Abstract
We quantified the effects of deep brain stimulation (DBS) of the subthalamic nucleus (STN) and medication on Parkinsonian rigidity using an objective measure of work about the elbow joint during a complete cycle of imposed 1-Hz sinusoidal oscillations. Resting and activated rigidity were analyzed in four experimental conditions: 1) off treatment; 2) on DBS; 3) on medication; and 4) on DBS plus medication. Rigidity at the elbow joint was also assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS). We tested ten patients who received STN DBS and ten age-matched neurologically healthy control subjects. The activated rigidity condition increased work in both Parkinson’s disease (PD) patients and control subjects. In PD patients, STN DBS reduced both resting and activated rigidity as indicated by work and the UPDRS rigidity score. This is the first demonstration that STN stimulation reduces rigidity using an objective measure such as work. In contrast, the presurgery dose of antiparkinsonian medication did not significantly improve the UPDRS rigidity score and reduced work only in the activated rigidity condition. Our results suggest that STN DBS may be more effective in alleviating rigidity in the upper limb of PD patients than medications administered at presurgery dosage level.
Index Terms: Arm, human, movement disorders, rigidity
I. Introduction
Rigidity is one of the three cardinal signs of Parkinson’s disease (PD), along with tremor and bradykinesia. At present, there is no cure for Parkinson’s disease although L-dopa alleviates Parkinsonian symptoms [1]. As the disease progresses, the efficacy of L-dopa decreases and the incidence and severity of the side effects increase [2]. Deep brain stimulation (DBS) of the subthalamic nucleus (STN) has been shown to modify several of the neurophysiological parameters associated with tremor and bradykinesia better than medication alone [3], [4]. STN DBS has also been shown to improve clinical measures of rigidity [2], [5]–[8].
In the clinic, rigidity is assessed by a neurologist who moves the subject’s limb. The level of involuntary resistance to the imposed movement is scored using a clinical rating scale, such as the Unified Parkinson’s Disease Rating Scale (UPDRS). The UPDRS is a subjective scale and the rigidity scores for the same patient may differ widely depending on the examiner [9]. As such, considerable effort has been extended over the years to objectively quantify Parkinsonian rigidity in a laboratory setting [10]–[17] or by using portable devices [9], [18]–[20]. In a seminal study, Webster [21] suggested that involuntary resistance to imposed movement can be quantified by measuring a resistive torque generated by the subject when the limb is passively moved by a computer controlled torque motor. Webster [22] also introduced the terms “resting rigidity” and “activated rigidity.” In the resting rigidity condition, the patient is asked to completely relax while the examiner passively moves the limb. Activated rigidity refers to a well-known clinical observation that resistance of the passively moved limb increases when the patient is asked to perform a concurrent active movement task with the other limb. In a number of previous studies, calculated mechanical work done by the rigid limb has been used as a quantitative measure of both resting and activated rigidity [11], [13]–[15], [23]–[25].
The purpose of the present study was to examine the effects of presurgery therapeutic doses of medication and STN DBS on resting and activated rigidity in patients with PD. We used both a subjective rating scale such as the UPDRS and a quantitative measure of mechanical work done by the passively moved limb. Patients were tested in four treatment conditions: 1) off treatment; 2) on DBS; 3) on medications (Meds); and 4) on DBS plus Meds.
II. Methods
A. Subjects
Ten subjects with sporadic PD whose ages ranged from 50 to 72 years old (mean age 64 years, sd = 6.34 years) and ten age and gender matched control subjects from 51 to 72 years old (mean age 64 years, sd = 6.67 years) participated in the rigidity experiments. A priori inclusion criteria were established for the study, and the first ten PD patients who received STN DBS and met the criteria were chosen for the study. Patients were examined by a neurologist specializing in movement disorders and included in the study if they: 1) had sporadic PD as outlined by the Parkinson’s Disease Society Brain Bank diagnostic criteria [26], [27] and 2) were deemed to have upper limb rigidity. The experiment was performed 3 to 38 months (average 18 months) after surgery. All subjects gave written informed consent to all experimental procedures, which were approved by the local Institutional Review Board (University of Illinois, Chicago) and Rush University Medical Center.
In nine cases, quadripolar DBS electrodes (model 3389, Medtronic Inc, Minnesota, USA) were implanted in the STN opposite to the most severely affected body side, and in one case electrodes were implanted bilaterally. For the patient with bilaterally implanted electrodes, the most affected limb was studied (right limb), and the right deep brain stimulator was deactivated 16 h prior to the inception of the testing and remained off throughout the entire four-day study [28]. Subsequently, only the left deep brain stimulator was manipulated during the four-day study. Surgery was performed as part of clinical care according to standard procedures [29], [30]. Briefly, the anatomical target was based on standard stereotactic coordinates while the patient was in the MRI scanner with a Leksell stereotactic head frame in place [31]. MRI images were subsequently reformatted and coordinates refined using a Stealth system. Intraoperatively, microelectrode penetrations were made through a burr hole to verify the neurophysiological target [29], [32]. Responses to sensory stimuli (passive movements of upper and lower limbs) were sought to identify the sensorimotor portion of the STN, where the DBS lead was subsequently placed ventral to this region. In our study, as part of the surgery protocol, we recorded the pattern of firing of the cells within STN that changed with the electrode path and estimated the length of STN from the electrode path to always be greater than 4 mm for the subjects in this study. In addition, the contacts for all but two of the patients in this study were at 0 and 1 (monopolar), and most estimates of current spread do not suggest that the thalamus could have been stimulated [33], [34]. Postoperatively, MRI demonstrated appropriate placement of the DBS lead.
Following surgery, stimulation parameters were optimized in order to reduce presurgery scores on the motor section of the UPDRS. The same neurologist rated the entire motor section of the UPDRS for each patient off treatment, on STN DBS, on medication, and on the combination of STN DBS and medication. Optimization took place during several visits over the ensuing months. At the time of the experiments, the frequency was 185 Hz for all patients, and in all but four cases the pulse width was 60 μ s. For subjects 3, 5, 6, and 9 the pulse width was 90 μ s. The stimulation intensity varied from 1.7 to 4.0 V (mean = 3.0 V, sd = 0.72). Stimulation voltage and electrode contacts for each patient are listed in Table I.
