Abstract
Intervertebral disc (IVD) degeneration is an often investigated pathophysiological condition because of its implication in causing low back pain. As human material for such studies is difficult to obtain because of ethical and government regulatory restriction, animal tissue, organs and in vivo models have often been used for this purpose. However, there are many differences in cell population, tissue composition, disc and spine anatomy, development, physiology and mechanical properties, between animal species and human. Both naturally occurring and induced degenerative changes may differ significantly from those seen in humans. This paper reviews the many animal models developed for the study of IVD degeneration aetiopathogenesis and treatments thereof. In particular, the limitations and relevance of these models to the human condition are examined, and some general consensus guidelines are presented. Although animal models are invaluable to increase our understanding of disc biology, because of the differences between species, care must be taken when used to study human disc degeneration and much more effort is needed to facilitate research on human disc material.
Keywords: Intervertebral disc degeneration, Animal models, In vivo, In vitro
Introduction
The main reasons for studying intervertebral disc (IVD) degeneration are related to the clinical link with back pain. Because of the difficulty of obtaining human material, particularly ‘normal’ human tissue, there have been many studies of disc degeneration in other species such as mouse, rat, sand rat, rabbit, dog, sheep, pigs, goats and apes. Models of disc degeneration are in the main used to address two aspects: (1) the scientific questions relating to disease progression and its treatment and (2) safety issues, the level of which is often prescribed and dictated by regulatory bodies or agencies. Thus models have been used to address questions relating to both the aetiopathogenesis and developing therapeutic strategies. There are many different models which have been used in many different species over the years. In this paper, we consider the relevance and limitations some of these may have. This is not meant to be a comprehensive description of all the models, but rather a paper to provoke thought and urge caution and careful consideration to researchers using animal models to study disc degeneration. A list of a large number of animal models is provided in Table 1 and some of the models have been reviewed by Lotz [81]. There are several important aspects to consider in relation to using animals for studying human disease. These include the development and anatomy of the spine in different species, and loading and size differences, and the mechanical, biochemical, nutritional, etc., interventions that produce a disease model. Each of these is discussed below.
Table 1.
In vivo animal models used to study disc degeneration
| Model type | Animal | Stimulus | References |
|---|---|---|---|
| Spontaneous | Mouse | Pintail mouse-genetic | Berry [15] |
| Mouse | Cmd aggrecan knockout | Watanabe et al. [152] and Watanabe and Yamada [153] | |
| Mouse | Inherited kyphoscoliosis | Mason and Palfrey [91] | |
| Mouse | Collagen II mutation | Sahlman et al. [128] | |
| Mouse | Collagen IX mutation | Kimura et al. [69] | |
| Mouse | Myostatin knockout | Hamrick et al. [49] | |
| Mouse | HLA-B27 transgenic spondylolisthesis | Weinreich et al. [154] | |
| Mouse | Defect at ank locus, ankylosing spondylitis | Sweet and Green [139] | |
| Mouse | twy mouse—IVD calcification and ankylosis | Furuya et al. [38] | |
| Rat | HLA B27 transgenic, spondylolisthesis | Hammer et al. [48] and Taurog et al. [141] | |
| Sand rat | Accelerated ageing | Moskowitz et al. [109] | |
| Dog | Chondrodystrophy | Braund [17] | |
| Baboon | Ageing | Lauerman et al. [75] | |
| Chemical | Rat | Norcross et al. [111] | |
| Rabbit | Chondroitinase ABC | Ando et al. [4] | |
| Dog | Yamada et al. [161] | ||
| Rabbit | Fibronectin fragments | Anderson et al. [3] | |
| Dog | Chymopapain, krill proteases | Melrose et al. [98, 103] and Suguro et al. [138] | |
| Antigen induced inflammation | Mouse (BALB/c) | Immunized with aggrecan and/or versican, develops spondylitis | Shi et al. [131], Mikecz et al. [105] and Glant et al. [46] |
| Mouse, rat | Static/dynamic compression of tail and thoracolumbar discs | Ariga et al. [6], Lotz et al. [82], Ching et al. [26], Iatridis et al. [58] and Stokes and Iatridis [137] | |
| Disc compression | Rabbit | Static compression | Kroeber et al. [73] |
| Pig | Compression injury, lumbar spine and caudal disc compression | Lundin et al. [83, 84] | |
| Bipedal | Mouse, rat | Amputation of upper limbs and tail | Higuchi et al. [50] and Cassidy et al. [22] |
| Endplate damage | Pig | Endplate perforation | Holm et al. [51] |
| Hyperactivity (running) | Dog | Long distance running training | Puustjarvi et al. [123, 124] and Saamanen et al. [127] |
| Fusion | Dog | Cole et al. [28, 29] | |
| Rabbit | Lumbar arthrodesis | Phillips et al. [122] | |
| Sheep | Foster et al. [37] | ||
| Spinal distraction | Rabbit | Controlled dynamic distraction | Kroeber et al. [72] |
| Spinal instability | Mouse | Resection of spinous processes, Resection of facet joints | Ariga et al. [5] and Peng et al. [120] |
| Rabbit | Stokes et al. [136] | ||
| Pig | Kaigle et al. [62] | ||
| Rabbit | Bilateral facet joint resection at L7S1 and rotational manipulation | Osterman and Osterman [116] | |
| Rat | Facetectomy/capsulotomy torsional lumbar injury | Latorre et al. [74] | |
| Rabbit | Hadjipavlou et al. [47] | ||
| Rabbit | Distraction, rib resection and spinal rotation | Thometz et al. [144] | |
| Rabbit | Surgical narrowing of intervertebral neural foramen, vibrational stimulation of dorsal root ganglia | Pedrini-Mille et al. [119] | |
| Disc lesions | Rabbit | Full depth anterior annular stab | Lipson and Muir [79, 80] |
| Rabbit | Multiple 5 mm stab incisions using 16, 18 or 21G needles | Kim et al. [66, 67], Masuda et al. [92] and Sobajima et al. [133] | |
| Rabbit | NP removal | Urayama [147] | |
| Rabbit | Surgical resection of NP | Takaishi et al. [140] | |
| Sheep | 3–5 mm outer anterolateral annular incision (rim-lesion) | Osti et al. [117] and Melrose et al. [93, 94, 96, 99, 100, 102] | |
| Sheep | Circumferential annular tear (delamellation) | Fazzalari et al. [35] and Thompson et al. [145] | |
| Pig | 5 mm outer annular incision | Kaapa et al. [60, 61] | |
| Dog | Full depth posterior annulotomy | Olsewski et al. [114] | |
| Chronic AF, NP, facet joint lesion model | Pig | Combined lesions in AF, NP, facet joint and capsule | Kaigle et al. [63] |
| Pinealectomy models of scoliosis | Chicken | Pinealectomy | Cheung et al. [23], Machida et al. [85, 86], Wang et al. [151], Kanemura et al. [64] and Turgut et al. [146] |
| Rat | Pinealectomy + bipedal | Machida et al. [87, 88] |
Differences with species, spinal level and age
Development of mammalian intervertebral discs
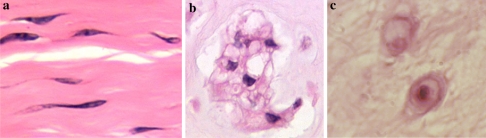
There is a common developmental pathway for the IVD seen in all mammals, with the vertebral column deriving from aggregation of the mesenchyme around the notochord. Following segmentation, motion segments are formed with large numbers of cells accumulating in the developing intervertebral annuli fibrosi, but fewer cells in the developing and more rapidly growing vertebral bodies. The cells of the annulus become highly orientated, laying down the disc matrix in a similar orientation to form the concentric annular lamellar structure. The notochordal cells disappear from all areas other than the future nucleus pulposus (NP) where they expand and produce a matrix rich in hyaluronan which is important to provide the swelling pressure to expand the NP and essential for its normal development [7, 12]. Although the overall process is the same, there are subtle but important differences which result, both in newborn and adult humans, compared to other mammalian species. Of particular importance, is the difference in cells which populate the central NP (Fig. 1): in humans, the discs contain some notochordal cells but the number of these decreases very rapidly after birth, such that by adulthood and possibly long before 4–10 years of age, there are no notochordal cells present in the human nucleus. The source of NP cells in the mature disc is unclear, suggestions include the inner annulus or even the cartilage endplates of the vertebral bodies [67, 68]. Most other species, including mouse, rat, cat, mink, dog, pig and rabbit, all have notochordal cells in the nucleus at birth, but unlike humans, retain them throughout much of their adult life, e.g. as reported in an 18-year-old cat [21]. Cows and sheep resemble humans in that they may have some notochordal cells at birth, but the numbers decrease rapidly and horses apparently have none at birth. Dogs are very interesting in that they fall into two types, chondrodystrophoid, e.g. dachshunds, which lose their notochordal cells after birth (and often develop disc related pathologies, e.g. prolapses later in life) and non-chondrodystrophoid dogs, which retain their notochordal cells throughout life (and have fewer disc related pathologies). Mice and rats retain a predominantly notochordal NP throughout life and rabbits have large numbers of notochordal cells at least until 12 months of age [55].
Fig. 1.
Cells of the intervertebral disc differ in morphology according to the region of origin, age and species: a Annulus fibrosus cells (bovine disc); b Notochordal cells (bovine disc); c Nucleus pulposus cells (human disc)
The relevance of notochordal cells in animal models used to study disc degeneration can be great since this is a completely different cell type in terms of morphology and function to the cells populating the adult human NP [54]. Notochordal cells are large, often highly vacuolated cells, but with many cytoskeletal markers. They produce a large amount of hyaluronan [134] and have many gap junctions, often forming groups or clumps of cells. In addition, notochordal cells have a marked influence on the metabolism of proteoglycans (PGs) by fibrochondrocytes within the NP [2, 113]. Although the exact role of these cells in forming the nucleus is largely unknown, it has been suggested that notochordal cells are progenitor and/or organizer cells. They may directly synthesize matrix proteins and eventually differentiate into the mature chondrocytic nucleus cells or they may help to recruit and co-ordinate other mesenchymal cells to synthesize the extracellular matrix. Hence results obtained from animals which retain notochordal cells well into adulthood, may have little relevance at all to the adult human situation.
