Abstract
There is a major controversy whether spinal trauma with vertebral endplate fractures can result in post-traumatic disc degeneration. Intervertebral discs, which are adjacent to burst endplates, are frequently removed and an intercorporal spondylodesis is performed. In any case, the biological effects within the discs following endplate factures are poorly elucidated to date. The aim of our investigations was therefore to establish a novel disc/endplate trauma culture model to reproducibly induce endplate fractures and investigate concurrent disc changes in vitro. This model is based on a full-organ disc/endplate culture system, which has been validated by the authors before. Intervertebral disc/endplate specimens were isolated from Burgundy rabbits and cultured in standard media (DMEM/F12, 10%FCS). Burst endplate fractures were induced in half of the specimens with a custom-made fracture device and subsequently cultured for 9 days. The biological effects such as necrotic or apoptotic cell death and the expression of pro-apoptotic genes and other genes involved in organ degeneration, e.g. matrix metalloproteinases (MMPs) were analyzed. Cell damage was assessed by quantification of the lactate dehydrogenase (LDH) activity in the supernatant. The expression of genes involved in the cellular apoptotic pathway (caspase 3) and the pro-apoptotic proteins FasL and TNF-α were monitored. The results demonstrate that LDH levels increased significantly post trauma compared to the control and remained elevated for 3 days. Furthermore, a constant up-regulation of the caspase 3 gene in both disc compartments was present. The pro-apoptotic proteins FasL and TNF-α were up regulated predominantly in the nucleus whereas the MMP-1 and -13 transcripts (collagenases) were increased in both disc structures. From this study we can conclude that endplate burst fractures result in both necrotic and apoptotic cell death in nucleus and annulus tissue. Moreover, FasL and TNF-α expression by nucleus cells may lead to continued apoptosis induced by Fas- and TNF-α receptor bearing cells. In addition TNF-α over-expression has potentially deleterious effects on disc metabolism such as over-expression of matrix proteinases. Taken together, the short term biological response of the disc following endplate fracture exhibits characteristics, which may initiate the degeneration of the organ.
Keywords: Endplate fracture, Disc degeneration, Disc culture model, Apoptosis, Matrix metalloproteinases
Introduction
There is an ongoing discussion on the question of whether severe vertebral fractures lead to post-traumatic disc degeneration or not. In the year 2000, Kerttula et al. published radiological follow-up data on magnetic resonance imaging (MRI) findings in young patients after vertebral fractures. It was found that there was a significantly increased rate of pathological signal changes after at least 1-year follow-up time (mean 3.7 years), especially when both adjacent endplates were fractured [28]. However, the study was critically commented by Dickson et al. and Alanay [3, 13] because of the limited number of enrolled patients (n = 14). The latter also cited a previous study by Oner et al. who investigated post-traumatic disc space changes with conventional X-rays and MRI. Based on their findings, the authors concluded that there was no evidence for a correlation between spine trauma and disc degeneration [41]. In contrast, Zucherman et al. analyzed MRI images from 379 patients to evaluate disc degeneration in the higher lumbar spine. They found that isolated high lumbar disc degeneration was not only associated with pre-existing endplate defects, such as in Scheuermann’s disease, but also with fractures below or above the respective intervertebral discs [23]. Similarly, Vornanen et al. have analyzed 2-year follow-up MRIs data from patients with spine fractures after Harrington rod stabilization. They could demonstrate that, unlike in distant immobilized segments, the discs adjacent to the fractures mostly exhibited a decreased signal intensity in T2-weighted MR images [50].
Although not painful in every case, post-traumatic disc degeneration potentially exhibits a substantial significance because problems such as increasing segmental kyphosis and other secondary degenerative changes like facet joint arthritis, spinal stenosis and degenerative scoliosis may result [1, 53].
The hypothesis that post-traumatic disc degeneration indeed exists is vaguely supported by a large number of animal models, which employ other mechanical means to induce the degenerative process. Basically there are three different methodical approaches described: stab incision models [4, 47], endplate perforation models [9, 22] and overload models [33, 40].
Sobajima et al. and Anderson et al. both demonstrated the initiation of disc degeneration on the basis of pathologic gene expression profiles using annular stab models in rabbits. In the first study, along with MRI changes, they found that the genes for the matrix components collagen type II and aggrecan, as well as the proteinase inhibitor TIMP-1 were decreased, whereas type I collagen was up-regulated 3 weeks after the incision procedure [47]. Anderson et al. presented degenerative disc changes on the histological level as well as an altered matrix gene transcription. In this context they pointed out the up-regulation of the genes for the collagenases MMP-1 and MMP-13 with the same injury mechanism [4]. Holm et al. drilled multiple holes through porcine endplates into the nuclei and observed subsequent disc degeneration with a loss of water content and morphological changes in the nucleus and annulus after 3 months [22]. In the third model, internal or external spinal fixators are attached to adjacent vertebra (motion segments) and a predefined compressive load is applied, as in the work by Lotz et al. where mouse tail discs were statically compressed with a custom-made device with an applied stress of up to 1.3 MPa. By DNA nick-end labelling (TUNEL) they demonstrated an increased rate of apoptotic disc cell death with compression time and magnitude [36].
