Abstract
A retrospective analysis was performed in a nine month period of the electrophysiological data, imaging and clinical findings of patients with monoradicular disc herniation compressing either the L5 or the S1 nerve root. The primary purpose of the analysis was to determine the distribution of pathological spontaneous activity in the medial paraspinal muscles on electromyographic examination in monoradicular L5 and S1 nerve root compression syndromes. Anatomically, the medial paraspinal muscles receive their innervation from a single root while the iliocostalis muscles and the longissimus muscle are thought to be innervated by multiple nerve roots. In the analysis, in single nerve root lesion of the L5 or S1 nerve root, electromyography of the medial paraspinal muscles revealed pathological spontaneous activity one to three vertebrae cranial to the disc herniation with extension to the opposite side of the lesion. In conclusion, the medial paraspinal muscles might be thought to be innervated by one single nerve root on anatomical studies, electrophysiologically the extension of axonal lesion signs of one single lumbar nerve root is much broader. The widespread distribution of the L5 and S1 nerve root must be taken into consideration on electromyographic examination of the medial paraspinal muscles.
Keywords: Paraspinal muscles, Electromyography, Disc herniation
Introduction
Electromyography is an important tool in the evaluation of the differential diagnosis including lumbosacral radiculopathy, polyneuropathy, motor neuron disease, affection of the lumbosacral plexus, radiculitis, myopathy and central nervous disease. Neurophysiological examination normally supports the majority of the findings of imaging studies disclosing a nerve root compression [17, 19]. In case of surgical intervention, confirmation of an axonal lesion on electromyography correlates with a successful surgical outcome, particularly when it is combined with imaging studies and psychological testing [20, 21].
In lumbosacral radiculopathy, examining a set of muscles of the lower extremities, the combination of positive and negative findings allows the determination of the damaged nerve root [4, 14, 15]. The inclusion of the paraspinal muscles in the electromyographic investigation raises the identification rate of an axonal lesion in lumbosacral radiculopathies [4].
The lumbar paraspinal muscles constitute a set of three muscle groups with different innervation patterns. Bogduk and colleagues have proposed an anatomical scheme which more precisely describes the location of multifidus muscle, longissimus muscle and iliocostalis muscle fascicles. The most medial multifidus muscle has been anatomically shown to be innervated by one nerve root while the more lateral localised muscles are innervated by multiple nerve roots [1, 2]. The multifidus muscle, thought to be innervated by one single nerve root, stretches multiple levels below the spinous process from which it originates [16]. Indeed, the anatomically based electrophysiological findings of Haig showing monosegmental innervation of the multifidus muscle have been corroborated by studies describing multisegmental denervation potentials two levels below the affected lumbar nerve root [10]. On the other hand, several authors suspected a polysegmental innervation of the medial paraspinal muscles as they detected spontaneous activity several segments caudal and adjacent to the lumbar nerve root lesion [6, 14, 22].
We questioned if there is a cranial extension of pathological spontaneous activity in the paraspinal medial muscles in acute monoradicular lumbar nerve compression syndromes affecting the L5 or the S1 nerve root. If the electromyographic examination reveals axonal lesions signs cranial and caudal to the level of the disc herniation, is there a need to extend the diagnostic procedure because of widespread paraspinal denervation in the case of an obvious monosegmental disc herniation? Since the electromyographic examination, especially of the paraspinal muscles, is uncomfortable for the patient, the aim of the electrodiagnostician should be to minimise the number of muscles studied.
Materials and methods
Patient selection. All examinations were performed in a routine setting for diagnostic investigation of acute and subacute back pain. Patients gave informed consent to each diagnostic procedure. The retrospective analysis was conducted among 11 patients showing an S1-syndrome and 10 showing an L5-syndrome presenting with lower back pain during a 9 month period (September 2005 to June 2006) using electrodiagnostic findings, imaging studies (magnetic resonance imaging, computed tomography, X-ray) and clinical data of patients presenting a monoradicular lumbar discogenic damage of the L5 or the S1 nerve root. Subjects with an additional foraminal or extraspinal herniation on the corresponding or adjacent level in addition to the mediolateral disc herniation, a herniation on another level, spondylolisthesis or lateral or central lumbar stenosis, other neuromuscular disease or a history of back surgery were excluded. In addition, there had to be a distal paresis confined to a damage of either the L5 or the S1 nerve root defined as a weakness of 4/5 or less on the Medical Research Council Scale. Subjects presenting a disc herniation on the level L5/S1 were only included if electromyographic examination revealed spontaneous activity in the gastrocnemius muscle and gluteus maximus muscle and/or gluteus medius muscle. In subjects presenting a disc herniation on the level L4/L5, pathological spontaneous activity had to be in the peroneus longus muscle and gluteus medius muscle or clinical weakness in the gluteus medius muscle in addition to the axonal lesion signs on electromyography in the peroneus longus muscle. For all patients, a standardised physical examination was completed that consisted of the following to exclude other neurological disease than monosegmental nerve root compression: (1) a neurologic examination including manual muscle testing and sensation and reflex assessment (2) a musculoskeletal examination, (3) straight leg raise test.