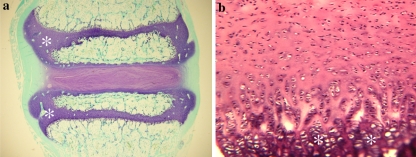
Another variation which occurs between species relates to the ossification centres. The central core of the vertical body forms the primary ossification centre in most mammals. Secondary centres arise in most mammals at the cranial and caudal aspects of the vertebral body, forming complete osseous plates above and below the physial plates. In humans, however, this secondary centre is restricted to the circumferential parts of the vertebrae (adj. to outer annulus), forming an epiphysial ring at the outer edge of the vertebral column. This fuses in the human at ∼25 years of age, whereas in many mammals, e.g. rodents, the complete epiphysis and adjacent physis persists throughout life, effectively within the body of the vertebrae. This results in the base of the cartilage endplate in humans acting as the growth region for the vertebral body, whereas in most other species (including sheep and cows) an epiphysial plate remains within the vertebral body (Fig. 2). However, there can even be variation between different colonies of the same strain of species, e.g. some Wistar rats have a complete centre of secondary ossification whilst in others it is incomplete, i.e. not a plate across the entire cross-section of the vertebra (C. B. Little and J. Ralphs, personal communication).
Fig. 2.
Growth plates (asterisks) occur within the vertebral bodies of many species, e.g. a sheep, unlike in the (b) human where they are restricted to the base of the cartilage endplate (CEP), interfacing between the disc and vertebral body
Anatomical variations
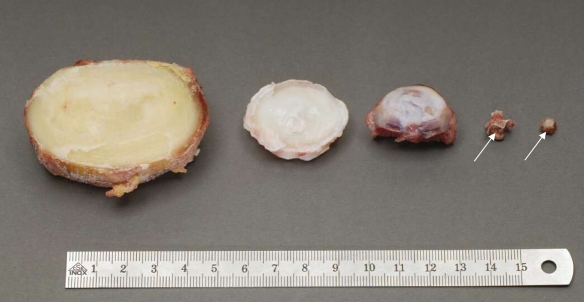
Besides the differences mentioned above, there are other anatomical variations between species. The shape, profiles and relative sizes (Fig. 3) of the IVDs and adjacent spinal tissues vary. In humans, for example, the antero-posterior diameter of the vertebral endplates steadily increases from the cervical to the lumbar region, while in large quadrupeds such as calves, sheep and pigs this diameter almost stays the same over the whole spine [157]. In consequence, compared to humans, these species have larger endplate diameters in the cervical but smaller diameters in the lumbar spine except for the calf, which has lumbar endplate diameters close to those of the human. In the lumbar spine, smaller endplate diameters are also found for primates (Orang-utan and Chimpanzee) and for the kangaroo [16]. Similarly to the endplate diameter, in large quadrupeds, disc height is more constant from cervical to lumbar regions and thus compared to humans it tends to be larger in the cervical but smaller in the lumbar spine. In addition to these quantitative measures, the shape of the IVD also differs from species to species. Human lumbar discs are convex on both their upper and lower surface. In contrast, in some animal species such as calf and pig, they are convex only in cranial but concave in caudal endplates forming a kind of cupola. Also the numbers of discs in different regions of the spine vary between species. In addition, the properties of the discs and vertebrae vary significantly in different locations within the spine of the same animal, e.g. caudal and lumbar discs of the rat [34]. Simple aspects, such as the size of the disc, can influence the appropriate choice of an animal model. Not only are there implications of scaling (see below) with small versus large discs, but some animals may be inappropriate just because the procedure being proposed is physically impossible in small species.
Fig. 3.
Relative sizes of discs in different species. From left to right: human lumbar, L4–L5; bovine tail, C1–C2; sheep thoracic, T11–T12; rat lumbar and tail (arrows show the intervertebral disc location)
The variation of properties (biomechanical, morphological and cellular) between regions within the disc itself is also an important factor to consider in choosing and designing the model. For example, the cell types vary with location, with those from the annulus differing not only in morphology but also in their metabolism, response to applied loads, etc., from the cells within the NP [57, 70, 125, 150].
Biomechanical differences
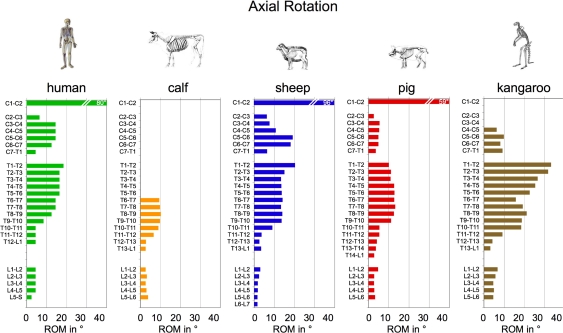
Variations of anatomical dimensions and properties as described above are important factors influencing the biomechanical behaviour of the spinal segments. Comparing the segmental range of motion of animal species and humans with each other, certain similarities but also certain differences can be detected [155, 158]. For example, the range of motion in axial rotation is smaller in the lumbar compared to the thoracic and cervical spine in all animal species and in humans (Fig. 4). However, in the lumbar spine the absolute values of range of motion are smaller for calf, sheep, and pig compared to the human. This difference is most pronounced in flexion/extension. Thus, while most of the flexion/extension motion of the trunk is produced in the lumbar segments in humans, this is not the case in calves, sheep and pigs.
Fig. 4.
Median range of motion (ROM) in degree in left plus right axial rotation for each single segment of the calf, sheep, pig and kangaroo spine compared to the human (human data from White and Panjabi [160])
Such flexibility data can play an important role in the study of IVD disorders since they are an indirect measure for disc deformation, and, thus, are one of the factors affecting degeneration and regeneration processes. For example, it is assumed that the higher flexibility of the lumbosacral segment could be one factor responsible for the reduced PG content found in this region [143]. Furthermore, the three-dimensional flexibility is an important parameter to study implant performance in vitro and in vivo. A certain animal species can therefore be inappropriate as a model for the human just because it has an inappropriate segmental flexibility.
Species differences in biochemistry
There are some inherent biochemical differences in the IVD that are associated with species such as the fact that rodent aggrecan core protein lacks an extended keratan sulphate attachment domain [9] making it quite distinct from other mammalian species. In a similar vein, analysis of aggrecan cleavage using neoepitope antibodies can be hampered because of notable species differences in the amino acid sequences at the common metalloproteinase cleavage sites that have been implicated in human disc degeneration [36]. Rodents also differ from other mammals including man, in that they do not express matrix metalloproteinase-1, a collagenase thought to be important in disease and tissue remodelling in other species.
It is also important to remember that the composition of the IVD varies with the disc location within the spine. Compositional studies on lapine, canine and ovine discs have been undertaken and have shown that the composition varies with ageing and with spinal level [95] such that lower levels of PGs were evident in IVDs at the juncture of a mobile and less mobile spinal motion segment, e.g. at the thoracolumbar and lumbosacral junction. A generalized and gradual decrease in PG levels is evident in all lumbar discs of the above species with aging, with such changes being more pronounced in the thoracolumbar and lumbosacral discs. The aggregation levels of disc aggrecan are also lower in these IVDs. It is noteworthy that such changes are less pronounced in the non-chondrodystrophic canine breeds and this may contribute to their relatively low incidence of disc disease.
Differences seen between species in changes with age and degeneration
The age of animals used for spine research must be closely monitored as naturally occurring changes, both histological and biochemical, occur with age in a number of species. In the dog, changes in the histology, radiographic appearance and biochemistry (decreased PG content and aggregation levels with hyaluronan, increased PG hydrodynamic size and relative keratan sulphate content, increased serine proteinase activity and decreased serine proteinase inhibitory protein levels) generally associated with pathology in human discs are also observed, particularly in chondrodystrophic breeds [28, 31, 41–45, 101, 102].
Similar biochemical changes have been reported with ageing in sheep and rabbits [89] in association with altered metabolism and PG synthesis by the disc fibrochondrocytes as occurs in humans [11]. In addition, disc calcification is a feature of aging in both dogs and sheep. In the latter, the distribution of the calcified deposits is restricted predominantly to the transitional zone between the annulus fibrosus (AF) and NP in the lumbar regions whereas in man the thoracic region is predominantly affected. In dogs, calcification occurs diffusely throughout the NP and their incidence also peaks in the thoracolumbar spine [17]. A recent observation also demonstrated that the biomechanical properties of lumbar discs in pigs changed significantly with age [118].
Loading on the spine and intervertebral discs
In humans the weight of the upper body acts on the lumbar spine. This is not the case in quadrupeds. Therefore, loading of the lumbar spine is often assumed to be larger in humans than in quadrupeds. This, however, may not be correct since muscle contraction and tension of passive structures such as ligaments also adds substantially to the loads acting on the spine [159]. This additional load may be higher in large quadrupeds such as calves, sheep or pigs than in humans since stabilizing a horizontally aligned spine requires higher muscle forces and passive tension than stabilizing an almost balanced vertically aligned spine, e.g. suspenion bridge versus an inverted pendulum. Hence, although there are no directly measured forces in the literature, and therefore no consensus, loading of the lumbar spine is probably not smaller but even larger for large quadrupeds than for humans. This would also be consistent with the up to fourfold higher bone mineral density found in sheep, calf and pig lumbar vertebral bodies compared to the human [156].
In small quadrupeds such as rabbit, mouse or rat, smaller muscular and ligamentous forces are needed to stabilize the spine. The spines of these animals are therefore probably loaded by much lower forces than that of the human. However, their intradiscal pressure might be similar to that of the human since the diameter of their discs on which this force is acting is much smaller.
Tail IVDs of animals mainly become loaded by muscle contraction or tension of passive structures. The more the muscles need to be contracted and the more the passive structures become tensioned, the more the loading of the tail discs will increase. Measurements of the in vivo loading of the spine and tail of various animals, however, are still lacking. Yet, this information would be crucial for most in vivo studies since the mechanical environment is known to influence the biological behaviour of the disc cells. Also, the loading of the disc plays an important role in in vivo or in vitro implant tests since it affects the performance of the implant for example in terms of its susceptibility to destruction or dislocation.