Nevertheless, the above-described in vivo animal models are substantially simplified because they mimic only very few aspects of the realistic spine trauma which is normally characterized by its dynamic nature and the endplate burst due to the viscoelastic properties of the disc during the impact involving high energies [29]. Furthermore, the induction of genuine traumatic disc degeneration in animal models with the reproducibility of the complex injury pattern after spine trauma with burst endplates and mechanical damage to the disc is technically and analytically challenging and exhibits ethical problems. Consequently, the resulting intradiscal biological events, which may or may not lead to disc degeneration are sparsely investigated [21] and the correspondence to non-traumatic disc degeneration is largely unknown.
Considering results which were obtained from disc herniation models [2, 31], the initial post-traumatic and degenerative changes are likely comparatively complex. Numerous pathobiological changes and inflammatory cascades, such as the induction of programmed cell death (apoptosis), chemotactic recruitment of leukocytes and the activation of matrix-degrading metalloproteinases (MMPs) can be expected [4, 27, 51].
The aim of our study was to establish the first in vitro disc trauma fracture culture model and furthermore to determine the influence of trauma on different aspects of disc biology. We hypothesized that disc trauma initiates biological cascades and the expression of genes which may lead to an ultimate degeneration of the organ. The model is based on our recently developed and validated full-organ disc/endplate system, which has been shown to be suitable for long-term culture [19, 20].
Methods
Induction of endplate fracture and disc trauma
Thoracolumbar and lumbar spines were harvested under sterile conditions within 12 h after sacrifice from four male adult Burgundy rabbits (4–5 kg, 4–6 months old) obtained from the Bern University animal facility. The disc/endplate specimens were dissected as described before [19, 20]. After harvest the specimens (n = 10 per animal) were washed with agitation overnight in DMEM/F12 media (Labforce, Nunningen, Switzerland) with 50 μg/ml gentamicin (Labforce) and 10% FCS and 20 mmol sodium citrate. Endplate fractures were induced after 24 h with a custom-made drop device (Fig. 1). In a separate pilot experiment, the required potential energy as well as the appropriate shape of the impact tip (flat surface) was defined by assessing the failure load of the endplate on a material testing machine, (200–300 N depending on the size of the disc specimen). The absorbed energy in this quasi-static test was approximately 0.12 J. Based on that we defined a potential energy of 1.2 J (factor ten) for the dynamic drop injury. The resulting fracture lines within the bone showed the pattern of endplate burst fractures. As a consequence, a herniation of the nucleus pulposus through the endplates could be observed in all cases (Fig. 2). After the procedure, the traumatized discs, as well as the control specimens, were transferred into six well plates containing 6 ml of standard DMEM/F12 cell culture medium, supplemented with 10% FCS, 25 μg/ml l-ascorbate, 50 μg/ml gentamicin and cultured for 9 days with daily media changes.
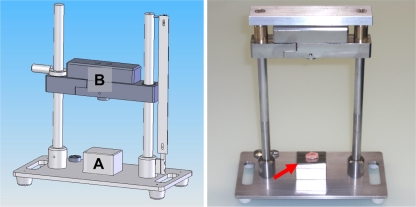
Fig. 1.
Vertebral endplate fracture device. Endplate fractures, and hence disc trauma, were induced with a dropped weight fracture device under sterile conditions. The specimens (red arrow) were placed on a centered metal surface (a) and the slider (b) equipped with a plain tip was dropped from a defined height. The transferred potential energy was calculated to be approx. 1.2 J (see “Methods”)
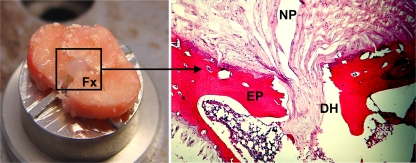
Fig. 2.
Fracture morphology. The induced endplate fractures demonstrated a typical burst pattern (Fx, left figure) with multiple fracture lines. This consistently resulted in a decompression of the nucleus pulposus. Histology demonstrated an osseous disc herniation (DH) of the gel-like nucleus pulposus (NP) through the vertebral endplate (EP, right figure)
Specimens were randomly assigned to the different assays (QPCR, caspase 3/7 activity assay, TUNEL, histology) with the respective control specimen taken from the same animal in each case. The culture media was collected daily and frozen at −80°C until further processing.
Assessment of cell viability
At days 1 and 6 of culture, specimens (n = 8) were digested with 0.1% pronase (1 h)(Calbiochem VWR International, Dietikon, Switzerland) and 0.025% collagenase (20 h)(Roche, Basel, Switzerland). The isolated disc cells were incubated for 20 min in microtiter plates (100 μl, 20.000 cells/well, 4 wells/disc) to allow sedimentation. Fifty-microliters of ethidium homodimer-1 (EthD-1, 2 μM)/calcein AM (1.6 μM) solution (Live/Dead® viability kit, Invitrogen, Basel, Switzerland) were added. Cytosolic esterases in active living cells convert the calcein AM into a green fluorescent dye and the double-stranded DNA in dead cells is intercalated by the ethidium homodimer which fluoresces red [42]. Methanol-treated cells were used as controls. Cell fluorescence was documented on an inverse microscope (Leica, DM IL; filters: I3 S 450–490 nm and N2.1 S 515–560 nm). Digital photos were taken with both filters (25×, five vision fields per well) and image analysis software (Quantity One®, Bio-Rad, Hercules, CA, USA) was used for relative quantification of live (green) and dead cells (red).