TABLE I.
Patient Characteristics
| Age (yrs) | Gender | Post-surgical UPDRS a Off Treatment | DBS Parameters | Anti-Parkinsonian Medications | |
|---|---|---|---|---|---|
| Item 22 | Stim Level (V) | Electrode Contacts | |||
| 66 | F | 3 | 2.8 | 1−C+ | Carbidopa/Levodopa CR 50/200 2 tabs
Amantadine 100mg 1 tab |
| 64 | F | 3 | 3.0 | 1−C+ | Carbidopa/Levodopa
25/250 1 tab Trihexyphenidyl 2mg 1 tab Carbidopa 25 mg 1 tab |
| 66 | F | 2 | 3.6 | 1−C+ | Carbidopa/Levodopa 25/100 1.5 tabs
Carbidopa/Levodopa CR 50/200 1 tab Amantadine 100 mg 1 tab Ropinirole 2 mg 1 tab |
| 67 | F | 1 | 2.3 | 0−C+ | Carbidopa/Levodopa 25/100 1 tab
Carbidopa/Levodopa CR 25/100 1 tab Amantadine 100mg 1 tab Pramipexole 1 mg 1 tab |
| 72 | F | 1 | 4.0 | 0−C+ | Carbidopa/Levodopa 25/100 1.5 tabs |
| 64 | F | 3 | 3.6 | 0−1−2−3+ | Carbidopa/Levodopa 25/100 1 tab
Amantadine 100 mg 1 tab Ropinirole 5 mg 1 tab |
| 50 | M | 3 | 2.5 | 1−C−+ | Carbidopa/Levodopa 25/100 1 tab
Carbidopa/Levodopa CR 50/200 1 tab Pergolide 1 mg 1 tab Amantadine 100 mg 1 tab |
| 64 | M | 1 | 1.7 | 1−C+ | Carbidopa/Levodopa 25/100 1 tab
Amantadine 100 mg 1 tab Pramipexole 0.5 mg 1 tab Entacapone 200 mg 1 tab |
| 57 | M | 2 | 3.0 | 2−C+ | Carbidopa/Levodopa 10/100 2 tabs
Entacapone 200 mg 1 tab Pramipexole 1 mg 0.5 tabs |
| 70 | M | 1 | 3.5 | 1−C+ | Carbidopa/Levodopa t 25/100 3 tabs |
UPDRS = Unified Parkinson’s Disease Rating Scale
B. Procedures
The patients were tested on four separate consecutive days in the morning under each of four treatment conditions: 1) off treatment; 2) on DBS; 3) on Meds; and 4) on DBS plus Meds. The order of testing conditions (1–4) was randomized across subjects. The control subjects were tested on one day in the morning. For the on Meds and on DBS plus Meds conditions (Table I), patients took the clinically determined preoperative medication dosage [35]. We used presurgery dosage levels instead of postsurgery dosage levels because most patients had a reduction in their medication postsurgery, and we wanted to make certain that each patient received a therapeutic benefit from their medication. We were interested in studying a therapeutic dose of levodopa rather than a suprathreshold dose since this is what patients use in daily life. The medication dose was the first dose taken prior to testing. For example, if the patient takes 2 Sinemet (Carbidopa/Levodopa) 4 times/day the amount listed in Table I would be 2 sinemet. This is the presurgery medication dose.
The stimulators were switched off and on by one of the experimenters using a Medtronic Console Programmer (Model 7432). In the off treatment condition, patients were always tested following 12 h without the specific treatment [36]. For instance, the night prior to the off treatment condition both treatments were withheld, and the night prior to the on DBS condition only medications were withheld. For the off treatment and on Meds conditions, each patient was examined using the Medtronic Console Programmer to ensure that the stimulator was off. For the on DBS, on Meds, and on DBS plus Meds conditions, the treatment(s) were active for at least 12 h prior to rigidity testing.
C. Experimental Setup
The subject was seated with the arm abducted 90°. The subject’s arm was placed in a supine position and lightly strapped to a rigid, lightweight manipulandum bar which can freely rotate in the horizontal plane, such that the axis of rotation aligns with the elbow (Fig. 1). A torque motor is mounted underneath the manipulandum such that the axes of rotation of the torque motor and manipulandum coincide. Servo control was used to have the motor move the limb along a sinusoidal trajectory. Joint angle was measured by a capacitive transducer mounted on a shaft of the torque motor. Joint torque was measured by a torque transducer (Sensotec) that is inserted between the shafts of the motor and manipulandum. Position and torque signals were low-pass filtered with an analog third order Bessel filter with a 50-Hz cutoff frequency. Muscle surface electromyograms (EMG) were recorded with Bagnoli electrodes (Delsys), taped over the bellies of the biceps brachii, brachioradialis, and triceps (lateral and long heads) muscles. The EMG signals were amplified and band-pass filtered (20–450 Hz). All signals were digitized at 1000 samples/s (National Instruments). Digitized EMG signals were full-wave rectified and then low-pass filtered with a second-order dual-pass Butterworth filter with a 50-Hz cutoff frequency. Digitized angle and torque signals were low-pass filtered with a second-order dual-pass Butterworth filter with a 4-Hz cutoff frequency.
Fig. 1.