The immobilization of a segment of the canine lumbar spine induces compositional changes in the discs adjacent to the immobilized segment relative to spinal level and age matched discs of non-operated control animals [19, 29, 30, 142]. Alterations in spinal biomechanics due to scoliosis are also important determinants of disc cell metabolism and consequently also affect disc composition [20, 40, 97]. The introduction of a controlled outer anterolateral annular surgical defect also alters the intrinsic spinal mechanics at this spinal level and changes in disc cell metabolism and composition are measurable in the affected disc [93, 94, 96].
Scaling of specific parameters
Most non-human discs used in research are of a different size (usually smaller) than their human counterparts. Therefore, scaling is required in the interpretation of experimental findings from animal models since many aspects of disc behaviour are size-dependent. This certainly includes the mechanical behaviour of IVDs. Size (disc height) also strongly affects solute diffusion in disc tissues and thus solute transport in nutritional studies. Scaling requires that we know the form of the relationship between the size (geometrical parameters) and the behaviour being tested. At the very least, one should know whether the relationship is linear, quadratic, etc.
Either an empirical or an analytical approach can be used to determine the form of this relationship. Empirically, a series of discs having different sizes is studied and the relationship is found by linear or non-linear regression analysis. Analytically, if known theoretical considerations define the relationship between the behaviour of interest and the size parameters, then the form of the scaling relationship can be predicted. Ideally, both approaches should be used and the theoretical relationship should be verified experimentally.
In the mechanical behaviour of a disc, the size of the animal is also important in determining the magnitude of loading that is habitually imposed in the disc. This factor should also be taken into account in interpretation of experimental findings in a model. Apart from size differences there may be other species-dependent differences in disc behaviour, including structural variations. For instance, the mechanical stiffness of a disc depends on the annulus lamellar structure and also on its material properties (elastic moduli, etc.). The annulus structure has a different effect on the stiffness in each of the degrees of freedom—compression, torsion, etc. Transport phenomena depend on physical size (particularly diffusion pathways) but also on anatomical differences between species in relation to endplate structure and blood supply.
In principle, if the tissue properties do not vary between discs, then only the geometrical dimensions need to be taken into account in the scaling. Some basic structures and the tissue material properties do not vary greatly between mammalian species, or with anatomical level and age. For example, there is (mostly anecdotal) evidence that the number of lamellae, the lamellar fibre angle, and sizes of trabeculae are relatively constant. Other variables such as the thickness of lamellar layers vary with the disc size and its level in the spinal column (cervical, thoracic, lumbar and caudal).
Model systems
In vitro animal models for studying disc degeneration and repair
Cell cultures
Growing disc cells in the laboratory is obviously one useful technique for studying their behaviour and attempting to mimic either different environments and insults or also the effect of various interventions. Whilst useful information can certainly be gained from such model systems, it is important also to appreciate the intrinsic limitations of these experimental systems. An advantage of cell culture is that it is a ‘simple’ model, taking the cell down to literally its bare minimum. This simplicity, however, can also be considered a disadvantage, in that one is studying the cell in a totally non-physiological situation; in vivo the disc cells are surrounded by a large volume of extracellular matrix, with negligible cell–cell contact. This is very different from cell culture systems, particularly when grown in monolayer. The shape and morphology of cells is also known to influence their behaviour enormously. Hence the type of culture system used is capable of eliciting very different responses from the cells, particularly monolayer compared to three-dimensional culture system utilizing some form of scaffold. Commonly used three-dimensional culture systems include gels such as calcium alginate (polyguluronic/mannuronic acid) in the form of micro-beads [25, 90], agarose and collagen gels. Other scaffolds have also been used in tissue engineering type applications on IVDs such as gelatin/chondroitin-6-sulfate/hyaluronan tri-copolymer, hyaluronan hydrogels, fibrin/collagen co-polymer gels, polyglycolic acid or porous calcium polyphosphate. The choice of ‘support systems’ to be used should be influenced, not only by what aspect will be investigated, e.g. whether matrix production will be studied or molecular biology (requiring easy dissociation of the cells), but also on the type of cells being studied. Alginate, for example, appears to be less than optimum for studying cells from the outer annulus for which collagen gels may be more suitable [52]. Despite these points, cell culture systems can be used to address certain issues, as long as the question being asked and the interpretation of the results are considered carefully, taking into account the intrinsic limitations of each respective experimental system.
Explant cultures of whole discs
An important alternative to in vivo studies is an in vitro explant model, in which controlled in vitro conditions can be applied to a more physiologically relevant model. The main advantage of in vitro explant systems compared to cell models is that the cells are not removed from their highly specialized extracellular matrix whose effect on cell behaviour is not well understood. There are various models in the literature, including small rodent discs either alone, without bone, or as motion segments; the disadvantage of these is that unless they are placed under load they swell markedly and lose glycosaminoglycans (GAGs) thus changing the extracellular environment. Bovine caudal discs have been proposed as a suitable biological and biomechanical model for the study of the human lumbar disc. Bovine caudal discs (14–22 mm in diameter and 5–10 mm thick) are close in size to the human lumbar disc and the musculature of the bovine tail maintains an in vivo pressure on the discs that is approximately the same as in the human lumbar disc in the prone position (0.1–0.3 MPa). However, caudal (tail) discs in mice have been shown to have significantly different mechanical properties compared with lumbar discs in the same animals [130]. As noted earlier, the biochemical content and metabolic behaviour of discs from different spinal levels vary markedly and this must be borne in mind when extrapolating results from tail discs to cervical or lumbar regions. Also, tail IVDs in most animals cannot be considered to be weight bearing in the same context as discs above the sacrum; one exception to this is the kangaroo which has caudal discs which are weight bearing. This would be an interesting system to evaluate but such experiments have yet to be undertaken. Oshima et al. cultured bovine caudal discs under 5 kg static load in order to maintain in vivo hydration levels and measured changes in metabolic activity by varying the load magnitude [115]. That study was limited to 12 h and used discs without endplates, but such a system appears to be promising, especially in terms of its applicability for mechano-biology studies of the IVD. Further studies have been conducted using bovine discs with and without endplates [77] and they have shown that it is possible to maintain cell viability and the biosynthetic responsiveness of large discs for up to 1 week in vitro when the discs are cultured under static load and without vertebral endplates. Modifications of this method are proposed, such as applying cyclic loading (and hence motion), to influence convective transport, or reducing clot formation without the vertebral endplate via the administration of anticlotting agents ante-mortem [39]. Explant models, possibly simulating disc degeneration, can be used by culturing bovine caudal motion segments (with vertebral endplates attached) either with enzymes injected into the nucleus or via serum deprivation. These have been reported to be successfully cultured for up to 28 days [59].
The development of an entire IVD organ culture of large dimensions will have a high impact on future research on disc degeneration and regeneration. Such an organ culture model will give us a unique opportunity to measure the effect of growth factors, cytokines and protease inhibitors introduced directly into the IVD by injection, on the metabolic activity (synthesis and degradation) of disc cells in a controlled environment nearly identical to the in vivo conditions. This model could also be used to evaluate tissue-engineering approaches designed to treat disc degeneration, such as the implantation of cells, either alone or embedded in a biomatrix, as well as to assess transfection efficiency (for gene therapy) in an environment similar to the natural one. As with all in vitro models, the full physiological interaction of systemic and local factors cannot be controlled and any pathophysiological pathways or therapeutic modalities identified would ultimately need verification in vivo. However, once the ideal parameters (i.e. optimal biological factors, which inhibit degeneration or induce repair) have been identified with the model, fewer animal experiments need to be performed to test these conditions in vivo, reducing the use of live animals in research to a minimum.
In vivo models for studying disc degeneration and repair
Along with the cautionary notes relating to species and spinal level discussed above, the actual mechanism for inducing disc degeneration can influence the interpretation of the results obtained from an experiment. In Table 1, many of the different mechanisms for inducing or studying disc disease and the species in which they have been undertaken are summarized. The text following Table 1 discusses some of these models in more detail to highlight potential deficiencies or strengths of individual models.
Mechanical models
The commonly used mechanical interventions involve increased or decreased loading, increased or decreased motion, or injury. In models that impose altered loading, one should distinguish between sustained (constant) forces, and cyclic loading of different frequencies. Many of the interventions that are intended to alter just loading or motion in fact unintentionally modify both (e.g. application of a loading apparatus also reducing the intervertebral motion). The relative influences of alterations of loading and motion on disc degeneration were previously reviewed by Stokes and Iatridis [137].
Tail models
The tail provides an attractive model because its discs are accessible to interventions, with minimal risk of damage to surrounding structures, and minimal interference with normal physiological function. The IVDs in the tails of mice, rats and cattle have been used. In vivo mechanical interventions include an early forced ‘curved tail’ model [78], external apparatus to apply axial compression [27, 76, 149] or asymmetrical compression [32, 104]. Degeneration subsequent to injection of protein digestive enzymes into caudal discs [111] has also been reported. It should be noted that the biomechanical studies with external apparatus normally impose some degree of restricted motion, as well as altered loading. In vitro studies of the tail discs include structural [121], cell mechanics [18] and physiological studies.
The use of caudal discs as surrogates for the human lumbar disc has been questioned at several levels:
Differing mechanical loading relative to human lumbar spine. The tail has been suspected to have lesser loading (especially compressive stress) than the human spine. However, Oshima et al. reported that the swelling pressure in bovine tail discs is similar to that in human lumbar discs, indicating that the prevailing compressive stress of the tail discs is probably of similar magnitude to lumbar discs. Anaesthesia [110, 135] produces a similar increase in disc thickness as that which results from applying distraction forces of 60% bodyweight (c. 0.25 MPa) to the tail, which suggests that the ambient loading is of similar magnitude.
Different anatomy (absence of posterior elements), and differing dimensions relative to human lumbar spine (often being smaller, with anterioposterio-lateral proportions being more circular and the cephalous-caudal dimension being ‘thicker’ than lumbar discs). In fact, Elliott and Sarver [34] and Sarver and Elliott [130] reported substantial dimensional and mechanical behaviour similarity between the lumbar discs of mice, rats and humans, after scale effects were taken into account. Summarizing their dimensional data, together with some anecdotal observations of radiographs by one of the authors, the ‘aspect’ ratio (height/mean diameter) of human lumbar discs averages 0.24, which is similar to values for mouse and rabbit lumbar spines, whereas the ratio for lumbar spines of rats, deer and pigs is as little as half this value. The ratio for caudal discs is in the range 0.23 (bovine and mice) to 0.35 (rat).