Quantification of lactate dehydrogenase activity
Lactate dehydrogenase activity (LDH) activity, as an indicator for cytotoxicity, was measured in cell-free culture supernatant with an enzymatic coupled redox reaction, which reduces tetrazolium salt to the coloured product formazan [12, 32]. The procedure was carried out according to the manufacturer’s protocol for the Cytotoxicity Detection Kit (LDH) (Roche, Basel, Switzerland). To evaluate the influence of the specimen harvest procedure on LDH levels, one additional experimental group was defined for LDH measurements. In addition to the normal specimens with an immediate fracture induction 1 day after specimen harvest, one further group of specimens was evaluated with a delayed fracture induction following 10 days recovery time in culture. Supernatants were collected daily and frozen (−80°C). For analysis, supernatants from the trauma and control group were incubated with 100 μl premixed test solution (100 μl/well, n = 6 discs/group). The colour reaction was allowed to develop for 15 min at room temperature. Light absorbance was measured at 450 nm (Spectra Max 190, Molecular Devices, Sunnyvale, CA, USA). Standard media was used as blank controls. The measured optical density values were normalized to the control from day 1 and presented in percent.
Quantitative real time PCR
RNA isolation from annulus and nucleus tissue was performed immediately on the day of collection. The disc material was snap-frozen with liquid nitrogen and pulverized with a mortar and pestle on RNase free surfaces. Half of the pulverized annulus tissue was stored at −80°C for later detection of apoptosis (see below). The samples for PCR (approx. 45 mg, n = 18) were covered with 1 ml of TRIzol reagent (Invitrogen) followed by further homogenization with a Polytron mixer (Kinematica, Newark, NJ, USA). RNA was processed following the manufacturer’s instructions including additional steps for elimination of proteoglycans with centrifugation for removal of the insoluble extracellular matrix and modified precipitation of RNA using 0.5 ml isopropanol.
One microgram of total RNA was used for the synthesis of the cDNA (iScript cDNA Synthesis Kit, BioRad). The complementary DNA template (1 μl) was mixed 1:20 with the Real Time reaction solution (SYBR Premix Ex Taq, Takara, Japan) which contained 0.25 μM specific primers as follows: house keeping gene: GAPDH: Forward: AAGGCCATCACCATCTTCCA Reverse: GGATGCGTTGCTGACAATCT, Chemokines: MCP-1: Forward: TCTGTGCCTGCTGCTCATAG Reverse: GCTCATTAGCCTCTTCACTG, IL-6: Forward: CTGGTGGTGGCTACCGCTTT Reverse: ATGGTCTCCAGGATGCTCCG, IL-8: Forward: CAACCTTCCTGCTGTCTCTG Reverse: GGTCCACTCTCAATCACTCT, Metalloproteinases: MMP-1: Forward: ATACCTGGAAAACTACTACAATCTG Reverse: TCTTCAGGGTTTCAGCATCT, MMP-2: Forward: CGCCGTCTCCCGTCATCAAA Reverse: TGAGGGTGTCCTTCAGCACG, MMP-9: Forward: CCACCTTGGTGGTCTTCCCA Reverse: TGCAGAAGTAGCAGCGGCAG, MMP-13: Forward: TGCCCCTCCTCAACAGTAAC Reverse: GAGCCCGCTGCATTCTTCTT; Apoptosis-associated genes: caspase 3: Forward: TGCATATTCCACAGCACCTG Reverse: GGGACTGGATGAACCAGGA, FasL: Forward: CAATGAGGGCAAACCAGACT Reverse: AAAAATGACTCTTGTCTGTGTACTCC, TNF-α: Forward: CAGCCTCTTCTCTTTCCTGCT Reverse: CCGATCACCCTGAAGTGC.
Real time PCR (IQ5, Biorad) was conducted with the following settings: denaturation 95°C—10 s (1 cycle), 40 amplification cycles: 95°C—5 s, 64°C—40 s; followed by melting curve analysis. During the optimization of the reaction conditions and melting temperatures, DNA agarose electrophoresis was used to validate the PCR products.
Assessment of apoptosis
Caspase 3/7 activity
To determine caspase-dependent apoptotic cell death, the pulverized annular samples (n = 18) were homogenized with the Polytron mixer (Kinematica) in 1 ml lysis buffer consisting of 25 mM HEPES (pH 7.5), 5 mM MgCl2, 5 mM DTT, 1 mM PMSF, 1 μg/ml benzamidine (Sigma) and 0.1% Triton × 100. The homogenates were centrifuged at 16,100×g for 5 min. Protein concentration was quantified according to Bradford [5] and the concentration in all samples adjusted to 220 μg/ml. Duplicates of 50 μl of the supernatants were transferred into 96 well plates and the Caspase Glo 3/7 assay was applied according to the manufacturer’s protocol (Promega, Mannheim, Germany). The generated light signal was detected after 0.5 h incubation with a luminometer (Infinite 200, Tecan, Switzerland). The background signal of the lysis buffer was used as a blank and subtracted from the read values. The caspase 3/7 activities of the annular samples were presented as relative change normalized to the controls, which were set 100 percent.
TUNEL
Terminal deoxynucleotidyl transferase (TdT) dUTP nick end labelling is well established for the demonstration of apoptosis. The assay detects single strand breaks within the DNA which are caused by the activation of intracellular DNases. The tissue fragments were fixed with 4% p-formaldehyde and the TUNEL procedure was applied on 6 μm kryosections on super-frost slides (Menzel, Braunschweig, Germany) according to the manufacturer’s labelling protocol (DeadEnd Colorimetric TUNEL System, Promega, Switzerland). DNase-treated (Sigma) sections were used as positive controls. Cell death was assessed by the quantification of labelled cells under the microscope.
