Abstract
Blood vessel clots are found around the nerve root in patients with lumbar disc herniation. Thrombosis formation in the experimental application of nucleus pulposus to the nerve root has been shown in histological studies. In addition, reduction of blood flow and nerve conduction velocity are induced by the application of nucleus pulposus, which mimics lumbar disc herniation. In patients with lumbar disc herniation, nerve root block, which is thought to increase nerve blood flow, improves radiculopathy. 5-HT2A receptor antagonists are used in chronic arterial occlusive diseases to improve blood flow and have been reported to work as well as nonsteroidal anti-inflammatory drugs in improving radiculopathy due to lumbar disc herniation in clinical studies. This study investigated the effects of a 5-HT2A receptor antagonist on blood vessel diameter and blood flow in a canine experimental model of lumbar disc herniation. A total of 13 dogs were used. The animals were divided into three experimental groups and surgery was performed 1 week before measurements. In the nucleus pulposus group (NP; n = 5), the nucleus pulposus was applied to the nerve roots from the ventral side. In the sham group (n = 5), nucleus pulposus was not applied. In the naïve group (n = 3), the animals did not undergo surgery. Measurements of vessel diameter and blood flow were done before and after administration of saline and drugs. The diameters and blood flow volume of the observed blood vessels were measured on video-recordings every 10 min for 65 min. In all groups, vessel diameter and blood flow did not change before or after administration of saline. In the NP and sham groups, vessel diameter and blood flow increased significantly after administration of 5-HTRA compared with the naïve group. 5-HTRA improved blood vessel diameter and blood flow in the nerve roots inflamed by the application of nucleus pulposus but not in the intact nerve roots. 5-HTRA might be a potential agent to improve blood flow in the nerve roots of patients with lumbar disc herniation.
Keywords: Nerve root, Blood flow, 5-HT, Disc herniation
Introduction
Lumbar disc herniation is the one of the most common diseases that induces low back pain and radiculopathy. Many studies have investigated the mechanisms of radicular pain due to lumbar disc herniation and both mechanical and chemical factors [9, 18, 19, 29] have been implicated. Serotonin is one of the chemical factors that play a role in inducing pain in the peripheral nerves and affecting blood vessels. Recently, a clinical study showed that a 5-HT2A receptor antagonist (5-HTRA) provided the same symptom relief as nonsteroidal anti-inflammatory drugs in patients with lumbar disc herniation [11]. In the clinical studies, 5-HTRA has been shown to improve circulation and is used to treat circulatory disorders associated with diabetes as well as chronic arterial occlusive diseases [8]. In addition, 5-HTRA is used to treat intermittent claudication due to lumbar spinal stenosis and has been shown to improve blood flow in the nerve root in experimental cauda equina compression [24]. It is also expected to improve blood flow in the nerve roots in the orthopaedic field [24].
It is reported that blood vessel clots are found around symptomatic nerve roots in patients with lumbar disc herniation [5]. In the canine experimental disc herniation model, there is thrombosis formation in nerve roots exposed to nucleus pulposus [13, 20]. It has also been found that blood flow decreased after nucleus pulposus was applied to nerve roots, which is related to a reduction in nerve conduction velocity [13, 18, 31]. This study focused on blood flow to the nerve roots in lumbar disc herniation. Our aim was to assess the effect of 5-HTRA on blood flow in a canine experimental model of lumbar disc herniation.
Materials and methods
A total of 13 female beagle dogs (15 months old) with an average body weight of 10.6 ± 0.4 kg (range 10.5–11.5 kg) were used in this study (Table 1). The experimental protocol was approved by the local animal ethics committee and conformed to Fukushima Medical University Guidelines, the Japanese Government Animal Protection and Management Law (No. 15), and the Japanese Government Notification on Feeding and Safekeeping of Animals (No. 6). All the dogs were anesthetized with an intra-muscular injection of 25 mg/kg body weight of ketamine hydrochloride (Ketalar 50 mg/ml; Parke -Davis, Morris Plains, NJ, USA) and 10 mg/ml body weight of pentobarbital sodium (Nembutal 50 mg/ml; Abbott Laboratories, Chicago, IL, USA). After endotracheal intubation, anesthesia was maintained by inhalation of nitrous oxide (3 l/min), oxygen (3 l/min), and halothane (1%, SIC Chemicals Ltd., Bristol, UK).
Table 1.
