Abstract
To study the effect of the degree of scoliosis, degree of hypokyphosis/lordosis and rotation of apical vertebra on individual lung volume (measured with CT scan) in asymptomatic adolescent idiopathic scoliosis (AIS) patients. Individual (right and left) lung volume, angle of kyphosis and rotation of apical vertebra, were measured in 77 asymptomatic AIS patients having right thoracic curve, using modern computed tomography (CT) scan. To compare, lung volumes were measured in 22 normal persons (control group). The ratio of “right to left lung volume (convex to concave side)” was obtained and compared among these groups. With increased Cobb’s angle, ratio of convex to concave lung volume increased. For Cobb’s angle more than 40°, it was increased significantly (P = 0.0042). A significant degree of correlation was found between axial rotation angle of apical vertebra and right to left lung volume ratio (P = 0.0067, r = 0.271). A significant inverse correlation was found between the angle of kyphosis and right to left lung volume ratio, i.e., as the angle of kyphosis decreased the convex to concave lung volume ratio increased (P = 0.0109, r = −0.255). In asymptomatic, AIS patients, with increase in degree of curvature, and rotation of apical vertebra, the ratio of convex to concave side lung volume increases; indicating concave side lung volume is comparatively more affected (decreased) than convex side lung volume. On the other hand with decrease in the angle of kyphosis the convex to concave lung volume ratio increases indicating kyphotic angle has an inverse relation to convex to concave lung volume ratio.
Keywords: Adolescent idiopathic scoliosis, Individual lung volume, Convex to concave lung volume ratio
Introduction
Nash and Nevins [18], Smith et al. [22], and Ogilvie and Schendel [19] suggested that severe degree of scoliosis affects the size and dimension of thoracic cage and hence the pulmonary function. After 90° of curvature, there is a severe affection of the lung volume and it is of restrictive type due to the reduction in vital capacity, a twice likely hood of early death from cor pulmonale [8, 13]. Differences of opinion exist about relationship of severity of scoliosis and degree of pulmonary compromise. Westgate and Moe [25] and Smith et al. [22] suggested a direct relation; while Gazioglu et al. [7], Muirhead and Conner [17] and Upadhyay et al. [24] found no correlation in idiopathic curves. These studies were based on assessment of the pulmonary function tests [9, 10]. With modern computed tomography (CT) scan it is now possible to measure lung volume, vertebral rotation and angle of kyphosis accurately [4–6, 24]. The purpose of our study was to document the effect of severity of curve, rotation and kyphosis on individual lung volumes, in adolescent idiopathic scoliosis (AIS). The lung volume is expected to be lower on the concave side due to the rotational effect of the curve causing crowding of ribs which leads to decreased hemi thoracic cavity and the lung volume.
Materials and methods
From December 2003 to April 2004, cross sectional study in 99 cases (77 AIS patients and 22 normal, control group) was performed with informed consent and approval from institutional review board. In AIS patient group, there were 63 females and 14 males; and their age varied from 10 to 22 years with mean of 13.8 years. The control group was randomly picked from the pediatric out-patient clinic with informed consent. There were 10 females and 12 males; and their age varied from 11 to 17 years with mean of 13.3 years.
Standard anterior–posterior and lateral radiographs, showing the complete spine, were obtained for all the patients and control group in standing position. Cobb’s angles were measured using anterior–posterior radiographs. All 77 curves were idiopathic, right sided, with apex in between T7 and T9 vertebra. Upper end vertebra varied from T1 to T3 and lower end vertebra varied from T11 to L1. Out of 77 curves 68 curves were King Type II and 9 were King Type III. Patients with left sided thoracic curve, King’s type I, IV and V or congenital scoliosis were excluded from the study.
Pulmonary function tests were conducted in the study group. All scoliosis patients were free of any respiratory complaint (asymptomatic) and all of them had normal PFT (FEV1/FVC > 80%. FEV1 > 80%). Patients with respiratory symptoms and abnormal PFT were also excluded from the study.