Histology
The tissue was fixed in 4% buffered p-formaldehyde and sequentially washed and dehydrated in 30% sucrose solution before being cut in 6 μm sections with a cryotome (Microm International, Walldorf, Germany). Staining was carried out with H&E and Alcian blue/Periodic Acid Schiff reaction (PAS). High-resolution photos were taken with a digital camera (Nikon 70D, Tokyo, Japan).
Statistics
The statistical analysis was performed either by use of the non-parametric ANOVA by ranks test (Kruskal–Wallis) or by the Mann–Whitney U test using Statistica 7.1 software (StatSoft, Inc., Tulsa, OK, USA). A significance value of p < 0.05 was specified. For PCR experiments, a difference of at least one PCR cycle was considered significant, based on serial dilution standards.
Results
Indicators for post-traumatic disc cell damage
Lactate dehydrogenase activity (LDH)
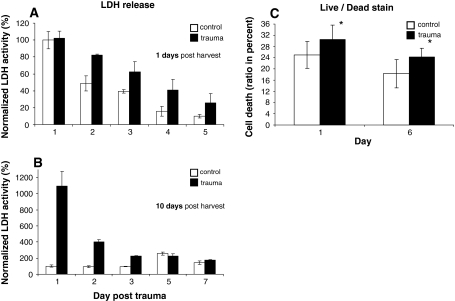
Increased LDH activity in the supernatant is an indicator for cytotoxic enzyme leakage through damaged cell membranes. If trauma was induced 24 h after harvest, LDH activities post trauma (t) were elevated in both groups without any significant difference between the groups (p = 0.79) (day 1 trauma 102.4 ± 4.9%, control 100 ± 10.3%). However, in the following course LDH activity in the trauma group (t) remained increased at each sampling point compared to the untreated control (c) (day 5: t, 26.5 ± 10.6%; c, 9.8 ± 2.1%; Fig. 3a). In contrast, if 10 days were allowed for recovery before the fractures were induced the trauma group exhibited a 10.9 ± 0.18-fold increase of LDH activity compared to the control (day 1: t, 1,093 ± 182%; c, 100 ± 14.3%; p < 0.05) which subsequently declined to reach control levels after 3 days (Fig. 3b).
Fig. 3.
Disc cell damage after trauma. Disc cell damage was assessed in the trauma group and the untreated control group with the quantification of lactate dehydrogenase (LDH) release in the supernatant at the indicated culture days after trauma (= day 0). Trauma was induced either 1 day (a) or 1 week (b) after the specimen harvest procedure. The results demonstrate significant cell damage due to specimen harvest in both groups, but a delayed decline of LDH activity in the trauma group (a). If 1 week was allowed for recovery of the specimens after the harvest procedure, a major increase could be recorded for the fracture group after trauma, whereas the control group did not show any cytotoxic LDH release (b). Additionally, disc cells were stained for cell viability with Live/Dead® stain (Molecular Probes). Here the ratio of dead cells (black bars) was increased compared to the control group (white bars). * p < 0.05 versus control
Live/dead stain
Cell count after viability staining with calcein and ethidium at day 1 post trauma demonstrated an increased ratio of dead cells in the trauma (t) group compared to the untreated control (c) (t: 30.5 ± 2.2%, c: 24.9 ± 1.6%, p < 0.05). After 6 days in culture the differences in cell viability were still evident (t: 24.3 ± 1.0%, c: 18.3 ± 1.1%, p < 0.05; Fig. 3c).
Apoptotic cell death and involved signalling
DNA nick end labelling (TUNEL)
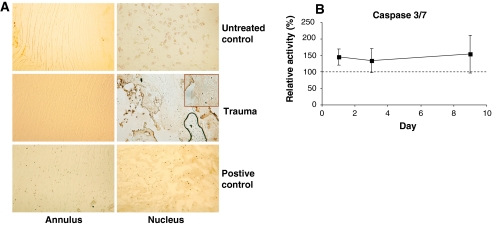
For in situ identification of apoptotic cells, e.g. DNA strand breaks, the TUNEL (terminal deoxynucleotidyl transferase nick end labelling) was used on histological sections. Semi-quantitative analysis demonstrated that disc specimens in the trauma group exhibited an increased number of apoptotic cells in the inner nucleus and more pronounced in the herniation part of the nucleus. The nuclei from the control group did not exhibit a significant number of TUNEL positive cells. There was no difference between trauma and control in the number of apoptotic cells in the annulus (Fig. 4).
Fig. 4.
Apoptosis after disc trauma. a Histological crysections were stained for apoptotic cell death with nick end labelling of the DNA (TUNEL). Traumatized discs from day 9 (trauma) demonstrated significantly higher numbers of TUNEL + cells in the herniating nucleus (inset) than the control group (untreated control). For the annulus, no significant difference in the number of apoptotic cells was detectable. DNAse-treated sections served as positive controls. b Protein extracts were prepared from the annuli fibrosi (n = 18) at the indicated time points and the lysate tested for caspase 3/7 activity. Values ± standard deviation were normalized to the untreated control (100%, dotted line) from the same day and presented in percent. Caspase 3/7 activity was increased in the trauma group over the entire culture time. p < 0.05 versus control
Caspase 3/7 enzyme activity
Enzyme activity measurements within disc cell lysates from both groups demonstrated a significant increase of caspase 3/7 activity to 145 ± 24% in the trauma group compared to the untreated controls (normalized to 100%) 24 h after trauma, which remained elevated at all sampling points (Fig. 4).