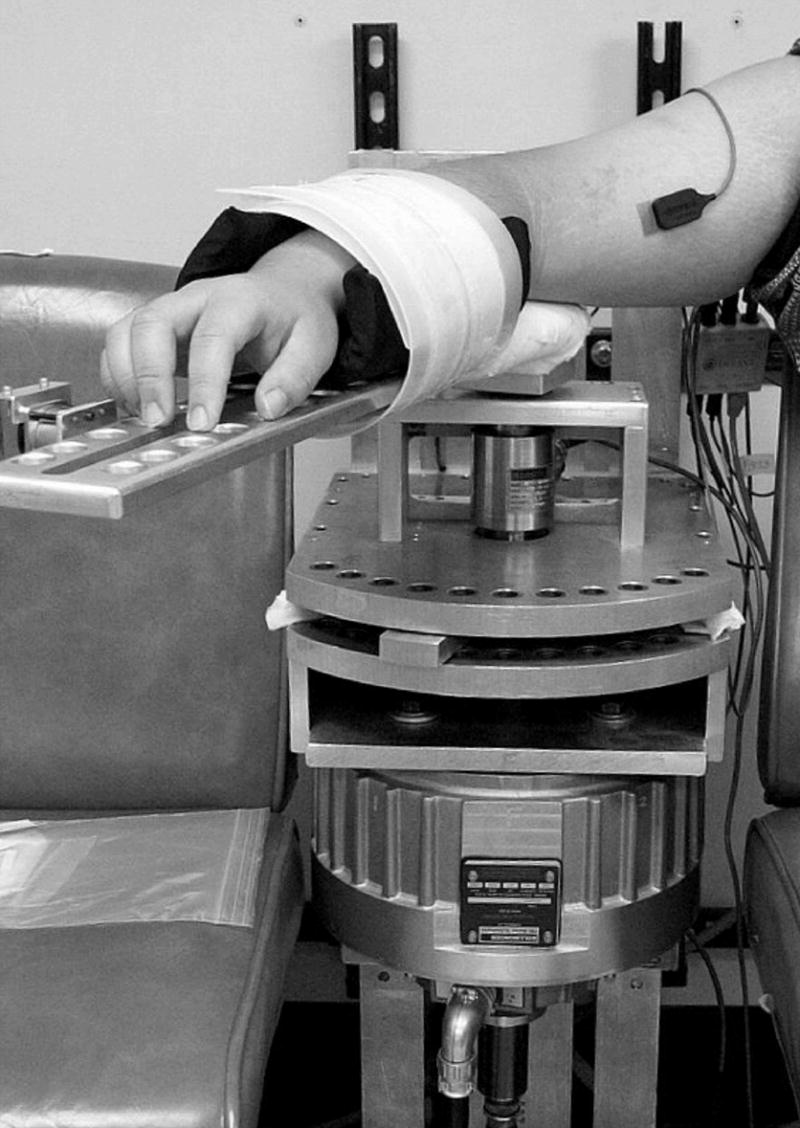
Experimental setup.
D. Experimental Protocol
The limb contralateral to the stimulator was tested. The subject was asked to relax the test limb. The motor moved the arm for 60 s along a 1-Hz sinusoidal trajectory over a range of 60° (± 30° from the middle point of the elbow flexed at 90°). The protocol included resting and activated rigidity conditions. The resting condition was conducted without mental or physical stressors in the form of secondary tasks. Additionally, during the resting condition, subjects were instructed to refrain from talking. In the activated rigidity condition, the subject was instructed to perform a concurrent secondary motor task with the hand ipslilateral to the stimulator. The motor task was a sustained isometric finger flexion. The isometric finger flexion task was chosen for two reasons. First, we wanted to examine the effect of a continuous descending motor command on rigidity in the contralateral limb. Second, we wanted to minimize any mechanical effects of limb movement on rigidity, and an isometric task which isolates the finger joint is unlikely to cause any carryover of mechanical perturbations to the contralateral limb.
The target force level for the secondary motor task was determined as follows. Subjects were initially asked to perform a maximum voluntary contraction (MVC) with their index finger in flexion at the second metacarpaphalangeal joint (MCP), and 5% of their MVC was calculated. Next, subjects performed the motor secondary task where they were required to produce and sustain a force which was equal to 5% of their MVC. An Entran calibrated load cell, with an excitation voltage of ± 10 V and a sensitivity of 190.50 mV/N, measured the amount of force exerted by the subjects during the MVC and during the secondary motor task. The load cell was affixed to the top of a stationary, rectangular, wooden box, which was positioned on top of the supportive surface for the limb ipsilateral to the stimulator. Subjects received visual feedback from a display computer monitor positioned in front of the subject. Subjects were instructed to push on the load cell to move the cursor to the target and then to maintain the cursor at the target until they heard an auditory “end” beep. A high control-to-display visual feedback gain level was used for this task, which meant that small changes in the amount of force produced caused large fluctuations in cursor movement. This visual feedback setup ensured that subjects had to attend to the display computer monitor in order to maintain the cursor at the target [37].
E. Rigidity Measures
Resistance to passive movement was quantified by calculating the average work (W) done by the motor over one cycle [21]. The work W was calculated by integrating the resistive torque T over angle q over 20 cycles of oscillations and dividing by the number of cycles. For each condition, a 20-s portion from the middle of one recorded trial was analyzed starting from t = 20 s. Work W was considered an objective measure of rigidity. Note that the torque and angle channels were low-pass filtered with a 4-Hz cutoff frequency (see Section II) so that a 4–8-Hz Parkinsonian tremor, that may be present in some of the subjects and in some of the conditions, did not affect W. In addition, rigidity in the same limb was subjectively assessed using scores on the motor section of the UPDRS: 0—absent; 1—slight or only with activation; 2—mild/moderate; 3—marked, full range of motion is achieved with difficulty; 4—severe, full range of motion is not achieved. The UPDRS testing was done for all ten subjects in the resting condition and for nine out of ten subjects in the activated rigidity condition.
F. Statistical Analysis
Analysis of variance (ANOVA) was used to analyze both UPDRS scores and work W. The effects of disease and activation condition were evaluated using a two-factor repeated measures ANOVA with one between groups factor and one within groups factor: (PD versus controls) × (resting versus activated). The effects of medication and STN DBS were evaluated using a three-factor repeated measures ANOVA: medication (off versus on) × DBS (off versus on) × condition (resting versus activated). Post hoc analysis using Tukey’s HSD test was done when a significant interaction between the main factors was found. All analyses were performed using Statistica (StatSoft).