Electromyographic, neurographic and imaging studies. Electromyography was performed using standard concentric needle electrodes with a recording surface of 0.007 mm2 and a Viking IV (Nicolet, Madison Wisconsin) electromyograph. For the detection of fibrillation potentials and positive sharp waves, sensitivity was set at 50 μV/division, with a bandpass of 5 Hz to 10 kHz, and a sweep of 10 ms/division. Motor conduction velocity of the peroneal nerve and sensory conduction velocity of the sural nerve contralateral to the affected side were measured using standard techniques, and the results were compared with the normal values of our laboratory. Distal and proximal muscles of the lower leg were considered abnormal on electromyography if they demonstrated positive sharp waves and fibrillation potentials in more than three of ten needle positions.
Muscles of the leg electromyographically examined included the tibialis anterior muscle on both sides, the gluteus medius and maximus muscle, the peroneus longus muscle, the gastrocnemius muscle (medial and lateral head), the rectus femoris muscle and the plantaris muscle on the affected side.
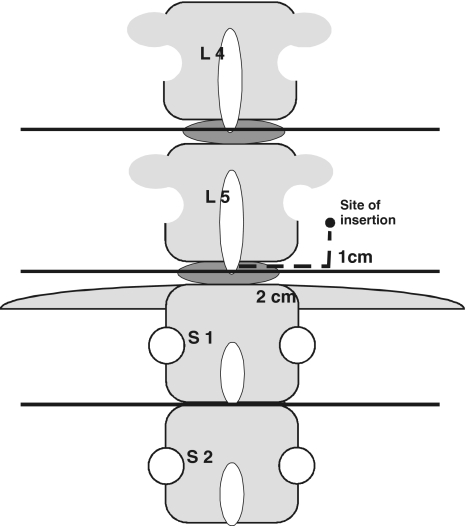
A modified MiniPM protocol of Haig, routinely performed in our lab, was used to examine the paraspinal lumbar muscles [7]. According to Haig, the needle position is 2, 5 cm lateral to the midline and 1 cm cranial to the tip of the L4, L5 or S1 spinous process. At each insertion side, a 50 mm monopolar EMG needle is directed at a 45° angle towards the midline, and inserted in approximately 5 mm intervals until it contacts the spinous process. The needle is withdrawn and directed cranially 45°, and the procedure is repeated. Again, it is withdrawn and redirected caudally 45° until contact with midline. We modified the protocol as follows: at each insertion site 2 cm lateral to the midline on the level of the spinous process and 1 cm cranial, a 50 mm monopolar needle is directed at a 45° angle towards the midline and inserted in approximately 5 mm intervals until it contacts the spinous process, then the needle was retracted and directed in a sagittal direction in 5 mm intervals (Fig. 1). A minimum of three sites at one lumbar/sacral level had to show pathological spontaneous activity to be regarded as an axonal lesion of the corresponding nerve root. Spontaneous activity had to be reproduced. Spontaneous activity in the proximity of bone was excluded. To define the level of the paraspinal insertion of the needle the spinous process of the vertebra L4 on the X-ray film was depicted and compared to the level of the iliac crest.
Fig. 1.