Animals’ condition
| No | Breed | Age (months) | Sex | Weight (kg) |
|---|---|---|---|---|
| 1 | Beagle | 15 | Female | 11.5 |
| 2 | Beagle | 15 | Female | 11 |
| 3 | Beagle | 15 | Female | 11 |
| 4 | Beagle | 15 | Female | 10.5 |
| 5 | Beagle | 15 | Female | 10.5 |
| 6 | Beagle | 15 | Female | 11 |
| 7 | Beagle | 15 | Female | 10.5 |
| 8 | Beagle | 15 | Female | 11 |
| 9 | Beagle | 15 | Female | 10 |
| 0 | Beagle | 15 | Female | 10.5 |
| 11 | Beagle | 15 | Female | 10.5 |
| 12 | Beagle | 15 | Female | 10 |
| 13 | Beagle | 15 | Female | 10 |
The animals were divided into three experimental groups: the nucleus pulposus group (NP; n = 5), the sham group (n = 5), and the naïve group (n = 3). In the NP group, the dogs were placed prone and a partial laminectomy of the caudal part of the sixth lumbar vertebrae was performed. The dura mater was gently retracted and the dorsolateral portion of the annulus fibrosus of the L6/L7 intervertebral disc was incised using an 18-gauge needle. This procedure resulted in visible leakage of nucleus pulposus into the epidural space to the nerve roots on the ventral side. In the sham group, the procedure was the same as the NP group but nucleus pulposus was not leaked to the nerve roots. In the naïve group, the animals did not undergo surgery.
Analysis was conducted 7 days after the initial operation, applying the same operational procedures (e.g., animal posture, anesthesia, and preoperative and postoperative management) as in the initial operation. A catheter was inserted and placed in the left cervical artery for continuous monitoring of blood pressure. Another catheter was placed in the abdominal aorta from the right femoral artery. This catheter was used for injection of ink, saline, and 5-HTRA. Preliminary experimental tests showed that the catheter was between the first and third lumbar arteries when its tip was placed on the same level as the distal bone edge of the twelfth rib. After insertion of the catheter, the ligamentum flavum between the laminae of the seventh lumbar and first sacral vertebrae were removed, and the cauda equina was exposed. The second or third sacral nerve root was identified just caudal to the compression site. The blood vessels were observed using a microscope equipped with a video-camera (Digital HI-SCOPE Video System; Hirox Co. Ltd., Tokyo Japan) at 400× magnification [22, 24–26] (Fig. 1).
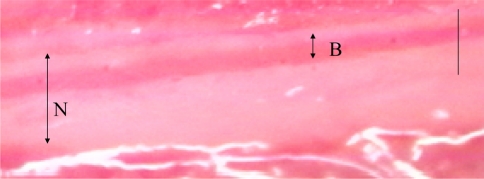
Fig. 1.
The observed blood vessel on the monitor. N nerve root, B blood vessel. Bar = 100 μm
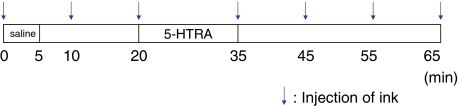
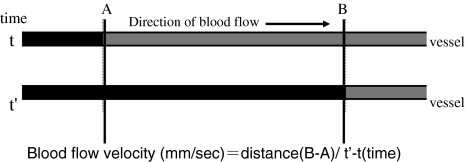
Sarpogrelate hydrochloride (5-HT2A receptor antagonist; 5-HTRA) (Mitsubishi Pharma Co. Tokyo, Japan) was used. In a clinical situation, the maximum serum concentration is 0.54 μg/ml after 100 mg sarpogrelate hydrochloride oral administration in adults. According to our previous paper, 0.05 μg/ml 5-HTRA was effective in improving blood flow in compressed nerve roots [24]. Therefore, we chose 0.05 μg/ml of 5-HTRA for this study. Saline was used for a vehicle of 5-HTRA. Blue ink was used to measure blood flow velocity. From the catheter in the abdominal aorta, 3 ml of blue ink (Pilot Co. Ltd., Tokyo, Japan) was injected manually for 1 s. When the ink flowed through the observed blood vessel, the color of the vessel changed from red to blue for 3 s. After confirming a flow of ink from the catheter to the observed blood vessels, recordings of the blood vessels were performed for 65 min. In all groups, intra-arterial saline (0.2 ml/min) was injected for 5 min using a catheter. After the administration of saline, the blood vessels were observed for 15 min. After 15 min, 5-HTRA (0.2 ml/min) was injected for 15 min and then the blood vessels were observed for 30 min without injection. Ink was injected before 5-HTRA and every 10 min thereafter (Fig. 2). The measurements of vessel diameter, blood flow velocity, and blood flow volume index in the observed blood vessels were performed by a blinded investigator using a video recording. The diameter of the blood vessels (μm) was measured for each ink injection using a monitor-connected video system equipped with a distance-measuring device. When two points were chosen, the distance-measuring device measured the distance between the two points automatically. Two points on the edge of blood vessel were chosen and measured three times at each time point. The average data of the diameter were used at each time point. The time (s) of the color changes from red to blue after injection of blue ink was measured using the iMovie software (Macintosh version) installed in a computer. The blood flow velocity (mm/s) was calculated by formula using time and distance (Fig. 3). The blood flow volume index was defined as (1/2 diameter)2 × (blood flow velocity) [22, 24–26]. The values of the diameter and blood flow volume index served as the baseline (100%) before injecting saline. All recordings of the diameter of vessels and the blood flow volume index were expressed as a percentage of baseline values.