III. Results
The muscle EMGs and torque-angle relations in the resting and activated rigidity conditions in a PD subject off treatment are illustrated in Fig. 2. In the resting condition, the imposed oscillations elicited small EMG bursts in the triceps muscle and the biceps EMG does not appear to be modulated at all (Fig. 2, column A, EMG panel). In contrast, in the activated rigidity condition, the same imposed movement elicited pronounced reciprocal EMG bursts in the biceps and triceps muscles (Fig. 2, column B, EMG panel). The area of the ellipse in the torque-angle plot, which is quantified as work W, is larger in the activated rigidity condition than in the resting condition (Fig. 2, torque-angle panels). The UPDRS score in this subject was 2 in the resting condition and 3 in the activated rigidity condition. These data illustrate that the secondary motor task resulted in an increase in rigidity reflected in a larger area of the ellipse and correspondingly higher W value than in the resting condition.
Fig. 2.
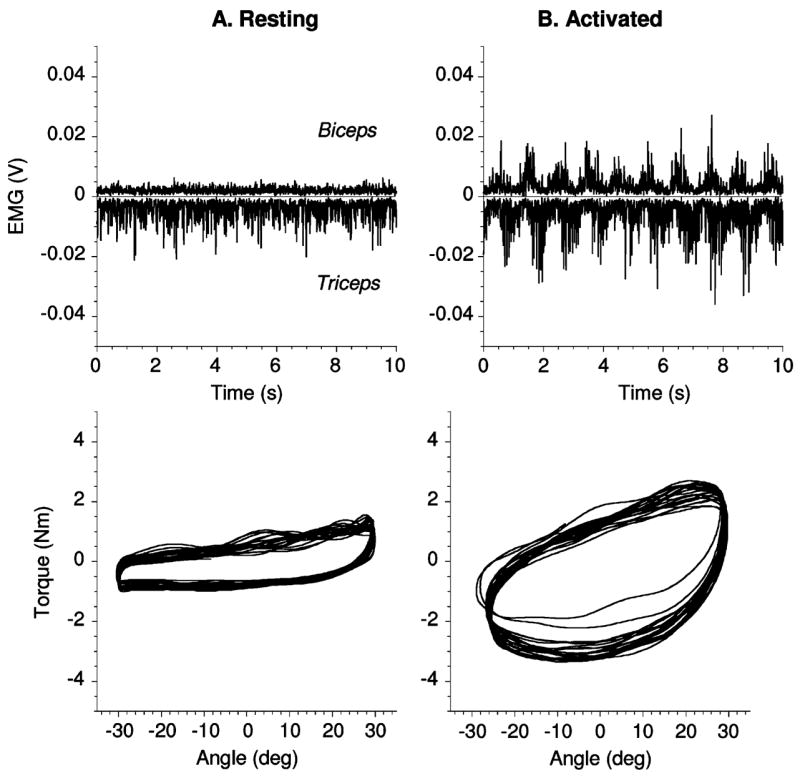
Data for one PD subject off treatment in resting (A) and activated (B) rigidity conditions are shown. Triceps EMG is inverted for better visualization. In torque-angle plots 20 s of data are shown. Estimated inertial torque was subtracted from measured torque in order to better represent resistive torque applied by the subject. Inertial torque was calculated by multiplying estimated moment of inertia of forearm and manipulandum bar 0.2 Kg*m 2 by angular acceleration. Note, this step does not affect W since work calculated for inertial torque over a complete cycle is zero.
We also compared calculated W for the neurologically healthy age-matched control subjects (Fig. 3, open squares) and PD patients off treatment (Fig. 2, filled circles) in the resting and activated rigidity conditions. The work calculated for the PD patients was significantly higher than the work for the control subjects (F1,9 = 6.04, p = .036). The data also clearly show that the secondary motor task resulted in an increased W in both control subjects and PD patients (F1,9 = 43.4, p = .0001). All interactions did not approach significance. These findings show that work is a valid operational measure of limb resistance that is sensitive to both disease and the activation maneuver.
Fig. 3.
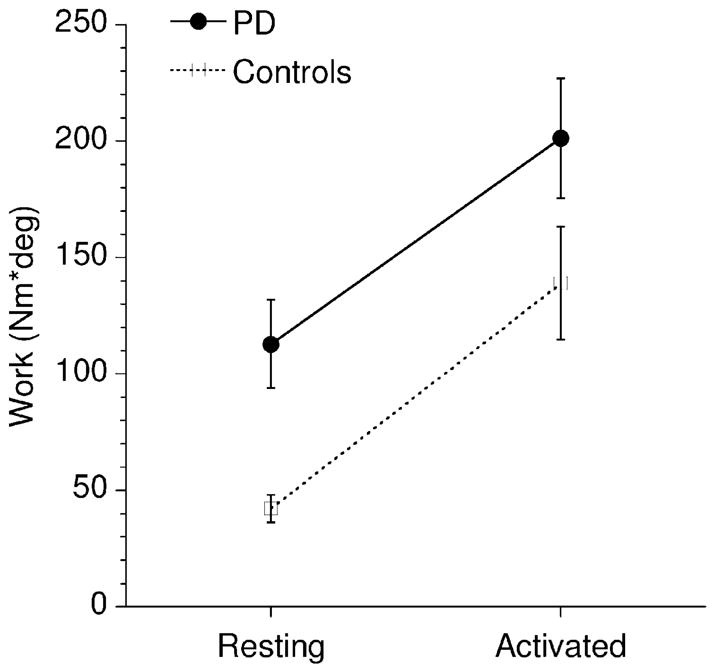
Effect of secondary motor task (activated rigidity) on work W in ten PD patients off treatment (filled circles) and ten age-matched neurologically healthy control subjects (open squares). Means and s.e.m. (bars) are shown.