Lung volume assessment
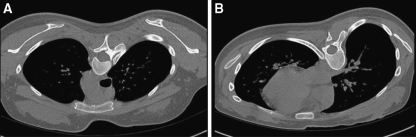
Computed tomography (CT) images of the thorax were obtained in all the 77 AIS patients and 22 control group, using a commercially available, 16 channel multi-detector row, scanner (Sensation 16, Siemens, Erlangen, Germany). Images were acquired from the lung apex to base, at full inspiration, in the low dose protocol (tube current 60 mA; tube voltage 120 kV). For all patients and control group, scanning was done at 1.5 mm collimation, and sections were obtained at 7 mm intervals (section thickness) (Fig. 1).
Fig. 1.
For all patients and control group, scanning was done at 1.5 mm collimation, and sections were obtained at 7 mm intervals. Sections at two different levels are shown here. a Section through superior mediastinum at level of spine of scapula. b Section through inferior mediastinum at infrascapular level
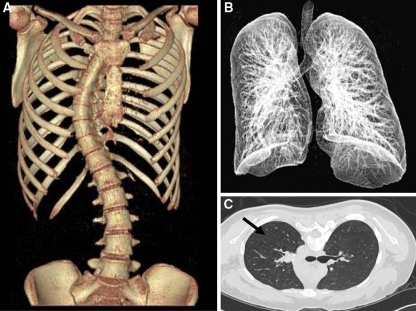
The individual right (convex side lung) and left (concave side lung) lung volumes (Fig. 2), angle of kyphosis and degree of rotation of the apical vertebra in axial plane (Figs. 3, 4) were measured in each case using the 3D image reconstruction software (RAPIDIA 2.7, INFINITT, South Korea) [3, 12, 26, 27] which has sensitivity of 94.25 with 1-mm collimation, 80% with 2.5 collimation described in the literature [26]. This program recognizes, the “Air density shade” of the lung, and volume for every section of the lung ; which then automatically calculate the volume for individual lung by summation of all section volumes (Fig. 2). All the CT scans were performed on the same machine and volumes were calculated by a radiologist who was unaware of purpose of the study.
Fig. 2.
The individual right and left lung volumes were measured in each case using the 3D image reconstruction software (RAPIDIA 2.7, INFINITT, South Korea). This program recognizes, the “Air density shade” of the lung (shown here with arrow), and volume for every section of the lung; which then automatically calculate the volume for individual lung by summation of all section volumes. a 3D CT scan image showing scoliosis of the spine with the rib cage. b 3D CT scan image of lung fields. c Cross sectional CT scan image used to calculate lung volume
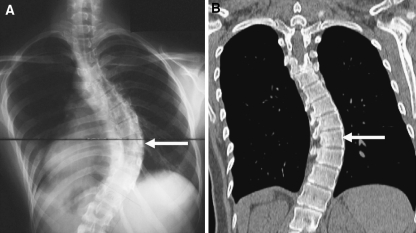
Fig. 3.
Apical vertebra determined on anterio-posterior radiogram and corresponding CT scan (shown with arrows) a Apical vertebra determined on anterio-posterior radiogram (depicted by arrow) b Apical vertebra determined on CT scan (depicted by arrow)
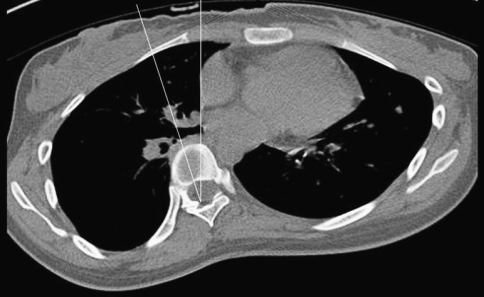
Fig. 4.
Degree of rotation of the “Apical Vertebra (shown in Fig. 3)” in axial plane was measured in each case using the 3D image reconstruction software. (The angle of rotation about the longitudinal axis relative to the sagittal plane is shown in this figure. It is the angle between “the line through the dorsal central aspect of the vertebral foramen and the middle of the vertebral body” and “the sagittal plane”)
Measurement of apical vertebral rotation performed on CT scan using Aaro-Dahlborn method (angle between line joining center of vertebral body to center of spinal canal and midsagittal plane) and angle of kyphosis measured on lateral X-ray of spine using Cobb’s method (angle between upper end plate of superior and lower end plate of inferior end vertebrae).