Caspase 3 gene expression
To verify the above findings on the gene expression level, quantitative PCR was performed with specific primers for the central down-stream caspase 3 gene. The measurements demonstrated an increased gene transcription level in the trauma group compared to the control for the annulus (A) and the nucleus (N) compartments 24 h post trauma (A: 2.6 ± 0.9-fold, N: 3.8 ± 0.6-fold). In the nucleus, the elevated gene expression persisted throughout the entire culture period whereas in the annulus a return to the control levels could be recorded at day 9 post trauma (A: 0.8 ± 0.5-fold, N: 6.7 ± 1.2-fold; Fig. 5).
Fig. 5.
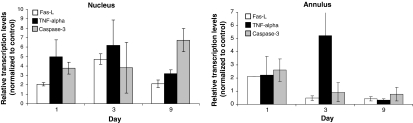
Transcription of apoptosis associated genes. Gene expressions of caspase 3, Fas ligand (Fas-L) and TNF-α were assessed separately for the nucleus and annulus (n = 36) at the indicated time points after disc trauma with quantitative real time PCR. The transcription levels ± standard deviation were normalized to the expression of GAPDH and depicted relative to the untreated controls. Differences of at least twofold increase/decrease were considered significant. In the trauma group in both disc compartments (nucleus, annulus) all tested genes were up-regulated post trauma
Gene expression of ligands for cell death receptors
The genes Fas-L and TNF-α encode for soluble or membrane bound ligands, which are able to bind and activate their respective cell death receptors (TNFα-R, Fas) on the cell membranes of target cells. In the annulus fibrosus (A), both genes were weakly up-regulated on the first day post trauma compared to the controls (A: Fas-L 2.1 ± 0.01-fold, TNF-α 2.1 ± 0.2-fold). However gene transcription of both genes subsequently dropped under control levels at day 9 (A: Fas-L 0.4 ± 0.2-fold, TNF-α 0.3 ± 0.1-fold). Similarly, in the nucleus pulposus (N) Fas-L and TNF-α were up-regulated 24 h after trauma (N: Fas-L 2.1 ± 0.2-fold, TNF-α 5.0 ± 1.8-fold) but in contrast to the annulus, remained up-regulated over the whole time (N: day 3 Fas-L 4.7 ± 0.6-fold, TNF-α 6.2 ± 2.7-fold; day 9 Fas-L 2.1 ± 0.4-fold, TNF-α 3.2 ± 0.4-fold, Fig. 6).
Fig. 6.
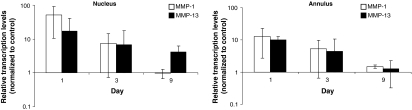
Transcription of collagenases. Gene expressions of the matrix metalloproteinases MMP-1 and MMP-13 were assessed separately for the nucleus and the annulus (n = 36) at the indicated time points after disc trauma with quantitative real time PCR. The transcription levels ± standard deviation were normalized to the expression of GAPDH and depicted on a log scale relative to the untreated controls. Differences of at least twofold increase/decrease were considered significant. The expression of both genes was up regulated at day 1 post trauma in both disc compartments and subsequently returned to almost control levels
Gene expression of collagenases
Both tested matrix metalloproteinases, MMP-1 and MMP-13, were strongly up-regulated in both disc compartments 24 h after trauma compared to the controls (A: MMP-1 12.6 ± 9.8-fold, MMP-13 10.1 ± 2.5-fold; N: MMP-1 51.3 ± 40.1-fold, MMP-13 16.9 ± 23-fold). In most groups a subsequent steady decrease of gene transcription was noticed until day 9 to reach control levels (A: MMP-1 1.5 ± 0.2-fold, MMP-13 1.2 ± 0.9-fold, N: MMP-1 1.0 ± 0.3-fold). In contrast, the expression of MMP-13 remained up-regulated at day 9 in the nucleus (N: MMP-13 ±2.1 fold, Fig. 6).
Discussion
The hypothesis to be tested in this study was that disc/endplate trauma initiates biological cascades within the intervertebral disc which can lead to degeneration of the organ. Due to the lack of appropriate animal models for endplate trauma, we used an in vitro organ culture approach, which is based on our recent work. There we established a whole-organ rabbit disc/endplate in vitro culture system with the possibility to keep the disc specimens in culture for more then 7 weeks without a significant loss of cell viability and proteoglycans from the extracellular matrix [19, 20]. For the current study, we constructed a dropped-weight device for the sterile induction of endplate burst fractures and an accompanying injury of the adjacent intervertebral disc. The applied energy of 1.2 J was defined as factor ten of the calculated energy obtained from “compression to failure” experiments on the material testing machine.
The induced endplate fractures exhibited a burst pattern with several intersecting fracture lines. This consistently resulted in a decompression and herniation of the nucleus through the endplate and epiphysis. Osseous disc herniation is a common finding in traumatic vertebral fractures and is due to the comparatively high dynamic stiffness of the disc tissue during the impact [26]. Oner et al. proposed a classification for MRI changes of the intervertebral discs after trauma. According to this classification, type 3 (Schmorl’s node) or type 5 (central endplate herniation) were induced in our in vitro study [41]. Przybyla et al. were investigating the influence of endplate fractures and annular tears on the intradiscal stress distribution and nucleus pressure. They found that endplate fractures lowered the nucleus pressure by 37% and substantially increased the stress on the posterior annulus with loading [44].