The changes in UPDRS rigidity scores for the tested upper limb (Fig. 4, top panels A, B) were analyzed. The activated rigidity condition resulted in significantly increased UPDRS scores (F1,8 = 46.6, p = .00013, compare the data in panels A and B, Fig. 4). STN DBS reduced UPDRS rigidity scores (F1,8 = 14.2, p = 0.005). However, we found that medication administered at the presurgery dosage level did not significantly lower UPDRS rigidity scores (F1,8 = 2.0,p = 0.195). All interactions did not approach significance.
Fig. 4.
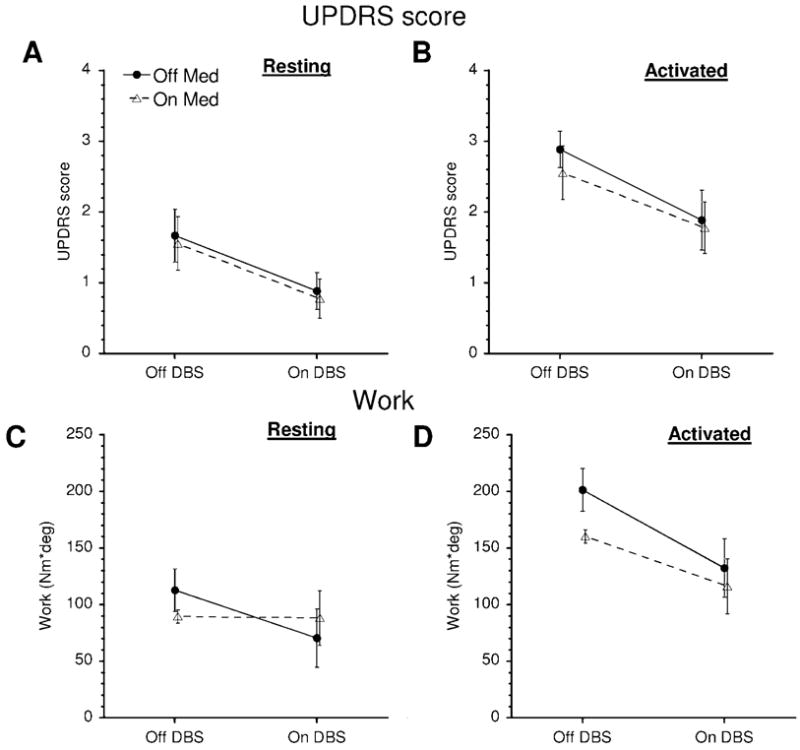
Effects of treatment on UPDRS scores (upper panels) and W (lower panels) in resting and activated rigidity conditions. Means and s.e.m. (bars) are shown.
Next, we analyzed changes in W (Fig. 4, bottom panels C, D). Similarly to the UPDRS scores, activated rigidity resulted in a significantly increased W (F1,9 = 16.7, p = 0.0028). STN DBS significantly lowered W (F1,9 = 8.79, p = 0.016), while the main effect of medication was not significant (F1,9 = 1.96, p = 0.2). Unlike the UPDRS scores, however, the W values showed a significant interaction between medication and secondary motor task (F1,9 = 5.57, p = 0.043). Medication significantly reduced W in the activated rigidity condition (Tukey’s HSD test, p = 0.022, see also Fig. 4, D) but not in the resting rigidity condition (Fig. 4, C, p = 0.9). A significant interaction was found between STN DBS and secondary motor task (F1,9 = 13.6, p = 0.005). In particular, STN DBS reduced W more in the activated rigidity than in the resting rigidity condition (Fig. 4, C, D). All other interactions did not approach significance.
IV. Discussion
In this paper, we have shown that STN DBS reduced both resting and activated rigidity as indicated by the subjective UPDRS rigidity score and objective W measure. Second, medication administered at the presurgery dosage level did not significantly improve the UPDRS rigidity score. This result can be partially explained by the fact that four out of ten patients in our study had low resting rigidity (Table I, Item 22). However, medication significantly lowered W in the activated rigidity condition. Since the activated rigidity was high in all subjects (Fig. 4, D), this result suggests that medication administered at presurgery dosage level does have a beneficial effect when rigidity is high. Third, the secondary motor task had a similar effect of increasing work W in both PD patients and age-matched neurologically healthy control subjects.
When rigidity in the upper extremity is assessed using the UPDRS, the patient is asked to relax and the examiner flexes/extends the patient’s limb in an approximately sinusoidal manner with a frequency that may range from less than 0.5 Hz up to 2 Hz [9], [38]. The purpose of objective measurements of rigidity such as W is to use a reproducible and easily quantifiable manipulation that simulates the salient features of the neurological exam. Webster and his colleagues [21], [22], [25] had a torque motor move the forearm over 100° with a constant velocity of 20 deg/s so that the full flexion-extension cycle took 10s to complete. In the later studies, faster oscillations with a frequency of 0.5 Hz over a shorter angular range of 40°–50° were used in the elbow experiments [14], [15], and rigidity in the wrist was tested using 1–1.5 Hz sinusoidal oscillations with a ± 30° displacement [13]. In a study of rigidity at the wrist, Teravainen and his colleagues [11] explored a range of waveform parameters: displacements from ± 15° to ± 30°, sinusoidal oscillations with frequencies from 0.2 Hz to 1.6 Hz, and a constant velocity waveform with a range of velocities from 24 to 190 deg/s. They concluded that a sinusoidal waveform was better than a constant velocity waveform in preventing the subject from assisting the movement and that rigidity was better detected using velocities of 140–190 deg/s and larger displacements. Therefore, in the present study we chose a 1-Hz sinusoidal waveform with a ±30° displacement and 190 deg/s velocity amplitude. While this waveform does not cover the extremes of the angular excursion where severe rigidity may be most pronounced, work (W) calculated for a ±30° displacement still captures the high level of resistance in severe rigidity.