Fig. 2.
Time course of injection. Saline was injected for 5 min, and 5-HTRA was injected for 15 min at 15-min intervals. The blood vessels were observed for 65 min. Blue ink was injected before 5-HTRA and every 10 min to measure the blood flow speed
Fig. 3.
The time (s) was measured using ink color changes (t′ − t). The distance from A to B was 0.82 mm on the monitor. The blood velocity (mm/s) was then calculated
Statistical analysis
Comparisons of the differences of the diameter of the blood vessels and blood flow index among each group over the entire observed time were evaluated by repeated measure ANOVA. Data were expressed as means ± SD. A value of P < 0.05 was considered significant. The diameter and ink speed were measured three times at each time point; therefore, intra-observer reliability (R) was evaluated by one-way ANOVA. R was calculated using a formula of R = (mean square between samples − mean square within samples)/mean square between samples + (times of measurement − 1) × mean square within samples. R-values of more than 0.8 were considered to be “good” and more than 0.9 to be “great”. The R-value was determined to be 0.939 and 0.896 for blood vessels and ink speed, respectively. Because intra-observer reliabilities of the diameter of blood vessels and blood speed in this study were great and good, the average data of three measurements were used.
Results
Throughout the observation period, neither paralysis nor dysfunction of the bladder was observed in any dog. The average body weight was 11 ± 0.4 kg when the analyses were performed. No wound infections occurred.
The blood pressure was not significantly different between pre and post administrations of 5-HTRA or saline in each group. The observed blood vessels were defined and we recorded seven vessels in the NP group, eight vessels in the sham group, and four vessels in the naïve group.
Diameter of blood vessels
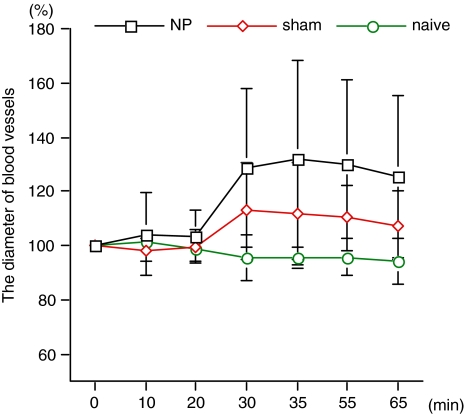
The diameters of the blood vessels did not change after administration of saline in any group and there were no significant differences in diameters among groups (Table 2). In the NP and sham groups, the vessel diameter increased after injection of 5-HTRA (Fig. 4). In the naïve group, the diameter did not change after administration of 5-HTRA. In the NP and sham groups, changes in blood vessel diameter during and after administration of 5-HTRA (from 20 to 65 min) were significantly higher than in the naïve group (P < 0.0001 and P = 0.0182, respectively). In particular, the diameter in the NP group was significantly increased compared with the sham group (P = 0.0027) (Table 3; Fig. 5)
Table 2.
Diameter of blood vessels (%) during and after administration of saline
| Group | Pre | 10 min | 20 min | P value vs. naïve | P value vs. sham |
|---|---|---|---|---|---|
| NP | 100 ± 0 | 100.4 ± 15.5 | 103.5 ± 9.5 | 0.3047 | 0.1337 |
| Sham | 100 ± 0 | 98.4 ± 4.2 | 99.9 ± 6.5 | 0.8184 | |
| Naïve | 100 ± 0 | 101.4 ± 2.1 | 98.6 ± 1.4 | 0.8184 |
Fig. 4.