Composition and metabolism. Recently Demers et al. [33] compared the composition and matrix synthesis in bovine tail discs and human lumbar discs of differing ages. They concluded that the bovine tail provided a good model of the young human, but was less well suited to studying age-related degeneration.
Hence, the tail has the advantage of being accessible for manipulation, but the disadvantage that the discs within it are distinctly different from lumbar discs in terms of biomechanics and composition, at least in rodents. The use of rats and mice must be questioned also because of the presence of notochordal cell. These are likely to respond very differently to mechanical loading compared with normal NP cells.
Bipedal mice
The strength of this model is that it is driven by overloading which some believe is involved in human disc disease. However its use cannot be supported on ethical grounds. Moreover, because of the notochordal nature of the NP in rodents, its value in predicting responses in humans is doubtful. It is also questionable whether laboratory housed animals with induced bipedalism really experience different spinal loading [8].
Injury models
In general these models are attractive in that the exact timing of the insult can be precisely controlled. In addition, damage to the AF and altered spinal mechanics are believed to play a critical role in human IVD degeneration.
Induction of spinal instability through various facetectomies and resection of spinous processes have in general had an uncertain time course and reliability in inducing disc degeneration and may stabilize with fibrosis over time. Fusion of a spinal segment results in induction of increased motion and degeneration in adjacent discs, but using this as a model of degeneration requires extensive surgery and is limited to induction of disease in a single level. There may also be an issue with the ability to readily transfer the same surgical technique and method between laboratories with extensive surgical procedures.
Perhaps the most extensively used surgical model has been to damage the AF. In 1948, the ‘stabbing’ of the IVD with a scalpel began to be used as a tool to induce disc degeneration in animals [65, 132]. Stab models may be classified into two types: the total annular stab model [80] and the superficial AF injury model [117]. Total annular injury induces NP avulsion and disc degeneration develops relatively quickly. It may, therefore be the model of choice for studying regeneration and the effect of therapy such as growth factors [66, 92, 133]. Degeneration after a superficial stab wound is much less rapid but involves actual active degeneration and may be superior for studying the pathophysiological processes [99, 100, 106–108]. In the rabbit, puncturing the AF with needles of defined gauges resulted in reproducible, degenerative changes within 2–8 weeks that could be quantitatively assessed through conventional radiography and magnetic resonance imaging (MRI), as well as histology. In contrast, the changes in endplate vascularity, facet joint osteoarthritis and metabolic pathology in NP and AF cells following partial thickness lesions in the sheep AF took 6–9 months to become apparent. The NP changes in the sheep model are much milder and less consistent compared with those in the rabbit following needle puncture. Because the rabbit disc contains notochordal cells that differ from human adult NP cells, possible species-related differences in the response to growth factors should be considered. However, in this rabbit model, the histology of the disc after puncture showed changes in the cell population from notochordal cells to chondrocytic cells. Therefore, in pilot pre-clinical studies, as a proof of concept, the use of the rabbit disc model may still be relevant and cost effective.
Biological models, directly influencing cells
Injection of degradative enzymes
One method which might seem appropriate to attempt to mimic degenerative disc disease in humans is proteolytic digestion or removal of GAG chains. Enzymes such as chymopapain or chondroitinases could be used to this effect. However, whilst this may result in a loss of GAG from the matrix, it would not be an exact replication of what happens naturally in the degenerating disc since there are no naturally occurring mammalian extracellular chondroitinases. On the other hand, depending on the question being asked of the study, this may not matter as a loss of PGs is a classic feature of disc degeneration. A further limitation of using enzyme degradation in an in vivo model is that, depending on the dose used, these models will heal with the cells synthesizing and replenishing the lost GAGs; thus they do not induce a true ‘degenerative’ process.
Altered nutrient supply
Although in vitro cell culture experiments [53] have demonstrated the effect of limited nutrition on cell viability and several studies have demonstrated the correlation between sclerosis of the endplate and disc degeneration [13, 14, 126], limited nutrition as a cause of disc degeneration has never been proven in intact discs. For this purpose, a canine model of insufficient blood supply to the disc was investigated by Hutton and colleagues [56]. To produce the blood supply disturbance, bone cement was injected into the vertebra adjacent to a single or to both endplates of experimental discs. After 70 weeks, they found no macroscopic degenerative changes in the experimental discs in comparison to control discs within the same animal. Unfortunately, the magnitude of nutrient transport inhibition within the disc created by the intervention was never measured. A similar study was also done in sheep to investigate the effect of multiple level vertebroplasty on disc health [71]. After 12 months, the authors observed histological changes consistent with a reparative response in early disc degeneration. Again they unfortunately did not assess nutrient transport alterations induced by their vertebroplasty intervention. Alternatively, another group is developing an ovine model for nutrient insufficiency induced disc degeneration [148]. In this model, the blood supply to the endplates is disrupted and a titanium foil is inserted to prevent vascular regeneration. To date, the group has been able to demonstrate that the method of intervention decreased significantly the number of perfused capillary buds in the endplate overlying the nucleus and that this was associated with inhibited diffusion of N2O into discs compared to control discs without the intervention. They also examined the mechanical effect of the intervention and found that there was no change in nucleus pressure profiles under physiological magnitudes of axial loads. Although the long-term effects of such an intervention must still be investigated, regardless of the results, it will provide unique information on the effects of limited nutrition on disc degeneration. Furthermore, such models will be invaluable in evaluating new biological interventions for disc degeneration and regeneration. As many of these molecular and cell-based therapies increase metabolic needs within the disc, their efficacy under limited nutrient conditions should be examined.
Genetic modification including the use of ‘knock-out’ mice
These are useful for studying the role of specific proteins or mutations in degeneration of the disc, e.g. the cmd mouse with haplo-insufficiency of aggrecan develops disc degeneration in the cervical spine. However, their use as more universally applicable models of disc degeneration, particularly in the light of the notochordal nature of mouse discs throughout life, is questionable.
Spontaneous models
These are very useful for evaluating natural degenerative pathophysiological processes. However, the inconsistent onset and long time frame over which they occur in genetically ‘normal’ animals does not make them useful for evaluating treatments.
Warnings and limitations relating to model systems
Since these models are being used to study the condition of disc degeneration in humans, we should consider what is generally meant by ‘disc degeneration’. It is however not surprising that there is no unified definition of disc degeneration as researchers working on IVD degeneration come from many backgrounds, e.g. biomechanical, biological, biochemical, biomedical, genetic, clinical, etc. It is true that disc degeneration in humans leads to many changes in the discs, e.g. reduced disc height, loss of signal on MRI, decreased water and PG content, increased cell death and cell cloning (presence of cell clusters), loss of collagen network integrity, disrupted mechanical function and morphological changes including fissuring, increased vascularization and innervation.
In a recent attempt to improve this lack of a consensus on the definition of disc degeneration, Adams and Roughley proposed the following definition—’The process of disc degeneration is an aberrant, cell-mediated response to progressive structural failure. A degenerate disc is one with structural failure combined with accelerated or advanced signs of aging. Early degenerative changes should refer to accelerated age-related changes in a structurally intact disc. Degenerative disc disease should be applied to a degenerate disc that is also painful’ [1].
The question remains: do any of the animal models used to date truly mimic a degenerate human disc as defined above. In reality, no one model results in an identical situation to that found in the human disorder. Hence there is NO ideal model which will answer all questions and address all aspects and is thus ‘a true model for disc degeneration’. A compromise is the best that can be achieved and the choice of the optimum model will depend on the questions being asked. This is a very important point and should be considered carefully at the outset of any study. The question(s) MUST be well defined and specific. For example, models using chymopapain result in PG loss but not necessarily all the other changes seen in disc degeneration in humans, yet such models can be used to investigate the effects of low PG on disc function [although long-term organ culture systems (up to 3–4 weeks) will soon be available that will have similar functions]. It is not, however, a model for investigating the aetiopathogenesis of disc degeneration or its repair, since in disc degeneration, PG loss is accompanied by morphological disorganization and other changes.
Potential problems relating animal models to human disc degeneration are:
Some induced animal models provide only an acute model of disc degeneration, which may be a very different condition from the chronic condition of disc degeneration in humans. For example, there may be no nutritional problems, or collagen disorganization or matrix fissures, or cell death in some acute models.
The age of the animals being used is very relevant. For convenience or cost purposes studies are often carried out using young, developing animals; outcomes may have little bearing on the situation in a skeletally mature individual and possibly even less on an older individual.
Markers for some important processes/factors are often species-specific and may not be available for study in some species.
Anatomical differences may lead to the wrong interpretation of results. For example, the endplate region is very different in man compared to most animals, yet this region is intimately involved in the nutritional pathway to the disc and appears to alter in disc degeneration in mankind.
Notochordal cells produce a disc with different biomechanics and different biology. Hence results from animals with notochordal cells may not translate well to adult humans and should be treated with great caution.
Breeds may differ, or even the same strain of animal kept in different centres—e.g. rat colonies bred in two different centres differed in their anatomy. Thus an important feature may vary depending on the source of the animal.
Different sizes and hence scale factors may limit the use of some animal models, for example if restricting nutritional pathways.
Animal studies may miss the vital importance of genetics, now believed to be responsible for up to 75% of disc degeneration in humans [10, 129]. It is difficult to include genetics in animal studies when the genes involved are not known. Another problem may be species differences (ADAMTS-5 is an example where it appears to be the primary aggrecanase in mice but evidence suggests that in other species both ADAMTS-4 and -5 may be involved in pathological processes). In addition, there is the limitation in studying one gene, for example, in ‘knock-out’ models, when disc degeneration in humans is likely to have multi-gene involvement.
Furthermore, one of the most important limitations in using animals to study disc degeneration relates to pain. Study of pain presents a great problem. Animal models of degeneration in general are not designed to assess this and yet this is the overriding clinical symptom which drives patients to seek treatment in the first instance. Neurological morphology and function can be studied to some extent but the important question of pain perception is much more difficult, particularly in the traditionally used small laboratory animals. The most relevant animals to study this are the more evolutionarily developed mammals, but they are ethically unacceptable animals with which to work.