Our data demonstrated that W differentiated between PD and healthy controls and between resting and activated rigidity (Fig. 3). Note that activated rigidity increased W in both PD and age-matched healthy controls which is contrary to the results of Relja et al. [15]. They reported that the activation maneuver did not affect the control subjects. The difference may be due to the fact that Relja and colleagues analyzed the data for 103 subjects aged 25–85 years old, while the age of control subjects in our study was 51–72 years old. Fung et al. [13] found that an activation maneuver increased the angular impulse in two out of ten controls aged 53–70 years old, while in our experiment W increased in nine out of ten controls. Our results are compatible with those of Kirollos and colleagues [14] who reported that an activation maneuver had a similar effect on PD and elderly controls but did not affect the young control subjects. We hypothesize that the effect of secondary motor task on resistance to imposed movement becomes more pronounced with age.
Activated rigidity is routinely used in clinical examination. If resting rigidity is not detected, then the patient is asked to do an activation maneuver with the other arm, e.g., clenching the fist, and the UPDRS score for the activated rigidity condition is assigned. If resting rigidity is detected, then the UPDRS score for resting rigidity is assigned and activated rigidity is not assessed. Because a UPDRS score may reflect either resting or activated rigidity the interpretation of treatment effects based on UPDRS data is complicated. Following the approach of Webster [22], several studies have examined resting and activated rigidity separately. Webster and Mortimer [25] found that L-dopa reduced W in resting but not activated rigidity while Relja et al. [15] found that L-dopa decreased both resting and activated rigidity. We found that medication administered in a presurgery dose did not significantly reduce UPDRS scores and significantly reduced work W only in the activated rigidity condition (Fig. 4).
Comparison of our findings with the published results is complicated by differences in medications and dosage administered in each study. Kirollos et al. [14] reported that two tabs of Sinemet CR (Carbidopa/Levodopa) reduced activated rigidity while one tab of Sinemet CR had no effect. Krack and colleagues [6] administered a dose of liquid L-dopa 50% higher than the usual morning dose of dopaminergic medication. They reported that with the DBS on, the scores on medication did not improve compared to off medication. However, a recent literature review [5] stated that one year after surgery, STN DBS reduced mean rigidity scores by 63% when patients were off medication and when STN DBS was combined with medication the rigidity scores were reduced by 73% when compared to preoperative off scores. It was also reported that in the preoperative period, medication reduced rigidity scores by 58% compared with the off scores. Thus, medication reduced rigidity by 58% before surgery while STN DBD alone reduced rigidity by 63% which suggests that, one year after surgery, STN DBS alone improved rigidity scores better than medication before surgery. A number of factors influence evaluation of the effect of medications on rigidity when STN DBS is on. In particular, medication may provide some additional improvement in rigidity in cases when the stimulation effect is less than optimal because of differences in the electrode placement or stimulation parameters. When the stimulation delivers the best results, however, medication may not further improve rigidity. Second, the differences in the dosage of medication administered during the test may have affected the UPDRS scores. Also, W may be a more sensitive measure of rigidity than the UPDRS which has a rather coarse scale from 0 to 4. Overall, both the previously published reports and our results suggest that once the STN stimulation is on, medication does not provide further significant improvement in rigidity.
Our results that STN DBS significantly improves UPDRS rigidity scores confirm previously published results [5], [6]. In addition, we found that STN DBS had a greater effect on W in activated rigidity than resting rigidity (Fig. 4, lower panels). The exact mechanisms of how STN DBS improves rigidity remain poorly understood. Meara and Cody [39] reported that an imposed movement at the wrist elicited phasic modulation in the EMGs which strongly increased in the activated rigidity condition. They suggested that the activation maneuver facilitates proprioceptive reflexes. We also observed large EMG bursts in the passively moved limb that were clearly elicited by the 1-Hz imposed oscillations (Fig. 2, B, EMG). The EMGs in the resting rigidity condition were much less modulated (Fig. 2, A, EMG). These observations support the idea that facilitation of proprioceptive reflexes underlies the increase in resistance in activated rigidity. An essential role of proprioceptive reflexes in rigidity was illustrated in experiments where rigidity was abolished by an intramuscular injection of novocain that selectively blocked the gamma fibers [40], [41]. It has been suggested that reduction of autogenic inhibition via spinal Ib interneurones is the key mechanism for rigidity at the spinal level [42], [43]. Spinal Ib interneurones are under descending control of the tegmentum and reticular formation, and abnormal activity of the basal ganglia in Parkinson’s disease indirectly influences the spinal Ib interneurones via connections between the basal ganglia and tegmentum [44]. STN DBS has been shown to restore the abnormally reduced autogenic inhibition yet it showed no correlation between the changes in rigidity and autogenic inhibiton [45]. Thus, although STN DBS has been shown to reduce Parkinsonian rigidity the neural mechanisms underlying the effect of stimulation remain unclear and a fertile ground for further research.
Acknowledgments
The authors would like to thank Medtronic for donating the Medtronic Program Console (Model 7432) used in this study. They also thank the staff at the Section for Movement Disorders, Department of Neurological Sciences, Rush University Medical Center for their help with patient recruitment and Dr. S. Leurgans for her assistance with some aspects of the statistical analyses.
This work was supported in part by the National Institutes of Health under Grant R01-NS052330, Grant R01-NS28127, Grant R01-NS40902, and Grant R01-NS52318.
Biographies
Mark B. Shapiro received the Ph.D. degree in electrical engineering from the University of Illinois, in 1997.
He completed an NIH postdoctoral fellowship in Motor Control and is currently a Research Assistant Professor at the University of Illinois at Chicago. His current interest is the integration of central and feedback mechanisms in control of human movement.
Dr. Shapiro is a member of the Society for Neuroscience and Society for Neural Control of Movement.

David E. Vaillancourt received the Ph.D. degree from Pennsylvania State University, College Park, in 2001.
He then completed a Postdoctoral Fellowship at the University of Illinois, Chicago, in clinical neurophysiology and neuroimaging, and was promoted to Assistant Professor in 2005. His current interests include using systems neuroscience techniques to understand the role of the basal ganglia, cerebellum, and cortex in motor control and movement disorders in humans. He has served on several National Institutes of Health study sections. He is on the editorial board of the Journal of Motor Behavior.

Molly M. Sturman received the Ph.D. degree in movement sciences from the University of Illinois at Chicago, in 2006.