The diameter of blood vessels after administration of 5-HTRA. In the NP and sham groups, changes in blood vessel diameter during and after administration of 5-HTRA (from 20 to 65 min) were significantly higher than in the naïve group (P < 0.0001 and P = 0.0182, respectively)
Table 3.
Diameter of blood vessels (%) during and after administration of 5-HTRA
| Group | 20 min | 35 min | 45 min | 55 min | 65 min | P value vs. naïve | P value vs. sham |
|---|---|---|---|---|---|---|---|
| NP | 103.5 ± 9.5 | 128.5 ± 29.1 | 131.6 ± 36.6 | 129.7 ± 31.3 | 125.4 ± 29.6 | <0.0001 | 0.0027 |
| Sham | 99.9 ± 6.5 | 114.2 ± 18.6 | 111.9 ± 18.7 | 111.4 ± 12.4 | 109.0 ± 12.8 | 0.0182 | |
| Naïve | 98.6 ± 1.4 | 95.6 ± 8.3 | 95.5 ± 4.0 | 95.7 ± 6.9 | 94.1 ± 8.2 | 0.0182 |
Fig. 5.
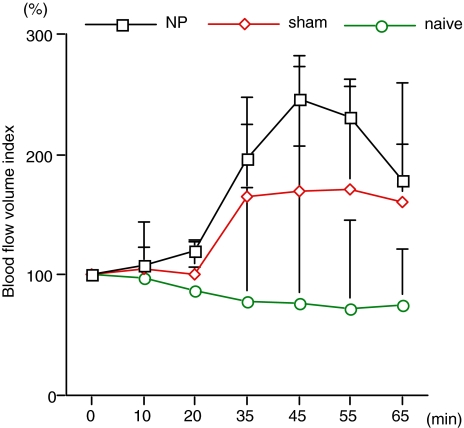
Blood flow after administration of 5-HTRA. In the NP and sham groups, the changes in blood flow during and after administration of 5-HTRA (from 20 to 65 min) were significantly higher than in the naïve group (P < 0.0001 and P = 0.0007, respectively)
Blood flow volume
Blood flow did not change after administration of saline in any group and there were no significant differences in blood flow among groups (Table 4). In the NP and sham groups, blood flow increased after injection of 5-HTRA (Fig. 2). In the naïve group, blood flow did not change after administration of 5-HTRA. In the NP and sham groups, the changes in blood flow during and after administration of 5-HTRA (from 20 to 65 min) were significantly higher than in the naïve group (P < 0.0001 and P = 0.0007, respectively). In particular, blood flow in the NP group was significantly increased compared with the sham group (P = 0.0278) (Table 5).
Table 4.
Blood flow index (%) during and after administration of saline
| Group | Pre | 10 min | 20 min | P value vs. naive | P value vs. sham |
|---|---|---|---|---|---|
| NP | 100 ± 0 | 107.5 ± 47.2 | 120.0 ± 20.1 | 0.0773 | 0.2576 |
| Sham | 100 ± 0 | 104.6 ± 6.7 | 100.6 ± 25.5 | 0.4064 | |
| Naïve | 100 ± 0 | 97.7 ± 12.9 | 87.2 ± 18.2 | 0.4064 |
Table 5.
Blood flow index (%) during and after administration of 5-HTRA
| Group | 20 min | 35 min | 45 min | 55 min | 65 min | P value vs. naïve | P value vs. sham |
|---|---|---|---|---|---|---|---|
| NP | 120.0 ± 20.1 | 196.6 ± 94.1 | 245.8 ± 130.1 | 231.6 ± 74 | 178.5 ± 47.0 | <0.0001 | 0.0278 |
| Sham | 100.6 ± 25.5 | 161.2 ± 77.0 | 165.2 ± 97.1 | 167.2 ± 79.9 | 155.9 ± 93.1 | 0.0007 | |
| Naïve | 87.2 ± 18.2 | 78.7 ± 25.3 | 75.8 ± 11.7 | 72.4 ± 13.1 | 74.5 ± 10.2 | 0.0007 |
Discussion
In the present study, vessel diameter and blood flow did not change before and after administration of saline in any group. This fact demonstrated that saline as a vehicle and ink to measure the blood flow velocity did not affect the blood vessel reaction in this experimental setting. In addition, all animals received saline before treatment; thus, blood vessels could be compared before and after administration of 5-HTRA both within each group and in each animal.