Some guidelines
In general, something useful can be learnt if specific and appropriate questions are asked. For example, ‘how does loss of PG alone affect disc biomechanics or nutrient transport?’ could be addressed in a model system utilizing PG depletion. In contrast, ‘how does disc degeneration affect PG loss?’ could not be, as chymopapain-induced PG loss does not necessarily equate with pathological disc degeneration. Hence a careful choice of the question to be addressed can result in useful information being gained.
In any studies using animals, everything relating to all aspects of the animals used should be described in the fullest detail, e.g. breed and strain of animal, sex, age, conditions of housing and feed, etc. This is because minor changes, such as housing conditions, have been shown to affect outcomes. In addition, the model chosen must be fully justified in regard to the outcome sought. Guidelines to specific aspects of disc degeneration to be studied are given below.
Aetiopathogenesis
Exercise caution about the choice of species used:
Bipedal rodents should not be used on ethical grounds.
Chickens are so different anatomically that they can provide little relevant information. It should be noted that while it is possible to induce scoliosis in young chickens, by pinealectomy and strict control of the day/night regime they are exposed to, such procedures fail to induce scoliosis in hamsters or rats [112]. It is possible however to induce scoliosis in bipedal rats using pinealectomy [87], but a recent study in the non-human primate (Rhesus monkey) showed that pinealectomy failed to induce scoliosis [24]. Thus, while the upright stance and pinealectomy appear to be important determinants in rats for the induction of radial spinal curvature the aetiological cues induced in chickens by such procedures differ from those cues evident in the non-human primate. Thus results obtained with the chicken model are unlikely to be applicable to the human condition.
Animals with notochordal discs should be used with caution and may provide more information on changes in the AF than in the NP.
The choice of species to be used in a model should be influenced by what markers and probes are available for that species, e.g. antibodies, etc., and in the case of gene studies, the species for which the genome is known (i.e. mouse, rat, dog, sheep, bovine and human).
The following might provide useful information on the aetiopathogenesis of IVD degeneration:
Noting that disc degeneration in humans is mostly genetic, use could be made of species which have naturally occurring disc degeneration such as dogs (chondrodystrophoid and non-chondrodystrophoid dogs), sand rats and pintail mice. How the degeneration arises in these species should be borne in mind when designing any study, however, as that may limit its usefulness. For example, it has been suggested that the prime pathology in sand rats may lie in the bone (with abnormal mineralization), whereas in the pintail mice the disorder may arise due to an abnormality of the Pax genes resulting in early apoptosis of the disc cells.
Knock-out and transgenic mice to study specific proteins or mutations (with caution because of the notochordal nature of animals, species differences and probable multi-gene involvement in humans).
Naturally aged animals (sheep and rabbits), compared to human could provide information.
Pathogenesis
Pathogenesis can be investigated by studies designed to examine specific questions such as the influence of nutrient occlusion, or altered mechanics or the effect of injury. However, the specific pathological mechanism and its elements must be similar between the animal model and human disc, e.g. solute transport routes and mechanisms (different in small rodents and humans but similar in larger quadrapeds and humans).
Developing or testing surgical procedures and prostheses
Since a minimum size is required to test procedures to be used in mankind, the use of rodents and rabbits is inappropriate for this aspect. Dogs, sheep, goats and pigs can all be useful for in vivo models of surgical procedures and bovine (calf) for in vitro work. However, caution must be exercised in relation to age, bone density, anatomy, disc region and innervation of the species and individuals being used.
Biological and pharmaceutical therapies
Animals are required for use in biocompatibility or toxicity tests and they can provide baseline information in other studies, for example, cell-based therapies (i.e. genetic and tissue engineering and growth factor addition). However, much work can be done in vitro in good explant culture models; there remains a great need for a good long-term explant model to be developed and validated. Some work has begun in this direction, being carried out in different species to date, including rat, rabbit, sheep and cow. The use of in vivo testing of small animals, including rabbits, could be useful to answer certain questions. However, these should preferably have no notochordal cells, as the presence of notochordal cells could have many influences, as discussed previously. Another property to consider in models being used to test pharmacological aspects is whether the animal is monogastric, since this will significantly affect absorption pharmacokinetics of any prospective drug or therapeutic regimen if administered orally and hence its applicability to man. As long as any limitations of the chosen model are appreciated, the results can be interpreted in a reliable fashion. Mechanical studies and the effect of nutrient supply on biological therapies should be tested on larger animals such as sheep or pig.
The clinical perspective
The overall aim of all involved in this area is to work towards some sort of therapy against disc degeneration and back pain. That therapy may be in the line of regeneration or in the line of prevention. However, there is no inherent good in succeeding against disc degeneration The only good is the intention to prevent or reduce back pain when it appears to be associated with disc degeneration. Pain is a symptom, not a disease. Its presence probably has as much to do with perception as with pathology. There is no direct measurement of pain, only of the perceived disability it causes. Not all disc degeneration is associated with back pain. Disc degeneration with age probably does not qualify as a disease in the first place, unless ageing itself is classed as a disease. The experience of pain in animals can be measured only in crude terms and only when severe.
There is therefore no means of measuring success or failure of some new technique of disc salvage and back pain control, through animal experimentation. Animal experimentation in this context therefore serves a limited purpose: proving the likely technical practicality of certain minimally invasive techniques, with an eye to human implementation. There is, in addition, the need to satisfy certain statutory agencies which demand a certain level of testing in animals before granting permission for a procedure to be applied to humans.
Taking all the above into account would suggest that pre-human animal work should involve some animal spine as close as possible to the human spine in size and biology. Only human work, however, will ultimately inform us about success against pain. Many of us have spent much of our disc research lives devoted to one animal model. Such investment of time, trouble and money, makes it extremely difficult to accept the need to change to a different animal model for the sake of greater relevance to the problem at hand. In theory, the most relevant animal model biologically for the next stage of experimentation should be a primate. However, primates should not be used for obvious ethical reasons.
In some sense it is probably quite immaterial which animal model is used, from mouse to primate, because no single animal model can sufficiently replicate the circumstances affecting the human adult lumbar disc sufficiently to ensure that some specific technique will work as well in humans; and no animals can explain how well they are getting on with their back pain. The only benefit of animal work really is to discover the practical problems likely to be encountered in human work, such as placing cells with or without gels into the disc and keeping them there, and keeping them alive. We would like to stress, that most of these investigations could be done using organ culture systems that are now available.
Various agencies, such as Medicines and Healthcare products Regulatory Agency (MHRA), National Institute for Health and Clinical Excellence (NICE), Central Office for Research Ethics Committees (COREC) and United States Food and Drug Administration (FDA), will want some evidence of successful animal work, in order to sanitize any permissions given to proceed to human work. That seems to be the accepted gateway, at least for the time being.
Conclusions
Clearly, animal models can be useful in studying some specific aspect of disc biology (with a clear scientific question). However, most of these animal models if used in the wrong way (to mimic human disc degeneration) do not serve to advance scientific knowledge. Rather they then just serve to spread incorrect information about the processes involved in disc degeneration and about the possibilities of repair. It is better not to do the experiment than to do it using the wrong model. A more fruitful exercise is to take the time instead to design better experiments. The FDA, and other regulatory bodies, should be concerned about the inappropriate use of animals and models which can lead to misinformation.
While animal work can be useful to help with the development of techniques and provide baseline data, work should ideally be done on human tissue. If we want to improve understanding of pathology and treatment of human IVDs, legislation should change to allow us easier access to human tissues, e.g. from pathological, cadaveric and organ donor source.
The fundamental question to be addressed is: is it more (un)ethical to use animal models, which we know do not represent any human disc pathological conditions, or to use human tissues with all the ethical issues? We collectively believe the answer is clear: human tissue needs to be made more available!
Footnotes
All of the authors contributed equally to this publication and are listed simply in alphabetical order.
Contributor Information
Mauro Alini, Email: mauro.alini@aofoundation.org.
Stephen M. Eisenstein, Email: Stephen.eisenstein@rjah.nhs.uk
Keita Ito, Email: keita.ito@aofoundation.org.
Christopher Little, Email: cblittle@med.usyd.edu.au.
A. Annette Kettler, Email: annette.kettler@uni-ulm.de.
Koichi Masuda, Email: kmasuda@rush.edu.
James Melrose, Email: jmelrose@med.usyd.edu.au.
Jim Ralphs, Email: ralphs@Cardiff.ac.uk.
Ian Stokes, Email: istokes@uvm.edu.