She is currently a postdoctoral fellow at the University of Illinois at Chicago in the Motor Control and Movement Disorders Group. Her current research focuses on how deep brain stimulation affects the neurophysiological characteristics of motor control in movement disorders such as Parkinson’s disease.
Dr. Sturman is a member of the Society for Neuroscience.

Leo Verhagen Metman received the M.D. and Ph.D. degrees from the University of Leiden, The Netherlands. He completed his neurology residency at Thomas Jefferson University Hospital, Philadelphia, PA.
He is Medical Director of the Neurosurgery Program for Movement Disorders at Rush University Medical Center, Chicago, IL. He specializes in the medical and surgical management of patients with Parkinson’s disease and other movement disorders including tremor and dystonia. He completed a fellowship in the Experimental Therapeutics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, before joining Rush in 1999.

Roy A. E. Bakay received the M.D. degree from Northwestern University, Chicago, IL, after which he completed residency training in neurological surgery at the University of Washington School of Medicine, Seattle. Following his residency, he completed a National Institutes of Health fellowship in neuronal plasticity.
He is currently the A. Watson Armour III and Sarah Armour Presidential Chair, Vice Chairman, and Residency Research Director in the Department of Neurosurgery at Rush University Medical Center, Chicago, IL. He enjoyed a successful career at Emory University School of Medicine, Atlanta, GA, before joining Rush in 2000. A leading authority on surgery for movement disorders, he specializes in functional and stereotactic neurosurgery and has authored numerous journal articles. He has served as an editor for various journals including Neurosurgery. He has received funding from the NIH to study pallidotomy and deep brain stimulation. His current research also includes neural tissue transplantation and gene therapy techniques.
Dr. Bakay is a member of numerous professional organizations including the American Association of Neurological Surgeons, the American Society for Neural Transplantation, the American Society of Stereotactic and Functional Neurosurgery and the Congress of Neurological Surgeons.

Daniel M. Corcos received the Ph.D. degree in motor control from the University of Oregon, in 1982.
He did a Postdoctoral Fellowship at Rush Medical Center, Chicago, IL, from 1983 to 1987. He was an Assistant Professor from 1987 to 1993, an Associate Professor with tenure from 1993 to 1997, and has been a Professor since 1997 at the University of Illinois, Chicago. He has served on several National Institutes of Health study sections. He was Executive Editor of the Journal of Motor Behavior from 1996 to 2004 and is currently the Editor for Rapid Communications. He is also on the Editorial Board for the Journal of Neuroengineering and Rehabilitation.

References
- 1.Mercuri NB, Bernardi G. The magic of l-dopa: Why is it the gold standard Parkinson’s disease therapy. Trends Pharmacol Sci. 2005;26:341–344. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, Larsen JP, Lees A, Oertel W, Poewe W, Rascol O, Sampaio C. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: Late (complicated) Parkinson’s disease. Eur J Neurol. 2006;13(11):1186–1202. doi: 10.1111/j.1468-1331.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 4.Sturman MM, Vaillancourt DE, Metman LV, Bakay RA, Corcos DM. Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson’s disease. Brain. 2004;127:2131–43. doi: 10.1093/brain/awh237. [DOI] [PubMed] [Google Scholar]
- 5.Hamani C, Richter E, Schwalb JM, Lozano AM. Bilateral subthalamic nucleus stimulation for Parkinson’s disease: A systematic review of the clinical literature. Neurosurgery. 2005;56:1313–21. doi: 10.1227/01.neu.0000159714.28232.c4. [DOI] [PubMed] [Google Scholar]
- 6.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 7.Kleiner-Fisman G, Herzog J, Fisman DN, Tamma T, Lyons KE, Pahwa R, Lang AE, Deuschl G. Subthalamic nucleus deep brain stimulation: Summary and meta-analysis of outcomes. Movement Disorders. 2006;21:S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 8.Schupbach WMM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, Czernecki V, Maltete D, Hartmann A, Mallet L, Pidoux B, Dormont D, Navarro S, Cornu P, Mallet A, Agid Y. Stimulation of the subthalamic nucleus in Parkinson’s disease: A 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76:1640–1644. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prochazka A, Bennett DJ, Stephens MJ, Patrick SK, Sears-Duru R, Roberts T, Jhamandas JH. Measurement of rigidity in Parkinson’s disease. Mov Disord. 1997;12:24–32. doi: 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- 10.Agate FJ, Jr, Doshay LJ, Curtis FK. Quantitative measurement of therapy in paralysis agitans. J Amer Med Assoc. 1956;160:352–4. doi: 10.1001/jama.1956.02960400010003. [DOI] [PubMed] [Google Scholar]
- 11.Teravainen H, Tsui JK, Mak E, Calne DB. Optimal indices for testing Parkinsonian rigidity. Can J Neurol Sci. 1989;16:180–3. doi: 10.1017/s0317167100028857. [DOI] [PubMed] [Google Scholar]
- 12.Kondraske GV, Potvin AR, Tourtellotte WW, Syndulko K. A computer-based system for automated quantitation of neurologic function. IEEE Trans Biomed Eng. 1984 May;31(5):401–414. doi: 10.1109/TBME.1984.325279. [DOI] [PubMed] [Google Scholar]