The blood vessels in the naïve group in this study did not change before or after administration of 5-HTRA. It is known that normal blood vessels keep their tension due to a balance of vasoconstrictor and vasodilator effects that occurs in smooth muscle and endothelial cells in blood vessels. There are seven groups of 5-HT receptors with 14 subtypes; the 5-HT1 and 5-HT2 receptors are associated with blood vessels. Nitric oxide, which is an important vasodilator [30], is produced in normal endothelial cells, mediated by the 5-HT1 receptor on endothelial cells, and affects vascular smooth muscle [2, 14, 28]. This vasodilator effect, and the vasoconstriction mediated by the 5-HT2A receptor on smooth muscle, maintains the tension in normal blood vessels. This tension can be maintained in various situations and may explain why, in this study, intact blood vessels in the naïve group did not change after the administration of 5-HTRA.
However, in the pathological lesions observed previously, blood vessels are vasoconstricted after administration of serotonin in canine coronary arteries [27]. It is known that vasospasm can be induced by serotonin through the 5-HT1 or 5-HT2 receptor [1, 3, 4, 10] and that 5-HTRA inhibits vasoconstriction by serotonin in the caudal artery in rats [6]. In the present study, the diameter and blood flow of blood vessels increased after administration of 5-HTRA in animals that underwent surgery. Surgery and surgical procedures such as the spread of blood around nerve roots could affect the nerve root, even though nucleus pulposus was not leaked in the sham group. Serotonin is released from platelets when inflammation and bleeding occur. In the NP and sham groups, the local concentration of serotonin might increase around the surgical area. However, in the NP group, the changes in vessel diameter and blood flow were highest compared with the sham and naïve groups. We could not determine whether serotonin was included in the nucleus pulposus or not under these inflammatory conditions. However, a previous study showed that plasma 5-hydroxyindoleacetic acid (5-HTAA), a metabolite of 5-HT, increased in animals in which the nucleus pulposus was harvested compared with sham animals [12]. This fact suggests that serotonin is associated with inducing symptoms seen in a lumbar disc herniation. The NP group in this study may have experienced increased inflammation and serotonin release compared with the sham group. We have also reported previously on the compression of the cauda equina in the canine model and showed that blood flow in the compressed nerve roots decreased with the dysfunction of endothelial cells after administration of serotonin, whereas blood flow in normal nerve roots did not decrease [25]. In addition, this reduction of blood flow was prevented by the 5-HTRA, indicating that this blood vessel reaction is associated with the 5-HT2A receptor [24]. The NP group in this study might have a dysfunction of endothelial cells compared with the sham group. This could explain why the effect of 5-HTRA was enhanced in the NP group rather than in the sham group.
In addition, 5-HT2A receptors are located on platelets, and serotonin can cause platelets to aggregate; thus the release of serotonin from aggregating platelets increases further platelet aggregation mediated by 5-HT2A. In a clinical situation, blood vessel clots are found around the nerve root in patients with lumbar disc herniation. Thrombosis formation in the experimental application of nucleus pulposus to the nerve root has been shown in histological studies [13, 20]. Therefore, 5-HTRA may prevent platelet aggregation and decrease the release of serotonin from platelets in patients with lumbar disc herniation.
There is no strong evidence regarding how much blood flow reduction influences the neuropathic pain. However, blood flow and nerve conduction velocity in the nerve root decrease after application of nucleus pulposus [13, 21, 31]. In addition, in the clinical setting, nerve root block is useful for improving radicular pain and maintains more duration than that of a local anesthetic effect. This is considered by the effect of blood flow increase. In an experimental study, intraradicular blood flow increased after nerve root infiltration [32]. Therefore, decreasing blood flow is associated with the dysfunction of the nerve root, and increasing blood flow might contribute to improving radiculopathy. In this study, blood flow increased after administration of 5-HTRA, and thus this compound might improve radiculopathy due to lumbar disc herniation.