Statistical analysis
Patients with AIS were divided into three groups as mild, moderate and severe groups. This division was based on the degree of Cobb’s angle as follows: mild group (group A): Cobb’s angle 11–25°; moderate group (group B): Cobb’s angle 26°–40°; severe group (group C): Cobb’s angle more than 40° (Table 1). Values of “Right to Left Lung Volume Ratio” were compared among the control group and group A, B, and C separately.
Table 1.
Division of patients into groups according to Cobb’s angle
| Group | Cobb’s angle in degrees | Number of patients | Age range (mean) |
|---|---|---|---|
| A. Mild | 11–25 | 23 | 11–17 (13.1) |
| B. Moderate | 26–40 | 27 | 10–19 (14.25) |
| C. Severe | >40 | 27 | 11–22 (15.1) |
| Control group | <10 | 22 | 11–17 (13.3) |
| Total | 99 | 10–22 (13.8) |
Kruskal–Wallis method was used for analysis of relationship between Cobb’s angle and lung volume ratio.
Pearson correlation analysis was used for analysis of relation between degree of rotation of apical vertebra and lung volume ratio, and for analysis of relation between angle of kyphosis and lung volume ratio.
Results
The results for relation between degree of severity of curve, and convex to concave lung volume ratio, are shown in Table 2.
Table 2.
The ratio of “right to left (convex to concave) lung volume” and its relation to degree of curvature
| Group | Cobb’s angle Mean ± SD (range) |
RLV Mean ± SD (mm3) |
LLV Mean ± SD (mm3) |
RLV/LLV ratio Mean ± SD |
AVR degree Mean ± SD (range) |
Kyphosis angle Mean ± SD (range) |
|---|---|---|---|---|---|---|
| Normal | 6.77 ± 2.76 (5–10) | 1805136.07 ± 579306.92 | 1615341.15 ± 528467.42 | 1.1229 ± 0.0667 | 2.77 ± 2.55 (0–6.77) | 21.65 ± 9.00 (6.7–38.65) |
| Group A | 19.30 ± 3.06 (14–24) | 1629315.2 ± 515026.7 | 1433697.93 ± 442201.88 | 1.1391 ± 0.0817 | 8.63 ± 4.09 (1.22–13.09) | 19.53 ± 7.28 (5.49–31.93) |
| Group B | 32.93 ± 3.09 (28–38) | 1621972.54 ± 429029.98 | 1388572.84 ± 379741.73 | 1.1786 ± 0.0954 | 9.50 ± 4.56 (1.98–16.89) | 12.86 ± 7.29 (2.39–28.9) |
| Group C | 45.78 ± 5.41 (40–64) | 1424311.4 ± 327768.99 | 1183196.72 ± 304095.29 | 1.2242 ± 0.1461 | 11.22 ± 5.73 (1.59–23.69) | 15.85 ± 8.12 (3.63–28.28) |
| Total (A + B + C) | 33.36 ± 11.43 (14–64) | 1556573.73 ± 432035.62 | 1331968.87 ± 387523.33 | 1.1823 ± 0.1158 | 9.83 ± 4.92 (1.22–23.69) | 15.98 ± 7.95 (2.39–31.93) |
From Table 2 it is clear that with increase in the degree of severity of the scoliosis (Cobb’s angle) from group A to C, the ratio of convex to concave lung volume increases. But this increase in ratio was not statistically significant for group A and B when compared with control. For group A and B (Cobb’s <40°) the values of P = 0.012; Pearson’s correlation +0.302 are statistically less significant than for group C (Cobb’s >40°) where this ratio is increased significantly P = 0.0042; Pearson’s correlation +0.437.
The Pearson’s correlation between left lung volume and Cobb’s angle is r = −0.345 when compared to the right lung volume r = −0.268 indicating that the concave side lung volume is significantly more affected than the convex volume. Similar correlation is seen even at low curves where the Pearson’s correlations are r = −0.207 and r = −0.143, respectively for left and right lung volumes.
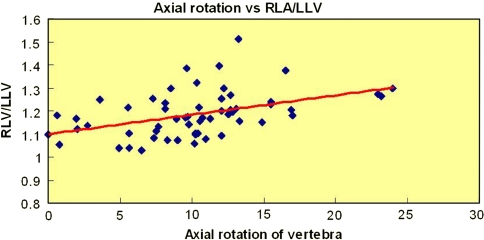
Relationship of, degree of rotation of apical vertebra, and ratio of convex to concave lung volume, is shown in scatter gram in Fig. 5. There was significant degree of correlation between axial rotation angle of apex, and right to left lung volume ratio (P = 0.0067 for apical rotation, Pearson’s correlation 0.271 for right to left lung volume ratio).
