and adolescence: a longitudinal MRI study. Nat Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, MacMaster FP, Stewart CM, Rosenberg DR. Decrease in Thalamic Volumes of Pediatric Patients With Obsessive-Compulsive Disorder Who Are Taking Paroxetine. Arch Gen Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- 13.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;21:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuorimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanna GL, Himle JA, Curtis GC, Gillespie BW. A Family Study of Obsessive-Compulsive Disorder With Pediatric Probands. Am J Med Gen. 2005;134B:13–19. doi: 10.1002/ajmg.b.30138. [DOI] [PubMed] [Google Scholar]
- 18.Herrman MJ, Jacob C, Unterecker S, Fallgatter AJ. Reduced response-inhibition in obsessive-compulsive disorder measured with topographic evoked potential mapping. Psychiatry Res. 2003;120:265–271. doi: 10.1016/s0165-1781(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 19.Hettema JM, Neale MC, Kendler KS. A Review and Meta-Analysis of the Genetic Epidemiology of Anxiety Disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 20.Kang DH, Kim JJ, Choi JS, Kim YI, Kim CW, Youn T, Han MH, Chang KH, Kwon JS. Volumetric Investigation of the Frontal-Subcortical Circuitry in Patients With Obsessive-Compulsive Disorder. J Neuropsychiatry Clin Neurosci. 2004;16:342–349. doi: 10.1176/jnp.16.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Life-Time Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 23.Lacerda AL, Dalgalarrando P, Caetano D, Camargo EE, Etchebehere EC, Soares JC. Elevated thalamic and prefrontal regional cerebral blood flow in obsessive-compulsive disorder: A SPECT study. Psychiatry Res. 2003;123:125–134. doi: 10.1016/s0925-4927(03)00061-1. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucey JV, Costa DC, Blanes T, Busatto GF, Pilowsky LS, Takei N, Marks IM, Ell PJ, Kerwin RW. Regional cerebral blood flow in obsessive-compulsive disordered patients at rest. Differential correlates with obsessive-compulsive and anxious-avoidant dimensions. Br J Psychiatry. 1995;167:629–634. doi: 10.1192/bjp.167.5.629. [DOI] [PubMed] [Google Scholar]
- 26.Mataix-Cols D, Wooderson S, Lawrenece N, Brammer MJ, Speckens A, Phillips ML. Distinct Neural Correlates of Washing, Checking, and Hoarding Symptom Dimensions in Obsessive-Compulsive Disorder. Arch Gen Psychiatry. 2004;61:546–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 27.MEDx. Sterling, Va: Sensor Systems; 1998. [Google Scholar]
- 28.Milham MP, Nugent AC, Drevets WC, Dickstein DP, Leibenluft E, Ernst M, Charney D, Pine DS. Selective Reduction in Amygdala Volume in Pediatric Anxiety Disorders: A Voxel-Based Morphometry Investigation. Biol Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Modell JG, Mountz JM, Curtis GC. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1989;1:27–36. doi: 10.1176/jnp.1.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- 31.Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J. Mapping Structural Brain Alterations in Obsessive-Compulsive Disorder. Arch Gen Psych. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 32.Rapoport JL, Wise SP. Obsessive-compulsive disorder: evidence for basal ganglia dysfunction. Psychopharmacol Bull. 1998;24:380–384. [PubMed] [Google Scholar]
- 33.Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of obsessive compulsive disorder. Biol Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg DR, Keshavan MS, O’Hearn KM, Dick EL, Bagwell WW, Seymour AB, Montrose DM, Pierri JN, Birmaher B. Frontostriatal measurement in treatment-naïve children with obsessive-compulsive disorder. Arch Gen Psychiatry. 1997a;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg DR, Dick EL, O’Hearn KM, Sweeny JM. Response inhibition deficits in obsessive-compulsive disorder: an indicator of dysfunction in frontostriatal circuits. J Psychiatry Neurosci. 1997b;22:29–38. [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry. 1998;35:26–37. [PubMed] [Google Scholar]
- 37.Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and Validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 39.Sowell ER. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationship during post-adolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinstrater O. MunsterT2T-Converter (3D Version) [online] 2003 http://neurologie.uni-muenster.de/T2T/t2tconv/conv3d.html.
- 41.Szeszko PR, MacMillian S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, Moore GJ, Rosenberg DR. Brain Structural Abnormalities in Psychotropic Drug-Naïve Pediatric Patients With Obsessive-Compulsive Disorder. Am J Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- 42.Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- 43.Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2002;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- 44.Valente AA, Miguel EC, Castro CC, Amaro E, Jr, Duran FL, Buchpiguel CA, Chitnis X, McGuire PK, Busatto GF. Regional Gray Matter Abnormalities in Obsessive-Compulsive Disorder: A Voxel-Based Morphometry Study. Biol Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain MR-images in children. Hum Brain Map. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilke M, Schmithorst VJ, Holland SK. Normative pediatric brain data for spatial normalization and segmentation differs from standard adult data. Magn Reson Med. 2003;50:749–757. doi: 10.1002/mrm.10606. [DOI] [PubMed] [Google Scholar]
- 47.Menzies L, Chamberain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofrontao-striatal model revisited. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neurbiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