The evaluation of trauma-induced cell death within the disc by quantification of the lactate dehydrogenase (LDH) activity in the supernatant demonstrated an increased membrane leakage in the trauma group, although potentially reversible, as an indicator for necrotic cell death [7, 37]. Interestingly, if disc trauma was induced just 1 day after disc harvest from the animal, LDH activity in the control was not significantly different from the trauma group. This implies that there is a substantial cell damage due to the harvest procedure itself and trauma does not significantly increase LDH leakage from disc cells or bone cells in the initial period. However, in the following course, the effect of trauma became visible in this parameter, as the decline of LDH activity was much slower in the trauma group. On the other hand, if sufficient time was allowed for the disc specimens to recover in vitro after isolation from the animal, there was a clear effect of trauma on the LDH levels. This finding is in accordance with the results of Bush et al. who investigated the role of mechanical stress on joint cartilage. The authors subjected cartilage explants to a single impact (100 g from 100 mm height) and demonstrated a subsequent increase of LDH release from the damaged cells. They furthermore pointed out that media osmolarity and thus the swelling conditions had an effect on chondrocyte cell death in vitro [6].
Besides LDH leakage, the cytotoxic effect of trauma on the disc cells was reflected also in the Live/Dead staining procedure. We found an approximately 8% difference in the dead cell ratio between the trauma group and the control group. As the Live/Dead stain utilizes the substrate calcein for intracellular esterases and an ethidium dimer which penetrates disrupted cell membranes and intercalates with the DNA in the cell nucleus, this method detects early necrotic rather than apoptotic cell death [39, 42]. In a recent study by Whiteside et al. the effect of impact loading on the viability of articular cartilage was investigated using confocal microscopy. They could demonstrate that the extent of chondrocyte cell death was proportional to the applied mean impact force [52].
To detect whether, in addition to the above-described organ damage, apoptosis is also initiated, the specimens were screened with quantitative PCR (qPCR) for gene expression of caspase 3 which plays a central role in the caspase pathway [10, 43]. QPCR demonstrated an increased gene transcription on day 1 after trauma in annulus and nucleus tissue. In the annulus the transcription levels quickly returned to control levels afterwards whereas the nucleus showed increasing values until day 9. Moreover, the increased transcription of the caspase 3 gene in the annulus could also be confirmed on the gene product level by the quantification of caspase 3/7 enzyme activity. The latter finding was also confirmed in a study by Heyde et al. who analyzed intradiscal changes in spine trauma patients undergoing discectomy and instrumented fusion [21]. Apart from in disc research, the involvement of the caspase pathway and a potential beneficial effect of therapeutic caspase inhibition was demonstrated in the area of joint cartilage injury studies [11, 14, 25].
To evaluate whether disc cells may undergo apoptosis derived from death receptor signals, the gene expression of two ligands for cell death receptors (e.g. Fas receptor, TNF-α receptor I/II) Fas-L and TNF-α were assessed. We found that both transcripts where up-regulated in the trauma group in both disc compartments but to a higher extent and in a continuous fashion in the nucleus samples. This finding implies that ongoing FasL and TNF-α expression by nucleus cells may lead to continued apoptosis induced by Fas- and TNF-α receptor bearing cells. In addition, TNF-α over-expression has numerous potentially deleterious effects on disc metabolism, such as over-expression of matrix proteinases [46]. The existence of Fas-L [48] and Fas-R on disc cells and their relevance in certain spine pathologies such as scoliosis and trauma have been described before [8, 21]. Park et al. studied the type of apoptotic pathway in the human annulus obtained from surgical specimens by depleting foetal calf serum in the culture medium. In this study they found that primarily the mitochondrial type II pathway of Fas-mediated apoptosis was activated. This is in agreement with the study from Rannou et al. in which mouse-tail discs where overloaded by static compression which resulted in a consecutive increase of the caspase 9 dependent mitochondrial pathway [45]. Interestingly, the nucleus seems to be much more susceptible to trauma as all tested genes were more highly expressed and also remained elevated whereas in the annulus most genes were only marginally (∼2-fold) increased for a short time.
Staining for apoptotic DNA strand breaks (nick ends) with the TUNEL technique partially confirmed the above results and gave information about the spatial distribution of cell death. The annuli from the trauma and the control group did not show any significant difference in number of apoptotic cells. In contrast, beside the inner nucleus, apoptotic cells were found predominantly in the herniated part of the nucleus in the trauma group with negative staining in the control nuclei. Huser et al. also demonstrated an increase of TUNEL positive cells, in this case for articular cartilage in an in vitro single-impact load model [24]. However, results from TUNEL staining have to be interpreted with care, as it sometimes lacks specificity and may also detect necrotic cell death [17].
The discrepancy in the annulus between pro-apoptotic gene expression and increased caspase 3 activity on one side and negative TUNEL staining on the other may be explained by intracellular counter-regulative processes (posttranscriptional gene silencing or posttranslational enzyme inhibition by anticaspases such as the inhibitor of apoptosis protein, IAP) and thus apoptosis may be finally prevented [35]. Furthermore the stress within the annulus in a single impact scenario is expected to be comparatively small as the non-compressible nucleus and the bursting endplates most likely absorb most of the transmitted energy and the annulus is known to be quite resistant to tensile forces [15].