There are some limitations to this study. The same blood vessel cannot be investigated before and after application of nucleus pulposus using this method. Therefore, the blood flow in this study did not show results before and after the leakage of the nucleus pulposus. According to a previous paper, blood flow in the nerve root was reduced 1 week after application of nucleus pulposus in a canine model [21]. Thus, the baseline of blood flow in the NP group could decrease after application of nucleus pulposus and the increased blood flow in these results suggests that changes in blood flow recover to normal levels. In either case, 5-HTRA is effective in improving blood flow. In addition, we investigated the treatment effect only 7 days after application of nucleus pulposus. A previous study in a canine model showed that blood flow decreases and nerve conduction velocity is reduced 7 days after application of nucleus pulposus, but these variables recover at 1 month after application of nucleus pulposus [21]. It is not clear if the effect of 5-HTRA will continue over a chronic period. This might be a limitation of this experimental model. Another limitation is the choice of the observed blood vessel. Because of difficulty of this procedure, we could find only one or two blood vessels on the monitor in one animal when the magnification was 400×. Even though blood vessels were observed, if we could not define the ink flow in the observed blood vessels on the monitor, we cannot measure blood flow. If we could observe two blood vessels fortunately, we could analyze two blood vessels in one animal. Thereby, the number of observed blood vessels was different in each group, which might affect the results.
Serotonin has effects not only in blood vessels but also in the nervous system. Serotonin is a neurotransmitter that inhibits and/or moderates pain in the central nervous system, whereas in the peripheral nervous system, serotonin induces pain. 5-HT1 and 5-HT3 receptors are known to be related to pain in peripheral nerves. In addition, it is reported that 5-HT2A receptors are expressed on dorsal root ganglion (DRG) neurons [17]. It has been reported that 5-HTRA is effective in the reduction of pain behavior in some experimental studies [7, 15, 16, 23]. However, we could not investigate pain behavior in this study. The improvement of blood flow may be directly or indirectly related to neuropathic pain relief. Thus, it is difficult to explain the relationship between pain behavior and blood flow. Based on the role of 5-HT2A receptors on blood vessels and in the peripheral nervous system, 5-HRTA may effect to improve blood flow and pain individually or interactively.
Conclusion
The present study, which focused on blood flow, found that 5-HTRA improves blood flow. 5-HTRA might be a potential agent to improve radiculopathy due to lumbar disc herniation.
Reference
- 1.Apperley E, Feniuk W, Humphrey PP, et al. Evidence for two types of excitatory receptor for 5-hydroxytryptamine in dog isolated vasculature. Br J Pharmacol. 1980;68:215–224. doi: 10.1111/j.1476-5381.1980.tb10410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen ML, Fuller RW, Wiley KS. Evidence for 5-HT2 receptor mediating contraction on vascular smooth muscle. J Pharmacol Exp Ther. 1982;218:421–425. [PubMed] [Google Scholar]
- 4.Feniuk W, Humphrey PP, Perren MJ, et al. A comparison of 5-hydroxytryptamine receptors mediating contraction in rabbit aorta and dog saphenous vein: evidence for different receptor types obtained by use of selective agonists and antagonists. Br J Pharmacol. 1985;86:697–704. doi: 10.1111/j.1476-5381.1985.tb08948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habtemariam A, Gronblad M, Virri J, et al. Blood clots in vessels surrounding nymptomatic nerve roots in disc herniation patients: a pilot study. Eur Spine J. 2002;11:447–451. doi: 10.1007/s00586-001-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara H, Osakabe M, Kitajima A. MCI-9042, a new antiplatelate agent is a selective S2-serotonergic receptor antagonist. Thromb Haemost. 1991;65:415–420. [PubMed] [Google Scholar]
- 7.Hsahizume H, Kawakami M, Yoshida M, et al. Sarpogeralte hydrochloride, a 5-HT2A receptor antagonist, attenuated neurogenic pain induced by nucleus pulposus in rats. Spine. 2007;32:315–320. doi: 10.1097/01.brs.0000253601.35732.c1. [DOI] [PubMed] [Google Scholar]
- 8.Hotta N, Nakamura J, Sumita Y, et al. Effects of the 5-HT2A receptor antagonist Sarpogrelate in diabetic patients with complications: a pilot study. Clin Drug Investig. 1999;18:199–207. doi: 10.2165/00044011-199918030-00004. [DOI] [Google Scholar]