Hans Joachim Wilke, Email: joachim.wilke@uni-ulm.de.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GD, Li X, Tannoury T, Beck G, Balian G. A fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine. 2003;28:2338–2345. doi: 10.1097/01.BRS.0000096943.27853.BC. [DOI] [PubMed] [Google Scholar]
- 4.Ando T, Kato F, Mimatsu K, Iwata H (1995) Effects of chondroitinase ABC on degenerative intervertebral discs. Clin Orthop Relat Res 318:214–221 [PubMed]
- 5.Ariga K, Miyamoto S, Nakase T, Okuda S, Meng WX, Yonenobu K, Yoshikawa H. The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine. 2001;26:2414–2420. doi: 10.1097/00007632-200111150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Ariga K, Yonenobu K, Nakase T, Hosono N, Okuda S, Meng WX, Tamura Y, Yoshikawa H. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs—an ex vivo study. Spine. 2003;28:1528–1533. [PubMed] [Google Scholar]
- 7.Aszodi A, Chan D, Hunziker E, Bateman JF, Fassler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey AS, Adler F, Min LS, Asher MA. A comparison between bipedal and quadrupedal rats: do bipedal rats actually assume an upright posture? Spine. 2001;26:E308–E313. doi: 10.1097/00007632-200107150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Barry FP, Neame PJ, Sasse J, Pearson D. Length variation in the keratan sulfate domain of mammalian aggrecan. Matrix Biol. 1994;14:323–328. doi: 10.1016/0945-053x(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 10.Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine. 2004;29:2679–2690. doi: 10.1097/01.brs.0000146457.83240.eb. [DOI] [PubMed] [Google Scholar]
- 11.Bayliss MT, Johnstone B, O’Brien JP. 1988 Volvo award in basic science. Proteoglycan synthesis in the human intervertebral disc. Variation with age, region and pathology. Spine. 1988;13:972–981. doi: 10.1097/00007632-198809000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Behrens A, Haigh J, Mechta-Grigoriou F, Nagy A, Yaniv M, Wagner EF. Impaired intervertebral disc formation in the absence of Jun. Development. 2003;130:103–109. doi: 10.1242/dev.00186. [DOI] [PubMed] [Google Scholar]
- 13.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30:167–173. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 14.Bernick S, Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine. 1982;7:97–102. doi: 10.1097/00007632-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Berry RJ. Genetical studies on the skeleton of the mouse 26. Pintail. Genet Res. 1960;1:439–451. doi: 10.1017/S0016672300000410. [DOI] [Google Scholar]
- 16.Boszczyk BM, Boszczyk AA, Putz R. Comparative and functional anatomy of the mammalian lumbar spine. Anat Rec. 2001;264:157–168. doi: 10.1002/ar.1156. [DOI] [PubMed] [Google Scholar]
- 17.Braund KG (1974) Some aspects of structure and function of the canine intervertebral disc. Ph.D. thesis, University of Sydney
- 18.Bruehlmann SB, Matyas JR, Duncan NA. ISSLS prize winner: collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine. 2004;29:2612–2620. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- 19.Bushell GR, Ghosh DP, Taylor TK, Sutherland JM, Braund KG. The effect of spinal fusion on the collagen and proteoglycans of the canine intervertebral disc. J Surg Res. 1978;25:61–69. doi: 10.1016/0022-4804(78)90159-2. [DOI] [PubMed] [Google Scholar]
- 20.Bushell GR, Ghosh P, Taylor TK, Sutherland JM. The collagen of the intervertebral disc in adolescent idiopathic scoliosis. J Bone Joint Surg Br. 1979;61-B:501–508. doi: 10.1302/0301-620X.61B4.500764. [DOI] [PubMed] [Google Scholar]
- 21.Butler WF. Comparative anatomy and development of the mammalian disc. In: Ghosh P, editor. The biology of the intervertebral disc. FL: CRC, Boca Raton; 1988. pp. 83–108. [Google Scholar]
- 22.Cassidy JD, Yong-Hing K, Kirkaldy-Willis WH, Wilkinson AA. A study of the effects of bipedism and upright posture on the lumbosacral spine and paravertebral muscles of the Wistar rat. Spine. 1988;13:301–308. doi: 10.1097/00007632-198803000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Cheung KM, Lu DS, Poon AM, Wang T, Luk KD, Leong JC. Effect of melatonin suppression on scoliosis development in chickens by either constant light or surgical pinealectomy. Spine. 2003;28:1941–1944. doi: 10.1097/01.BRS.0000083140.80750.93. [DOI] [PubMed] [Google Scholar]
- 24.Cheung KM, Wang T, Poon AM, Carl A, Tranmer B, Hu Y, Luk KD, Leong JC. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine. 2005;30:2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 25.Chiba K, Andersson GB, Masuda K, Momohara S, Williams JM, Thonar EJ. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine. 1998;23:1821–1827. doi: 10.1097/00007632-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Ching CT, Chow DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech. 2003;18:182–189. doi: 10.1016/s0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 27.Ching CT, Chow DH, Yao FY, Holmes AD. Changes in nuclear composition following cyclic compression of the intervertebral disc in an in vivo rat-tail model. Med Eng Phys. 2004;26:587–594. doi: 10.1016/j.medengphy.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Cole TC, Burkhardt D, Frost L, Ghosh P. The proteoglycans of the canine intervertebral disc. Biochim Biophys Acta. 1985;839:127–138. doi: 10.1016/0304-4165(85)90029-7. [DOI] [PubMed] [Google Scholar]
- 29.Cole TC, Burkhardt D, Ghosh P, Ryan M, Taylor T. Effects of spinal fusion on the proteoglycans of the canine intervertebral disc. J Orthop Res. 1985;3:277–291. doi: 10.1002/jor.1100030304. [DOI] [PubMed] [Google Scholar]
- 30.Cole TC, Ghosh P, Hannan NJ, Taylor TK, Bellenger CR. The response of the canine intervertebral disc to immobilization produced by spinal arthrodesis is dependent on constitutional factors. J Orthop Res. 1987;5:337–347. doi: 10.1002/jor.1100050305. [DOI] [PubMed] [Google Scholar]
- 31.Cole TC, Ghosh P, Taylor TK. Variations of the proteoglycans of the canine intervertebral disc with ageing. Biochim Biophys Acta. 1986;880:209–219. doi: 10.1016/0304-4165(86)90082-6. [DOI] [PubMed] [Google Scholar]
- 32.Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1:239–245. doi: 10.1016/s1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 33.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793–2799. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 34.Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29:713–722. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- 35.Fazzalari NL, Costi JJ, Hearn TC, Fraser RD, Vernon-Roberts B, Hutchinson J, Manthey BA, Parkinson IH, Sinclair C. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine. 2001;26:2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 36.Flannery CR, Little CB, Caterson B. Molecular cloning and sequence analysis of the aggrecan interglobular domain from porcine, equine, bovine and ovine cartilage: comparison of proteinase-susceptible regions and sites of keratan sulfate substitution. Matrix Biol. 1998;16:507–511. doi: 10.1016/s0945-053x(98)90021-x. [DOI] [PubMed] [Google Scholar]
- 37.Foster MR, Allen MJ, Schoonmaker JE, Yuan HA, Kanazawa A, Park SA, Liu B. Characterization of a developing lumbar arthrodesis in a sheep model with quantitative instability. Spine J. 2002;2:244–250. doi: 10.1016/s1529-9430(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 38.Furuya S, Ohtsuki T, Yabe Y, Hosoda Y. Ultrastructural study on calcification of cartilage: comparing ICR and twy mice. J Bone Miner Metab. 2000;18:140–147. doi: 10.1007/s007740050104. [DOI] [PubMed] [Google Scholar]
- 39.Gantenbein B, Grünhagen T, Lee CR, Donkelaar CC, Alini M, Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine. 2006;31:2665–2673. doi: 10.1097/01.brs.0000244620.15386.df. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh P, Bushell GR, Taylor TK, Pearce RH, Grimmer BJ. Distribution of glycosaminoglycans across the normal and the scoliotic disc. Spine. 1980;5:310–317. doi: 10.1097/00007632-198007000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh P, Melrose J, Cole TC, Taylor T. A comparison of the high buoyant density proteoglycans isolated from the intervertebral discs of chondrodystrophoid and non-chondrodystrophoid dogs. Matrix. 1992;12:148–155. doi: 10.1016/s0934-8832(11)80056-9. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh P, Taylor TK, Braund KG. The variation of the glycosaminoglycans of the canine intervertebral disc with ageing. I. Chondrodystrophoid breed. Gerontology. 1977;23:87–98. doi: 10.1159/000212177. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh P, Taylor TK, Braund KG. Variation of the glycosaminoglycans of the intervertebral disc with ageing. II. Non-chondrodystrophoid breed. Gerontology. 1977;23:99–109. doi: 10.1159/000212178. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh P, Taylor TK, Braund KG, Larsen LH. The collagenous and non-collagenous protein of the canine intervertebral disc and their variation with age, spinal level and breed. Gerontology. 1976;22:124–134. doi: 10.1159/000212129. [DOI] [PubMed] [Google Scholar]
- 45.Gillett NA, Gerlach R, Cassidy JJ, Brown SA. Age-related changes in the beagle spine. Acta Orthop Scand. 1988;59:503–507. doi: 10.3109/17453678809148772. [DOI] [PubMed] [Google Scholar]
- 46.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 47.Hadjipavlou AG, Simmons JW, Yang JP, Bi LX, Ansari GA, Kaphalia BS, Simmons DJ, Nicodemus CL, Necessary JT, Lane R, Esch O. Torsional injury resulting in disc degeneration: I. An in vivo rabbit model. J Spinal Disord. 1998;11:312–317. [PubMed] [Google Scholar]
- 48.Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 49.Hamrick MW, Pennington C, Byron CD. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF-8 (myostatin) J Orthop Res. 2003;21:1025–1032. doi: 10.1016/S0736-0266(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 50.Higuchi M, Abe K, Kaneda K. Changes in the nucleus pulposus of the intervertebral disc in bipedal mice. A light and electron microscopic study. Clin Orthop Relat Res. 1983;175:251–257. [PubMed] [Google Scholar]
- 51.Holm S, Holm AK, Ekström L, Karladani A, Hansson T. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64–71. doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Horner HA, Roberts S, Bielby RC, Menage J, Evans H, Urban JPG. Cells from different regions of the intervertebral disc—effect of culture system on matrix expression and cell phenotype. Spine. 2002;27:1018–1028. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]