Our analysis for gene expression has shown that the two collagenase genes, MMP-1 and MMP-13, were up-regulated in the trauma group. At this time it is difficult to estimate the relative importance and contribution of each specific MMP for the degenerative course. Collagenases such as MMP-1 (collagenase 1, interstitial collagenase), MMP-8 (neutrophil collagenase) and MMP-13 (collagenase 3) seem to be of particular importance as they are able to cleave intact triple-helical collagen molecules [16, 30, 34] and thus make them accessible to other matrix proteinases such as gelatinases. Gelatinases, like MMP-2 and MMP-9 [38, 49], act synergistically with collagenases, as they can cleave the denatured form of collagen. Immuno-histochemical studies on human discs have shown that, unlike in children, a few MMP-1 positive cells are already present in young adulthood [51]. Furthermore, there was a strong correlation between morphologic degenerative changes like clefts and tears and the number of MMP-1+ cells [34, 51]. Substrate specificity analysis has revealed that MMP-13 preferentially cleaves type II collagen [30]. LeMaitre et al. found that, unlike MMP-1 which seem to be constitutively expressed in adults, the MMP-13 gene is exclusively transcribed in aging and degenerating discs [34]. These data were confirmed by Guehring et al. who have shown in an experimental rabbit model that compression-induced disc degeneration is accompanied by an up-regulation of both collagenase genes [18].
Taken together, our study has demonstrated that disc trauma results in a number of biological changes such as necrotic and apoptotic cell death and up-regulation of the gene transcription of several genes, which are involved either in the apoptotic pathway itself or serve for signalling. This is accompanied by the induction of collagenases (MMP-1 and MMP-13), which are known to be involved in disc degeneration. However, there are several limitations to the study. Drawing conclusions from animal models as well as from in vitro systems is always problematic. Furthermore, an increased gene transcription, as demonstrated with quantitative PCR, does not necessarily result in a functioning gene product, as mechanisms like post-transcriptional modifications or gene silencing may interfere. With matrix metalloproteinases, only a small aspect of the characteristics of disc degeneration was studied. Moreover, other important factors in the process of host response in vivo, the fracture hematoma and the infiltrating white blood cells (WBC), were not taken into account. They are also known to secrete a number of potentially harmful mediators, such as MMPs, nitric oxide or TNF-α. Furthermore, later neo-vascularization of the prolapse may have an effect on disc degeneration. Finally, it remains unknown if the observed post-traumatic changes are exceptionally due to the physical damage of the disc or rather to the decompression aspect of the nucleus. And thus also the influence of endplate fracture healing on later disc and endplate function is entirely unknown.
Acknowledgments
The authors thank the AO Foundation, Davos Switzerland for funding and Ladina Ettinger for excellent technical assistance
References
- 1.Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–948. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahsan R, Tajima N, Chosa E, Sugamata M, Sumida M, Hamada M. Biochemical and morphological changes in herniated human intervertebral disc. J Orthop Sci. 2001;6:510–518. doi: 10.1007/s007760100006. [DOI] [PubMed] [Google Scholar]
- 3.Alanay A. Re: post-traumatic findings of the spine after earlier vertebral fracture in young patients. Spine. 2000;25:2847–2848. doi: 10.1097/00007632-200011010-00027. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291–1296. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Bush PG, Hodkinson PD, Hamilton GL, Hall AC. Viability and volume of in situ bovine articular chondrocytes-changes following a single impact and effects of medium osmolarity. Osteoarthritis Cartilage. 2005;13:54–65. doi: 10.1016/j.joca.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Chang JK, Wu SC, Wang GJ, Cho MH, Ho ML. Effects of non-steroidal anti-inflammatory drugs on cell proliferation and death in cultured epiphyseal-articular chondrocytes of fetal rats. Toxicology. 2006;228:111–123. doi: 10.1016/j.tox.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Fellenberg J, Wang H, Carstens C, Richter W. Occurrence and regional distribution of apoptosis in scoliotic discs. Spine. 2005;30:519–524. doi: 10.1097/01.brs.0000154652.96975.1f. [DOI] [PubMed] [Google Scholar]
- 9.Cinotti G, Della Rocca C, Romeo S, Vittur F, Toffanin R, Trasimeni G. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine. 2005;30:174–180. doi: 10.1097/01.brs.0000150530.48957.76. [DOI] [PubMed] [Google Scholar]
- 10.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang AC, Warren AP, Kim HT. Beneficial effects of intra-articular caspase inhibition therapy following osteochondral injury. Osteoarthritis Cartilage. 2006;14:526–532. doi: 10.1016/j.joca.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 13.Dickson RA, Butt WP. Post-traumatic findings of the spine after earlier vertebral fractures in young patients. Spine. 2003;28:1749–1750. doi: 10.1097/00007632-200308010-00027. [DOI] [PubMed] [Google Scholar]
- 14.D’Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr., Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 15.Galante JO. Tensile properties of the human lumbar annulus fibrosus. Acta Orthop Scand Suppl. 1967;100:101–191. doi: 10.3109/ort.1967.38.suppl-100.01. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg GI, Wilhelm SM, Kronberger A, Bauer EA, Grant GA, Eisen AZ. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986;261:6600–6605. [PubMed] [Google Scholar]