- 13.Fung VS, Burne JA, Morris JG. Objective quantification of resting and activated parkinsonian rigidity: A comparison of angular impulse and work scores. Mov Disord. 2000;15:48–55. doi: 10.1002/1531-8257(200001)15:1<48::aid-mds1009>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Kirollos C, Charlett A, O’Neill CJ, Kosik R, Mozol K, Purkiss AG, Bowes SG, Nicholson PW, Hunt WB, Weller C, Dobbs SM, Dobbs RJ. Objective measurement of activation of rigidity: Diagnostic, pathogenetic and therapeutic implications in Parkinsonism. Br J Clin Pharmacol. 1996;41:557–64. doi: 10.1046/j.1365-2125.1996.38313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Relja MA, Petravic D, Kolaj M. Quantifying rigidity with a new computerized elbow device. Clin Neuropharmacol. 1996;19:148–56. doi: 10.1097/00002826-199619020-00003. [DOI] [PubMed] [Google Scholar]
- 16.Xia R, Rymer WZ. The role of shortening reaction in mediating rigidity in Parkinson’s disease. Experimental Brain Res. 2004;156:524–528. doi: 10.1007/s00221-004-1919-9. [DOI] [PubMed] [Google Scholar]
- 17.Caligiuri MP, Galasko DR. Quantifying drug-induced changes in Parkinsonian rigidity using an instrumental measure of activated stiffness. Clin Neuropharmacol. 1992;15:1–12. doi: 10.1097/00002826-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Patrick SK, Denington AA, Gauthier MJ, Gillard DM, Prochazka A. Quantification of the UPDRS rigidity scale. IEEE Trans Neural Syst Rehabil Eng. 2001 Mar;9(1):31–41. doi: 10.1109/7333.918274. [DOI] [PubMed] [Google Scholar]
- 19.Caligiuri MP. Portable device for quantifying Parkinsonian wrist rigidity. Movement Disorders. 1994;9:57–63. doi: 10.1002/mds.870090109. [DOI] [PubMed] [Google Scholar]
- 20.Ghika J, Wiegner AW, Fang JJ, Davies L, Young RR, Growdon JH. Portable system for quantifying motor abnormalities in Parkinson’s disease. IEEE Trans Biomed Eng. 1993 Feb;40(2):276–283. doi: 10.1109/10.216411. [DOI] [PubMed] [Google Scholar]
- 21.Webster DD. A method for measuring the dynamic characteristics of muscle rigidity, strength, and tremor in the upper extremity. IRE Trans Med Electron. 1959;6:159–164. [Google Scholar]
- 22.Webster DD. Dynamic measurement of rigidity, strength, and tremor in Parkinson patients before and after destruction of mesial globus pallidus. Neurology. 1960;10:157–63. doi: 10.1212/wnl.10.2.157. [DOI] [PubMed] [Google Scholar]
- 23.Xia R, Markopoulou K, Puumala SE, Rymer WZ. A comparison of the effects of imposed extension and flexion movements on Parkinsonian rigidity. Clin Neurophysiol. 2006;117(10):2302–2307. doi: 10.1016/j.clinph.2006.06.176. [DOI] [PubMed] [Google Scholar]
- 24.Mortimer JA, Webster DD. Evidence for a quantitative association between EMG stretch responses and Parkinsonian rigidity. Brain Res. 1979;162:169–73. doi: 10.1016/0006-8993(79)90768-6. [DOI] [PubMed] [Google Scholar]
- 25.Webster DD, Mortimer JA. Failure of L-dopa to relieve activated rigidity in Parkinson’s disease. Adv Exp Med Biol. 1977;90:297–313. doi: 10.1007/978-1-4684-2511-6_21. [DOI] [PubMed] [Google Scholar]
- 26.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: A clinicopathologic study. Neurology. 1992;42:1142–6. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 27.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJG. How do Parkinsonian signs return after discontinuation of subthalamic DBS. Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Starr PA, Vitek JL, Bakay RA. Ablative surgery and deep brain stimulation for Parkinson’s disease. Neurosurgery. 1998;43:989–1013. doi: 10.1097/00006123-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 31.Cohn MC, Hudgins PA, Sheppard SK, Starr PA, Bakay RA. Pre- and postoperative MR evaluation of stereotactic pallidotomy. AJNR Amer J Neuroradiol. 1998;19:1075–80. [PMC free article] [PubMed] [Google Scholar]
- 32.Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: Technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027–43. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 33.McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589–95. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, Lang AE. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 1998;51:850–5. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- 36.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 37.Vaillancourt DE, Haibach PS, Newell KM. Visual angle is the critical variable mediating gain-related effects in manual control. Exp Brain Res. 2006;173:742–50. doi: 10.1007/s00221-006-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meara RJ, Cody FW. Stretch reflexes of individual parkinsonian patients studied during changes in clinical rigidity following medication. Electroencephalogr Clin Neurophysiol. 1993;89:261–8. doi: 10.1016/0168-5597(93)90105-x. [DOI] [PubMed] [Google Scholar]
- 39.Meara RJ, Cody FW. Relationship between electromyographic activity and clinically assessed rigidity studied at the wrist joint in Parkinson’s disease. Brain. 1992;115(Pt 4):1167–80. doi: 10.1093/brain/115.4.1167. [DOI] [PubMed] [Google Scholar]
- 40.Walshe F. Observation on the nature of the muscular rigidity of paralysis agitans, and its relationship to tremor. Brain. 1924;47:159–177. [Google Scholar]
- 41.Rushworth G. Spasticity and rigidity: An experimental study and review. J Neurol Neurosurg Psychiatry. 1960;23:99–118. doi: 10.1136/jnnp.23.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delwaide PJ. Parkinsonian rigidity. Funct Neurol. 2001;16:147–56. [PubMed] [Google Scholar]
- 43.Delwaide PJ, Pepin JL, Maertens A, de Noordhout Short-latency autogenic inhibition in patients with Parkinsonian rigidity. Ann Neurol. 1991;30:83–9. doi: 10.1002/ana.410300115. [DOI] [PubMed] [Google Scholar]
- 44.Delwaide PJ, Pepin JL, De Pasqua V, de Noordhout AM. Projections from basal ganglia to tegmentum: A subcortical route for explaining the pathophysiology of Parkinson’s disease signs. J Neurol. 2000;247:II75–II81. doi: 10.1007/pl00007765. [DOI] [PubMed] [Google Scholar]
- 45.Potter M, Illert M, Wenzelburger R, Deuschl G, Volkmann J. The effect of subthalamic nucleus stimulation on autogenic inhibition in Parkinson disease. Neurology. 2004;63:1234–1239. doi: 10.1212/01.wnl.0000140287.42535.37. [DOI] [PubMed] [Google Scholar]