- 53.Horner HA, Urban JPG. 2001 Volvo award winner in basic science studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 54.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 55.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutton WC, Murakami H, Li A, Elmer WA, Yoon ST, Minamide A, Akamaru T, Tomita K. The effect of blocking a nutritional pathway to the intervertebral disc in the dog model. J Spinal Disord Tech. 2004;17:53–63. doi: 10.1097/00024720-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Iatridis JC, MacLean JJ, Roughley PJ, Alini M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88(Suppl 2):41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 59.Johnson WEB, Menage J, Evans H, Gaguilo B, Roberts S, Eisenstein SM (2004) Serum-deprivation is associated with decreased cellularity and a loss of proteoglycan in organ cultures of bovine intervertebral discs. In: Proceedings of the 30th annual meeting, 5-5-1930, Porto, p 223
- 60.Kaapa E, Han X, Holm S, Peltonen J, Takala T, Vanharanta H. Collagen synthesis and types I, III, IV, and VI collagens in an animal model of disc degeneration. Spine. 1995;20:59–66. doi: 10.1097/00007632-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Kaapa E, Holm S, Han X, Takala T, Kovanen V, Vanharanta H. Collagens in the injured porcine intervertebral disc. J Orthop Res. 1994;12:93–102. doi: 10.1002/jor.1100120112. [DOI] [PubMed] [Google Scholar]
- 62.Kaigle AM, Holm SH, Hansson TH. Experimental instability in the lumbar spine. Spine. 1995;20:421–430. doi: 10.1097/00007632-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 63.Kaigle AM, Holm SH, Hansson TH. 1997 Volvo Award winner in biomechanical studies. Kinematic behavior of the porcine lumbar spine: a chronic lesion model. Spine. 1997;22:2796–2806. doi: 10.1097/00007632-199712150-00002. [DOI] [PubMed] [Google Scholar]
- 64.Kanemura T, Kawakami N, Deguchi M, Mimatsu K, Iwata H. Natural course of experimental scoliosis in pinealectomized chickens. Spine. 1997;22:1563–1567. doi: 10.1097/00007632-199707150-00006. [DOI] [PubMed] [Google Scholar]
- 65.Key JA, Ford LT. Experimental intervertebral disc lesions. J Bone Joint Surg Am. 1948;30A:621. [PubMed] [Google Scholar]
- 66.Kim KS, Yoon ST, Li J, Park JS, Hutton WC. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine. 2005;30:33–37. doi: 10.1097/01.brs.0000149191.02304.9b. [DOI] [PubMed] [Google Scholar]
- 67.Kim KW, Ha KY, Park JB, Woo YK, Chung HN, An HS. Expressions of membrane-type I matrix metalloproteinase, Ki-67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs: a cartilage endplate-fracture model using an intervertebral disc organ culture. Spine. 2005;30:1373–1378. doi: 10.1097/01.brs.0000166155.48168.0e. [DOI] [PubMed] [Google Scholar]
- 68.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–990. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 69.Kimura T, Nakata K, Tsumaki N, Miyamoto S, Matsui Y, Ebara S, Ochi T. Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int Orthop. 1996;20:177–181. doi: 10.1007/s002640050058. [DOI] [PubMed] [Google Scholar]
- 70.Kluba T, Niemeyer T, Gaissmaier C, Grunder T. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine. 2005;30:2743–2748. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- 71.Krebs J, Ferguson SJ, Goss BG, Aebli N (2006) Degenerative changes of intervertebral discs after vertebroplasty. In: Proceedings of the 33rd annual meeting of the international society for the study of the lumbar spine, Bergen, p 31
- 72.Kroeber M, Unglaub F, Guehring T, Nerlich A, Hadi T, Lotz J, Carstens C. Effects of controlled dynamic disc distraction on degenerated intervertebral discs: an in vivo study on the rabbit lumbar spine model. Spine. 2005;30:181–187. doi: 10.1097/01.brs.0000150487.17562.b1. [DOI] [PubMed] [Google Scholar]
- 73.Kroeber MW, Unglaub F, Wang HL, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 74.Latorre A, Albareda J, Castiella T, Lasierra JM, Seral F. Experimental model of multidirectional disc hernia in rats. Int Orthop. 1998;22:44–48. doi: 10.1007/s002640050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lauerman WC, Platenberg RC, Cain JE, Deeney VF. Age-related disk degeneration: preliminary report of a naturally occurring baboon model. J Spinal Disord. 1992;5:170–174. [PubMed] [Google Scholar]
- 76.Lee CR, Grad S, MacLean JJ, Iatridis JC, Alini M. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk. Anal Biochem. 2005;341:372–375. doi: 10.1016/j.ab.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Lee CR, Iatridis JC, Poveda L, Alini M. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine. 2006;31:515–522. doi: 10.1097/01.brs.0000201302.59050.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindblom K. Intervertebral-disc degeneration considered as a pressure atrophy. J Bone Joint Surg Am. 1957;39-A:933–945. [PubMed] [Google Scholar]
- 79.Lipson SJ, Muir H. Vertebral osteophyte formation in experimental disc degeneration. Morphologic and proteoglycan changes over time. Arthritis Rheum. 1980;23:319–324. doi: 10.1002/art.1780230309. [DOI] [PubMed] [Google Scholar]
- 80.Lipson SJ, Muir H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29:2742–2750. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 82.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. 1998 Volvo Award winner in biomechanical studies—compression- induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 83.Lundin O, Ekstrom L, Hellstrom M, Holm S, Sward L. Injuries in the adolescent porcine spine exposed to mechanical compression. Spine. 1998;23:2574–2579. doi: 10.1097/00007632-199812010-00012. [DOI] [PubMed] [Google Scholar]
- 84.Lundin O, Ekstrom L, Hellstrom M, Holm S, Sward L. Exposure of the porcine spine to mechanical compression: differences in injury pattern between adolescents and adults. Eur Spine J. 2000;9:466–471. doi: 10.1007/s005860000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine. 1993;18:1609–1615. doi: 10.1097/00007632-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J. Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Joint Surg Br. 1995;77:134–138. [PubMed] [Google Scholar]
- 87.Machida M, Murai I, Miyashita Y, Dubousset J, Yamada T, Kimura J. Pathogenesis of idiopathic scoliosis. Experimental study in rats. Spine. 1999;24:1985–1989. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 88.Machida M, Saito M, Dubousset J, Yamada T, Kimura J, Shibasaki K. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J. 2005;14:843–848. doi: 10.1007/s00586-004-0806-1. [DOI] [PubMed] [Google Scholar]
- 89.Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine. 2000;25:166–169. doi: 10.1097/00007632-200001150-00005. [DOI] [PubMed] [Google Scholar]
- 90.Maldonado BA, Oegema TR., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 91.Mason RM, Palfrey AJ. Intervertebral disc degeneration in adult mice with hereditary kyphoscoliosis. J Orthop Res. 1984;2:333–338. doi: 10.1002/jor.1100020405. [DOI] [PubMed] [Google Scholar]
- 92.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 93.Melrose J, Ghosh P, Taylor TK, Hall A, Osti OL, Vernon-Roberts B, Fraser RD. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665–676. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 94.Melrose J, Ghosh P, Taylor TK, Latham J, Moore R. Topographical variation in the catabolism of aggrecan in an ovine annular lesion model of experimental disc degeneration. J Spinal Disord. 1997;10:55–67. [PubMed] [Google Scholar]
- 95.Melrose J, Ghosh P, Taylor TK, McAuley L. Variation in the composition of the ovine intervertebral disc with spinal level and in its constituent proteoglycans. Vet Comp Orthop Traumatol. 1994;7:70–76. [Google Scholar]
- 96.Melrose J, Ghosh P, Taylor TK, Vernon-Roberts B, Latham J, Moore R. Elevated synthesis of biglycan and decorin in an ovine annular lesion model of experimental disc degeneration. Eur Spine J. 1997;6:376–384. doi: 10.1007/BF01834063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melrose J, Gurr KR, Cole TC, Darvodelsky A, Ghosh P, Taylor TK. The influence of scoliosis and ageing on proteoglycan heterogeneity in the human intervertebral disc. J Orthop Res. 1991;9:68–77. doi: 10.1002/jor.1100090110. [DOI] [PubMed] [Google Scholar]
- 98.Melrose J, Hall A, Macpherson C, Bellenger CR, Ghosh P. Evaluation of digestive proteinases from the Antarctic krill Euphasia superba as potential chemonucleolytic agents. In vitro and in vivo studies. Arch Orthop Trauma Surg. 1995;114:145–152. doi: 10.1007/BF00443388. [DOI] [PubMed] [Google Scholar]
- 99.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 100.Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- 101.Melrose J, Taylor TK, Ghosh P. Variation in intervertebral disc serine proteinase inhibitory proteins with ageing in a chondrodystrophoid (beagle) and a non-chondrodystrophoid (greyhound) canine breed. Gerontology. 1996;42:322–329. doi: 10.1159/000213810. [DOI] [PubMed] [Google Scholar]
- 102.Melrose J, Taylor TK, Ghosh P. The serine proteinase inhibitory proteins of the chondrodystrophoid (beagle) and non-chondrodystrophoid (greyhound) canine intervertebral disc. Electrophoresis. 1997;18:1059–1063. doi: 10.1002/elps.1150180705. [DOI] [PubMed] [Google Scholar]
- 103.Melrose J, Taylor TK, Ghosh P, Holbert C, Macpherson C, Bellenger CR. Intervertebral disc reconstitution after chemonucleolysis with chymopapain is dependent on dosage. Spine. 1996;21:9–17. doi: 10.1097/00007632-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 104.Mente PL, Aronsson DD, Stokes IAF, Iatridis JC. Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res. 1999;17:518–524. doi: 10.1002/jor.1100170409. [DOI] [PubMed] [Google Scholar]
- 105.Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–318. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- 106.Moore RJ, Crotti TN, Osti OL, Fraser RD, Vernon-Roberts B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine. 1999;24:519–525. doi: 10.1097/00007632-199903150-00003. [DOI] [PubMed] [Google Scholar]
- 107.Moore RJ, Osti OL, Vernon-Roberts B, Fraser RD. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine. 1992;17:874–878. doi: 10.1097/00007632-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Moore RJ, Vernon-Roberts B, Osti OL, Fraser RD. Remodeling of vertebral bone after outer anular injury in sheep. Spine. 1996;21:936–940. doi: 10.1097/00007632-199604150-00006. [DOI] [PubMed] [Google Scholar]
- 109.Moskowitz RW, Ziv I, Denko CW, Boja B, Jones PK, Adler JH. Spondylosis in sand rats: a model of intervertebral disc degeneration and hyperostosis. J Orthop Res. 1990;8:401–411. doi: 10.1002/jor.1100080312. [DOI] [PubMed] [Google Scholar]
- 110.Neufeld JH, Machado T, Margolin L. Variables affecting disc size in the lumbar spine of rabbits: anesthesia, paralysis, and disc injury. J Orthop Res. 1991;9:104–112. doi: 10.1002/jor.1100090113. [DOI] [PubMed] [Google Scholar]