- 17.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- 18.Guehring T, Omlor GW, Lorenz H, Bertram H, Steck E, Richter W, Carstens C, Kroeber M. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration—an in vivo animal study. Spine. 2005;30:2510–2515. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 19.Haschtmann D, Stoyanov JV, Ettinger L, Nolte LP, Ferguson SJ. Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine. 2006;31:2918–2925. doi: 10.1097/01.brs.0000247954.69438.ae. [DOI] [PubMed] [Google Scholar]
- 20.Haschtmann D, Stoyanov JV, Ferguson SJ. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res. 2006;24:1957–1966. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 21.Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A. Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol. 2006;6:5. doi: 10.1186/1472-6890-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm S, Holm AK, Ekstrom L, Karladani A, Hansson T. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64–71. doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hsu K, Zucherman J, Shea W, Kaiser J, White A, Schofferman J, Amelon C. High lumbar disc degeneration. Incidence and etiology. Spine. 1990;15:679–682. doi: 10.1097/00007632-199007000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Huser CA, Davies ME. Validation of an in vitro single-impact load model of the initiation of osteoarthritis-like changes in articular cartilage. J Orthop Res. 2006;24:725–732. doi: 10.1002/jor.20111. [DOI] [PubMed] [Google Scholar]
- 25.Huser CA, Peacock M, Davies ME. Inhibition of caspase-9 reduces chondrocyte apoptosis and proteoglycan loss following mechanical trauma. Osteoarthritis Cartilage. 2006;14:1002–1010. doi: 10.1016/j.joca.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Izambert O, Mitton D, Thourot M, Lavaste F. Dynamic stiffness and damping of human intervertebral disc using axial oscillatory displacement under a free mass system. Eur Spine J. 2003;12:562–566. doi: 10.1007/s00586-003-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato T, Haro H, Komori H, Shinomiya K. Sequential dynamics of inflammatory cytokine, angiogenesis inducing factor and matrix degrading enzymes during spontaneous resorption of the herniated disc. J Orthop Res. 2004;22:895–900. doi: 10.1016/j.orthres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Kerttula LI, Serlo WS, Tervonen OA, Paakko EL, Vanharanta HV. Post-traumatic findings of the spine after earlier vertebral fracture in young patients: clinical and MRI study. Spine. 2000;25:1104–1108. doi: 10.1097/00007632-200005010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Kifune M, Panjabi MM, Arand M, Liu W. Fracture pattern and instability of thoracolumbar injuries. Eur Spine J. 1995;4:98–103. doi: 10.1007/BF00278920. [DOI] [PubMed] [Google Scholar]
- 30.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 31.Kohyama K, Saura R, Doita M, Mizuno K. Intervertebral disc cell apoptosis by nitric oxide: biological understanding of intervertebral disc degeneration. Kobe J Med Sci. 2000;46:283–295. [PubMed] [Google Scholar]
- 32.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 33.Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 35.LeBlanc AC. Natural cellular inhibitors of caspases. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:215–229. doi: 10.1016/S0278-5846(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 36.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 37.Mesner PW, Jr, Kaufmann SH. Methods utilized in the study of apoptosis. Adv Pharmacol. 1997;41:57–87. doi: 10.1016/S1054-3589(08)61054-6. [DOI] [PubMed] [Google Scholar]
- 38.Morgunova E, Tuuttila A, Bergmann U, Isupov M, Lindqvist Y, Schneider G, Tryggvason K. Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science. 1999;284:1667–1670. doi: 10.1126/science.284.5420.1667. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien MC, Healy SF, Jr, Raney SR, Hurst JM, Avner B, Hanly A, Mies C, Freeman JW, Snow C, Koester SK, Bolton WE. Discrimination of late apoptotic/necrotic cells (type III) by flow cytometry in solid tumors. Cytometry. 1997;28:81–89. doi: 10.1002/(SICI)1097-0320(19970501)28:1<81::AID-CYTO10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 40.Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, Guehring T. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration–an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006;24:385–392. doi: 10.1002/jor.20055. [DOI] [PubMed] [Google Scholar]
- 41.Oner FC, Rijt RR, Ramos LM, Dhert WJ, Verbout AJ. Changes in the disc space after fractures of the thoracolumbar spine. J Bone Joint Surg Br. 1998;80:833–839. doi: 10.1302/0301-620X.80B5.8830. [DOI] [PubMed] [Google Scholar]
- 42.Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993;106(Pt 2):685–691. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- 43.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 44.Przybyla A, Pollintine P, Bedzinski R, Adams MA. Outer annulus tears have less effect than endplate fracture on stress distributions inside intervertebral discs: relevance to disc degeneration. Clin Biomech (Bristol, Avon) 2006;21(10):1013–1019. doi: 10.1016/j.clinbiomech.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 47.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 48.Takada T, Nishida K, Doita M, Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine. 2002;27:1526–1530. doi: 10.1097/00007632-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 49.Tryggvason K, Huhtala P, Hoyhtya M, Hujanen E, Hurskainen T. 70 K type IV collagenase (gelatinase) Matrix Suppl. 1992;1:45–50. [PubMed] [Google Scholar]
- 50.Vornanen M, Bostman O, Keto P, Myllynen P. The integrity of intervertebral disks after operative treatment of thoracolumbar fractures. Clin Orthop Relat Res. 1993;December(297):150–154. [PubMed] [Google Scholar]
- 51.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whiteside RA, Jakob RP, Wyss UP, Mainil-Varlet P. Impact loading of articular cartilage during transplantation of osteochondral autograft. J Bone Joint Surg Br. 2005;87:1285–1291. doi: 10.1302/0301-620X.87B9.15710. [DOI] [PubMed] [Google Scholar]
- 53.Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14:491–504. [PubMed] [Google Scholar]