Fig. 5.
Relationship of, degree of rotation of apical vertebra, and ratio of convex to concave (right to left) lung volume, is shown here in scatter gram. There was significant degree of correlation between axial rotation angle of apex, and right to left lung volume ratio (=0.271; P = 0.0067)
There was a significant degree of inverse correlation between angle of kyphosis and right to left lung volume ratio (P < 0.0109, Pearson’s correlation −0.255) in total group. Thus as the angle of kyphosis decreased the right to left lung volume ratio increased. Comparing the sex, there was no difference (P = 0.2278 unpaired t test) in RLV/LLV ratio in both AIS and control group.
Discussion
Smith et al. [22] mentioned that the earliest demonstrable pulmonary change in asymptomatic AIS is a decrease of lung volume. They also stated that the decrease in lung volume is slight in asymptomatic adolescents, and severe in untreated adults with established respiratory insufficiency. Their findings were based on measurements of pulmonary function tests and there was no mentioning about how the individual lung volume (convex or concave side) is affected.
Loyd et al. [14], Gamsu et al. [6], Ogilvie and Schendel [19] attempted in past, to obtain the lung volume by using plain radiography. These initial studies were based on the measurement of surface areas of the lung fields; or combinations of linear measurements from the radiograms; or use of technique of summed elliptical cylindroids from plain postero-anterior and lateral radiograms. In these studies [10, 15], three-dimensional data were interpreted from two-dimensional plain radiograms and these studies rely upon certain assumptions about shape and symmetry of the thoracic cavity. Also they could not explain the relationship between the degree of curvature and its effect on individual lung volume in idiopathic scoliosis.
With developments in imaging technology, CT scan has been be used for measuring the lung volume in scoliosis [4, 8, 16–19, 22, 24]. These authors and Brown et al. [1], Dougherty et al. [5] have shown that lung volume can be absolutely and accurately measured in scoliosis patients using CT scan.
However most of these studies were focused in assessment of post-operative increase or decrease in lung volume, to study the effect of particular implants or technique used in scoliosis correction surgeries [11, 20, 21]. None of these studies gave any idea about effect of degree of curvature to individual lung volume in idiopathic scoliosis patients.
The results (Table 2) have shown that with increase in the degree of severity of the scoliosis (Cobb’s angle) the concave side lung volume is decreased to greater extent as compared to convex side, as evident from the increase in ratio of convex to concave side lung volume, with the increase in the severity of the curve. In other words there is direct relation with increase in degree of curvature and decrease in the lung volume on concave side.
Although the increase in ratio was not statistically significant in mild and moderate group, it was definitely significant (P = 0.0042) for severe group (group C having Cobb’s angle more than 40°), indicating again that concave side lung is affected to great extent as compared to convex lung, in this group.
The possible reason why concave lung is more affected than convex lung could be that concave side lung is compressed to great extent, or the space available is less for concave side lung, due to loss of the vertical height of the lung, on the concave side than convex side. Campbell et al. [2] while studying congenital scoliosis has stated that with progression of deformity, there is less space available for lung on concave side due to longitudinal growth inhibition. We think that, similar mechanism must be acting in idiopathic scoliosis where the vertebral height is decreased on the concave side due to greater pressure on the vertebral ring apophysis, resulting in decreased lung volume on concave side. Vertebral rotation also play a role in decreasing the lung volume suggested by Takahashi et al. [23] can be the other factor for decreased lung volume.
We found a significant degree of correlation between degree of rotation of apical vertebra, and convex to concave lung volume ratio (Fig. 5). This shows that, as the rotation of apical vertebra (axial plane) increases, the ratio of convex to concave side lung volume increases; meaning thereby concave side lung volume is more affected (decreased) as compared to convex one, with the rotation of apical vertebra. In other words there is direct relation with increase in degree of rotation of apical vertebra and decrease in the lung volume on concave side.