- 111.Norcross JP, Lester GE, Weinhold P, Dahners LE. An in vivo model of degenerative disc disease. J Orthop Res. 2003;21:183–188. doi: 10.1016/S0736-0266(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 112.O’Kelly C, Wang X, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine. 1999;24:35–43. doi: 10.1097/00007632-199901010-00009. [DOI] [PubMed] [Google Scholar]
- 113.Oegema TR, Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine. 2000;25:2742–2747. doi: 10.1097/00007632-200011010-00005. [DOI] [PubMed] [Google Scholar]
- 114.Olsewski JM, Schendel MJ, Wallace LJ, Ogilvie JW, Gundry CR. Magnetic resonance imaging and biological changes in injured intervertebral discs under normal and increased mechanical demands. Spine. 1996;21:1945–1951. doi: 10.1097/00007632-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 115.Oshima H, Ishihara H, Urban JP, Tsuji H. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res. 1993;11:332–338. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- 116.Osterman K, Osterman H. Experimental lumbar spondylolisthesis in growing rabbits. Clin Orthop Relat Res. 1996;332:274–280. [PubMed] [Google Scholar]
- 117.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 118.Park C, Kim YJ, Lee CS, An K, Shin HJ, Lee CH, Kim CH, Shin JW. An in vitro animal study of the biomechanical responses of anulus fibrosus with aging. Spine. 2005;30:E259–E265. doi: 10.1097/01.brs.0000162531.49297.43. [DOI] [PubMed] [Google Scholar]
- 119.Pedrini-Mille A, Weinstein JN, Found EM, Chung CB, Goel VK. Stimulation of dorsal root ganglia and degradation of rabbit annulus fibrosus. Spine. 1990;15:1252–1256. doi: 10.1097/00007632-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Peng BG, Hou SX, Shi Q, Jia LS. The relationship between cartilage end-plate calcification and disc degeneration: an experimental study. Chin Med J. 2001;114:308–312. [PubMed] [Google Scholar]
- 121.Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat. 2005;207:299–312. doi: 10.1111/j.1469-7580.2005.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion—an experimental rabbit model. J Bone Joint Surg Br. 2002;84B:289–294. doi: 10.1302/0301-620x.84b2.11937. [DOI] [PubMed] [Google Scholar]
- 123.Puustjarvi K, Lammi M, Helminen H, Inkinen R, Tammi M. Proteoglycans in the intervertebral disc of young dogs following strenuous running exercise. Connect Tissue Res. 1994;30:225–240. doi: 10.3109/03008209409061974. [DOI] [PubMed] [Google Scholar]
- 124.Puustjarvi K, Lammi M, Kiviranta I, Helminen HJ, Tammi M. Proteoglycan synthesis in canine intervertebral discs after long-distance running training. J Orthop Res. 1993;11:738–746. doi: 10.1002/jor.1100110516. [DOI] [PubMed] [Google Scholar]
- 125.Rannou F, Poiraudeau S, Foltz V, Boiteux M, Corvol M, Revel M. Monolayer anulus fibrosus cell cultures in a mechanically active environment: local culture condition adaptations and cell phenotype study. J Lab Clin Med. 2000;136:412–421. doi: 10.1067/mlc.2000.109755. [DOI] [PubMed] [Google Scholar]
- 126.Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11:747–757. doi: 10.1002/jor.1100110517. [DOI] [PubMed] [Google Scholar]
- 127.Saamanen AM, Puustjarvi K, Ilves K, Lammi M, Kiviranta I, Jurvelin J, Helminen HJ, Tammi M. Effect of running exercise on proteoglycans and collagen content in the intervertebral disc of young dogs. Int J Sports Med. 1993;14:48–51. doi: 10.1055/s-2007-1021145. [DOI] [PubMed] [Google Scholar]
- 128.Sahlman J, Inkinen R, Hirvonen T, Lammi MJ, Lammi PE, Nieminen J, Lapvetelainen T, Prockop DJ, Arita M, Li SW, Hyttinen MM, Helminen HJ, Puustjarvi K. Premature vertebral endplate ossification and mild disc degeneration in mice after inactivation of one allele belonging to the Col2a1 gene for Type II collagen. Spine. 2001;26:2558–2565. doi: 10.1097/00007632-200112010-00008. [DOI] [PubMed] [Google Scholar]
- 129.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 130.Sarver JJ, Elliott DM. Mechanical differences between lumbar and tail discs in the mouse. J Orthop Res. 2005;23:150–155. doi: 10.1016/j.orthres.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 131.Shi S, Ciurli C, Cartman A, Pidoux I, Poole AR, Zhang Y. Experimental immunity to the G1 domain of the proteoglycan versican induces spondylitis and sacroiliitis, of a kind seen in human spondylarthropathies. Arthritis Rheum. 2003;48:2903–2915. doi: 10.1002/art.11270. [DOI] [PubMed] [Google Scholar]
- 132.Smith JW, Walmsley R. Experimental incision of the intervertebral disc. J Bone Joint Surg Br. 1951;33-B:612–625. doi: 10.1302/0301-620X.33B4.612. [DOI] [PubMed] [Google Scholar]
- 133.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine. 2005;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 134.Stevens JW, Kurriger GL, Carter AS, Maynard JA. CD44 expression in the developing and growing rat intervertebral disc. Dev Dyn. 2000;219:381–390. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1060>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 135.Stokes IA, Aronsson DD, Spence H, Iatridis JC. Mechanical modulation of intervertebral disc thickness in growing rat tails. J Spinal Disord. 1998;11:261–265. [PubMed] [Google Scholar]
- 136.Stokes IA, Counts DF, Frymoyer JW. Experimental instability in the rabbit lumbar spine. Spine. 1989;14:68–72. doi: 10.1097/00007632-198901000-00014. [DOI] [PubMed] [Google Scholar]
- 137.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29:2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suguro T, Oegema TR, Jr, Bradford DS. Ultrastructural study of the short-term effects of chymopapain on the intervertebral disc. J Orthop Res. 1986;4:281–287. doi: 10.1002/jor.1100040304. [DOI] [PubMed] [Google Scholar]
- 139.Sweet HO, Green MC. Progressive ankylosis, a new skeletal mutation in the mouse. J Hered. 1981;72:87–93. doi: 10.1093/oxfordjournals.jhered.a109459. [DOI] [PubMed] [Google Scholar]
- 140.Takaishi H, Nemoto O, Shiota M, Kikuchi T, Yamada H, Yamagishi M, Yabe Y. Type-II collagen gene expression is transiently upregulated in experimentally induced degeneration of rabbit intervertebral disc. J Orthop Res. 1997;15:528–538. doi: 10.1002/jor.1100150408. [DOI] [PubMed] [Google Scholar]
- 141.Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, Sayad A, Stagg AJ, Fox GM, Le O’Brien A, Rehman M, Zhou M, Weiner AL, Splawski JB, Richardson JA, Hammer RE. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–223. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 142.Taylor TK, Ghosh P, Braund KG, Sutherland JM, Sherwood AA. The effect of spinal fusion on intervertebral disc composition: an experimental study. J Surg Res. 1976;21:91–104. doi: 10.1016/0022-4804(76)90067-6. [DOI] [PubMed] [Google Scholar]
- 143.Taylor TK, Melrose J, Burkhardt D, Ghosh P, Claes LE, Kettler A, Wilke HJ. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine. 2000;25:3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 144.Thometz JG, Liu XC, Lyon R. Three-dimensional rotations of the thoracic spine after distraction with and without rib resection: a kinematic evaluation of the apical vertebra in rabbits with induced scoliosis. J Spinal Disord. 2000;13:108–112. doi: 10.1097/00002517-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 145.Thompson RE, Pearcy MJ, Barker TM. The mechanical effects of intervertebral disc lesions. Clin Biomech. 2004;19:448–455. doi: 10.1016/j.clinbiomech.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 146.Turgut M, Basaloglu HK, Yenisey C, Ozsunar Y. Surgical pinealectomy accelerates intervertebral disc degeneration process in chicken. Eur Spine J. 2006;15:605–612. doi: 10.1007/s00586-005-0972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Urayama S. Histological and ultrastructural study of degeneration of the lumbar intervertebral disc in the rabbit following nucleotomy, with special reference to the topographical distribution pattern of the degeneration. Nippon Seikeigeka Gakkai Zasshi. 1986;60:649–662. [PubMed] [Google Scholar]
- 148.van der Werf MJ, Lezuo P, Maissen O, Ito K (2006) Decreased diffusion as a result of perfusion block in the ovine lumbar spine: a future model for disc degeneration. In: Proceedings of the 52nd annual meeting of the orthopaedic research society, Chicago, IL, p 31
- 149.Walsh AJL, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–337. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 150.Wang JY, Baer AE, Kraus VB, Setton LA. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine. 2001;26:1747–1751. doi: 10.1097/00007632-200108150-00003. [DOI] [PubMed] [Google Scholar]
- 151.Wang X, Jiang H, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K. Characterization of the scoliosis that develops after pinealectomy in the chicken and comparison with adolescent idiopathic scoliosis in humans. Spine. 1997;22:2626–2635. doi: 10.1097/00007632-199711150-00010. [DOI] [PubMed] [Google Scholar]
- 152.Watanabe H, Nakata K, Kimata K, Nakanishi I, Yamada Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci USA. 1997;94:6943–6947. doi: 10.1073/pnas.94.13.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watanabe H, Yamada Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconj J. 2002;19:269–273. doi: 10.1023/A:1025344332099. [DOI] [PubMed] [Google Scholar]
- 154.Weinreich S, Hoebe B, Ivanyi P. Maternal age influences risk for HLA-B27 associated ankylosing enthesopathy in transgenic mice. Ann Rheum Dis. 1995;54:754–756. doi: 10.1136/ard.54.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 156.Wilke HJ, Kettler A, Gosh P, Claes L (1999) Is the lumbar sheep spine an adequate model for the human lumbar spine?—a comparison of biomechanical properties, macroscopic and microscopic anatomy and bone mineral density. In: Proceedings of the 26th annual meeting, Hawaii, p 124
- 157.Wilke HJ, Kettler A, Wenger KH, Claes LE. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247:542–555. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 158.Wilke HJ, Krischak ST, Wenger KH, Claes LE. Load-displacement properties of the thoracolumbar calf spine: experimental results and comparison to known human data. Eur Spine J. 1997;6:129–137. doi: 10.1007/BF01358746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wilke HJ, Rohlmann A, Neller S, Graichen F, Claes L, Bergmann G. ISSLS prize winner: a novel approach to determine trunk muscle forces during flexion and extension: a comparison of data from an in vitro experiment and in vivo measurements. Spine. 2003;28:2585–2593. doi: 10.1097/01.BRS.0000096673.16363.C7. [DOI] [PubMed] [Google Scholar]
- 160.White AA, Panjabi MM (1990) Clinical Biomechanics of the Spine. 2nd edn. JB Lippincott Company
- 161.Yamada K, Tanabe S, Eueno H, Oinuma A, Ytakahashi T, Mityauchi S, Shigeno S, Hirose T, Miyahara K, Sato M. Investigation of the short term effect of chemonucleolysis with chondroitinase ABC. J Vet Med Sci. 2001;63:521–525. doi: 10.1292/jvms.63.521. [DOI] [PubMed] [Google Scholar]