- 9.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ishida T, Kawashima S, Hirata K, et al. Serotonin-induced hypercontraction through 5-hydroxytryptamine 1B receptors in atherosclerotic rabbit coronary arteries. Circulation. 2001;103:1289–1295. doi: 10.1161/01.cir.103.9.1289. [DOI] [PubMed] [Google Scholar]
- 11.Kanayama M, Hashimoto T, Shigenobu K, et al. New treatment of lumbar disc herniation involving 5-hydroxytryptamine 2A receptor inhibitor: a randomized controlled trial. J Neurosurg Spine. 2005;2:441–446. doi: 10.3171/spi.2005.2.4.0441. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Kikuchi S, Konno S et al (2007) Participation of 5-hydroxytryptamine released to nerve root inflammation in lumbar disc herniation. ISSLS 34th meeting abstract, P115
- 13.Kayama S, Konno S, Kikuchi S, et al. Incision of the annulus fibrosus induces nerve root morphlogic, vascular, and functional changes. Spine. 1996;21:2539–2543. doi: 10.1097/00007632-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Leysen JE, DeChaffoy Courcelles D, Clerck F. Serotonin-S2 receptor binding sites and functional correlates. Neuropharmacology. 1984;23:1493–1501. doi: 10.1016/0028-3908(84)90093-5. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T. Effects of a 5-HT2A receptor antagnist, sarpogrelate on thermal or inflammatory pain. Eur J Pharmacol. 2005;516:18–22. doi: 10.1016/j.ejphar.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Nitanda A, Yasunami N, Tokumo K, et al. Contribution of the peripheral 5-HT2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Nuerochem Int. 2005;47:394–400. doi: 10.1016/j.neuint.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto K, Imbe H, Morikawa Y, et al. 5-HT2A receptor sybtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99:133–143. doi: 10.1016/S0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- 18.Olmarker K, Blomquist J, Rydevik B, et al. Inflammatogenic properties of nucleus pulposus. Spine. 1995;20:665–669. doi: 10.1097/00007632-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 19.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induced neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. [PubMed] [Google Scholar]
- 20.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity. Spine. 2001;26:863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Otani K, Arai I, Kikuchi S, et al. Nucleus pulposus-induced nerve root injury: relationship between blood flow and motor nerve conduction velocity. Neurosugery. 1999;45:614–619. doi: 10.1097/00006123-199909000-00034. [DOI] [PubMed] [Google Scholar]
- 22.Otani K, Kikuchi S, Konno S. Blood flow measurement on experimental chronic cauda equina compression in dogs: changes in blood flow at various conductions. J Spinal Disord. 2001;14:343–346. doi: 10.1097/00002517-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M, Obata H, Kawahara K, et al. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain. 2006;122:130–136. doi: 10.1016/j.pain.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Sekiguchi M, Konno S, Kikuchi S. Effects of 5-HT2A receptor antagonist on blood flow in chronically compressed nerve roots. J Peripher Nerv Syst. 2004;9:263–269. doi: 10.1111/j.1085-9489.2004.09401.x. [DOI] [PubMed] [Google Scholar]
- 25.Sekiguchi M, Konno S, Anzai H. Nerve vasculature changes induced by serotonin under chronic cauda equina compression. Spine. 2004;27:1634–1639. doi: 10.1097/00007632-200208010-00008. [DOI] [PubMed] [Google Scholar]
- 26.Sekiguchi M, Konno S, Kikuchi S. Effects on improvement of blood flow in the chronically compressed cauda equina: comparison between a selective prostaglandin E receptor (EP4) agonist and a prostaglandin E1 derivate. Spine. 2006;31:869–872. doi: 10.1097/01.brs.0000209256.96186.a7. [DOI] [PubMed] [Google Scholar]
- 27.Shimokawa H, Aarhus LL, Vanhoutte PM. Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ Res. 1987;61:256–270. doi: 10.1161/01.res.61.2.256. [DOI] [PubMed] [Google Scholar]
- 28.Vanhoutte PM. Serotonergic antagonists and vascular disease. Cardiovasc Drugs Ther. 1990;4:7–12. doi: 10.1007/BF00053420. [DOI] [PubMed] [Google Scholar]
- 29.Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- 30.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 31.Yabuki S, Kikuchi S, Olmarker K, et al. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglion. Spine. 1998;23:2517–2523. doi: 10.1097/00007632-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Yabiki S, Kikuchi S. Nerve root infiltration and sympathetic block. An experimental study of intraradicular blood flow. Spine. 1995;20:901–906. doi: 10.1097/00007632-199504150-00004. [DOI] [PubMed] [Google Scholar]