We found a significant degree of inverse correlation between the angle of kyphosis and right to left lung volume ratio, as the angle of kyphosis increases the lung volume ratio decreases. The possible explanation to this is that as the angle of kyphosis decreases there is a progressive decrease in the distance between the sternum anteriorly and the spine posteriorly which gets translated into a progressive decrease in the lung volume, with the concave lung volume being more affected than the convex lung volume.
The strength of the present study include the fact that it was a cross-sectional study of a very homogenous population in terms of age (all aged between 10 and 22 years), and diagnosis (AIS with right thoracic curve King’s type II or III). All patients were free from any respiratory complaint and had normal pulmonary function tests; thereby eliminating errors due to respiratory diseases. Another positive point was that, all the CT scans were performed on the same machine and volumes were calculated by the same person unaware of the purpose of the study.
We conclude, in asymptomatic AIS patients, with increase in degree of curvature and apical rotation the concave side lung volume decreases considerably than convex lung. On the other hand there is inverse correlation between the angle of kyphosis and convex to concave lung volume ratio indicating that as the angle of kyphosis decreases the concave lung volume is more affected than convex lung volume. This study may form a baseline for further studies on symptomatic scoliosis patients to anticipate and treat the respiratory complications effectively.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00586-008-0609-x
References
- 1.Brown MS, McNitt-Gray MF, Goldin JG, Greaser LE, Hayward UM, Sayre JW, Arid MK, Aberle DR. Automated measurement of single and total lung volume from CT. J Comput Assist Tomogr. 1999;23(4):632–640. doi: 10.1097/00004728-199907000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Campbell RM, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg [Am]; 2003;85(3):399–408. doi: 10.1302/0301-620X.85B3.13429. [DOI] [PubMed] [Google Scholar]
- 3.Chung JW, Yoon CJ, Jung S, Kim HC, Lee W, Kim Y, Jae HJ, Park JH. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004;15:249–256. doi: 10.1097/01.rvi.0000109402.52762.8d. [DOI] [PubMed] [Google Scholar]
- 4.Disler DG, Marr DS, Rosenthal DI. Accuracy of volume measurements of computed tomography and magnetic resonance imaging phantoms by three dimensional reconstruction and preliminary clinical application. Invest Radiol. 1994;29:739–745. doi: 10.1097/00004424-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty L, Asmuth JC, Gefter WB. Alignment of CT lung volumes with an optical flow method. Acad Radiol. 2003;10:249–254. doi: 10.1016/S1076-6332(03)80098-3. [DOI] [PubMed] [Google Scholar]
- 6.Gamsu G, Shames DM, McMahon J, Greenspan RH. Radiographically determined lung volumes at full inspiration and during forced expiration in normal subjects. Invest Radiol. 1975;10:100–108. doi: 10.1097/00004424-197503000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gazioglu BK, Goldstein LA, Femi-Pearse D, Yu PN. Pulmonary function in idiopathic scoliosis. Comparative evaluation before and after orthopedic correction. J Bone Joint Surg [Am] 1968;50-A(7):1391–1399. [Google Scholar]
- 8.Gollogly S, Smith JT, Campbell RM. Determining lung volume with three-dimensional reconstructions of CT scan data. A pilot study to evaluate the effects of expansion thoracoplasty on children with severe spinal deformities. J Pediatr Orthop. 2004;24:323–328. [PubMed] [Google Scholar]
- 9.Holbert JM, Brown ML, Sciurba FC, Keenan RJ, Landreneau KJ, Holzer AD. Changes in lung volume and volume of emphysema after unilateral lung reduction surgery: analysis with CT lung densitometry. Radiology. 1996;201:793–797. doi: 10.1148/radiology.201.3.8939233. [DOI] [PubMed] [Google Scholar]
- 10.Kauczor HU, Heussel CP, Fischer B, Klamm R, Mildenberger P, Thelen M. Assessment of lung volumes using helical CT at inspiration and expiration: comparison with pulmonary function tests. Am J Radiol. 1998;171:1091–1095. doi: 10.2214/ajr.171.4.9763003. [DOI] [PubMed] [Google Scholar]
- 11.Kovač V, Puljiz A, Smerdelj M, Pecina M. Scoliosis curve correction, thoracic volume changes, and thoracic diameters in scoliotic patients after anterior and after posterior instrumentation. Int Orthop (SICOT) 2001;25:66–69. doi: 10.1007/s002640100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W, Kim HS, Kim SJ, Kim HH, Chung JW, Kang HS, Hong SH, Choi JY. CT arthrography and virtual arthroscopy in the diagnosis of the anterior cruciate ligament and meniscal abnormalities of the knee joint. Korean J Radiol. 2004;5:47–54. doi: 10.3348/kjr.2004.5.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonstein JE, Bradford DS, Winter RB, Ogilvie JW. Natural history of spinal deformity––MOE”S text book of scoliosis and other spinal deformities. 3rd edn. Philadelphia: WB Saunders Company; 1995. pp. 87–93. [Google Scholar]
- 14.Loyd HM, String ST, DuBois AB. Radiographic and plethysmographic determination of total lung capacity. Radiology. 1966;86:7–14. doi: 10.1148/86.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Lugo N, Becker J, Bosse H, Campbell W, Evans B, Sagy M. Lung volume histograms after computed tomography of the chest with three dimensional imaging as a method to substantiate successful surgical expansion of the rib cage in achondroplasia. J Pediatr Surg. 1998;33:733–736. doi: 10.1016/S0022-3468(98)90201-9. [DOI] [PubMed] [Google Scholar]
- 16.Malcolm JR, Wind GG, Allely EB, Dam BE. Microcomputer reconstruction for analysis of spinal deformity and lung volume in hypokyphotic scoliosis. Spine. 1990;15(9):871–873. doi: 10.1097/00007632-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Muirhead A, Conner AN. The assessment of lung function in children with scoliosis. J Bone Joint Surg [Br] 1985;67-B(5):699–702. doi: 10.1302/0301-620X.67B5.4055863. [DOI] [PubMed] [Google Scholar]
- 18.Nash CL, Nevins K. A lateral look at pulmonary functions in scoliosis. J Bone Joint Surg [Am] 1974;56-A(4):440. [Google Scholar]
- 19.Ogilvie JW, Schendel MJ. Calculated thoracic volume as related to parameters of scoliosis correction. Spine. 1988;13(1):39–42. doi: 10.1097/00007632-198801000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sakic K, Pecina M, Pavicic F. Cardiorespiratory function in surgically treated thoracic scoliosis with respect to degree and apex of scoliotic curve. Respiration. 1992;60(5):312. doi: 10.1159/000196082. [DOI] [PubMed] [Google Scholar]
- 21.Sakic.k, Pecina M, Pavicic F. Pulmonary function in adolescents with idiopathic scoliosis. Int Orthop (SICOT) 1992;16:207–212. doi: 10.1007/BF00182695. [DOI] [PubMed] [Google Scholar]
- 22.Smith JP, King TC, Weber BJ, Cole JR, Briscoe WA, Levine DB. Lung function in idiopathic scoliosis: adolescence to old age. J Bone Joint Surg [Am] 1974;56(4):440. [Google Scholar]
- 23.Takahashi S, Suzuki N, Asazuma T, Kono K, Toyama Y. Factors of thoracic cage deformity that affect pulmonary function in adolescent idiopathic thoracic scoliosis. Spine. 2007;32(1):106–112. doi: 10.1097/01.brs.0000251005.31255.25. [DOI] [PubMed] [Google Scholar]
- 24.Upadhyay SS, Ho EKW, Gunawardene WMS, Leong JCY, Hsu LCS. Changes in residual volume relative to vital capacity and total lung capacity after arthrodesis of the spine in patients who have adolescent idiopathic scoliosis. J Bone Joint Surg [Am] 1993;75-A(1):46–52. doi: 10.2106/00004623-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Westgate HD, Moe JH. Pulmonary function in kyphoscoliosis before and after correction by the Harrington instrumentation method. J Bone Joint Surg [Am] 1969;51(5):935–946. [PubMed] [Google Scholar]
- 26.Won HJ, Choi Kim BI SH, Kim Y, Youn BJ, Han JK. Protocol optimization of multidetector computed tomography colonography using pig colonic phantoms. Invest Radiol. 2005;40:27–32. [PubMed] [Google Scholar]
- 27.Wood KB, Schendel MJ, Dekutoski MB, Boachie-Adijei O, Heithoff KH. Thoracic volume changes in scoliosis surgery. Spine. 1996;21(6):718–723. doi: 10.1097/00007632-199603150-00012. [DOI] [PubMed] [Google Scholar]