Site of the needle insertion at each level. The insertion site was performed 2 cm lateral to the spinous process and 1 cm cranial. At first the needle was directed in 5 mm intervals in a sagittal direction and then in a 45° medial angulation
Results
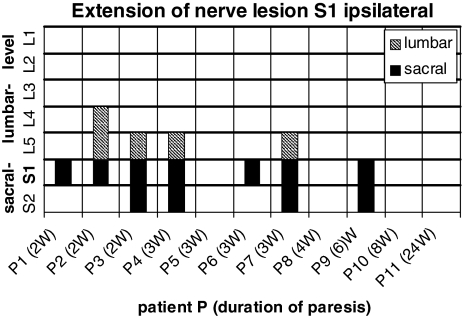
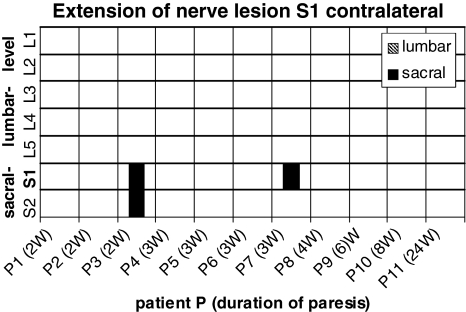
Eleven patients were selected presenting a paresis of the foot flexors and spontaneous activity on electromyography confined to a compression of the S1 nerve root (Table 1). All patients showed a mediolateral disc herniation at one side between the 5th lumbar vertebra and the first sacral vertebra on computer tomography or magnetic resonance imaging. The 11/11 patients revealed spontaneous activity in the gastrocnemius muscle, 9/11 in the gluteus maximus muscle and the remaining two patients showed atrophy of the gluteus maximus muscle. Paravertebral electromyography on the ipsilateral side of the paresis showed axonal damaging in 7/11 subjects at the level of the S1 vertebra, in cranial extension up to the L4 vertebra and in caudal direction down to the S2 vertebra (Fig. 2). On the opposite side of the paresis there were signs of axonal lesion paravertebral at the level of the S1 vertebra and in the caudal direction down to the S2 vertebra in two patients (Fig. 3).
Table 1.
Data of the 11 patients with a nerve compression injury to the S1 nerve root
| Age (range) years | Female : Male | Duration of the paresis; medium range (weeks) | MRC foot flexors (medium range) | Weakness of hip abduction (patients) | Atrophy of the ipsilateral gluteus maximus muscle (patients) | Nerve conduction studies normal : N.suralis/mot. peroneus (patients) | Axonal lesion signs in the ipsilateral gastrocnemius muscle (patients) | Axonal lesion signs in the ipsilateral gluteus maximus muscle (patients) | Axonal lesion signs in the rectus femoris/tibialis anterior muscle, peroneus longus muscle/contralateral tibialis anterior muscle (patients) | Straight leg raise test positive |
|---|---|---|---|---|---|---|---|---|---|---|
| 47 year (31–70) | 5:6 | 5.8 (2–24) | 3.4/5 (2/5–4/5) | 5/9a | 6/10b | 11/11 | 11/11 | 9/11 | 0/11 | 11/11 |
MRC Medical Research Council
aND in two patients
bND in one patient
Fig. 2.
Distribution of pathological spontaneous activity on the ipsilateral side in 11 patients with a S1 nerve root lesion in cranial and caudal extension. The filled bars correspond to axonal lesion signs in the sacral segments, the hatched bars to axonals lesion signs in the lumbar segments of each patient. Duration of the paresis in weeks (w)
Fig. 3.
Distribution of pathological spontaneous activity on the contralateral side in 11 patients with a S1 nerve root lesion in cranial and caudal extension. The filled bars correspond to axonal lesion signs in the sacral segments, the hatched bars to axonals lesion signs in the lumbar segments of each patient. Duration of the paresis in weeks (w)
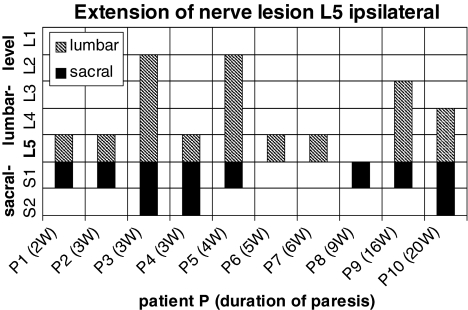
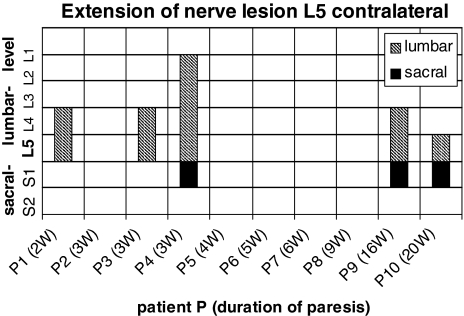
Ten patients presenting a paresis of the foot extensors were selected confined to an L5 nerve root lesion by imaging studies and on electromyographic findings (Table 2). Computed tomography or magnetic resonance imaging revealed a mediolateral disc herniation on one side between the 4th and the 5th lumbar vertebra. In one patient, disclosing six lumbar vertebrae on X-ray, there was an exclusively extraspinal extension of the disc herniation cranial to the last but one lumbar vertebra. Spontaneous activity was detected in the tibialis anterior muscle and in the peroneus longus muscle in 10/10 subjects. The 6/10 revealed spontaneous activity in the gluteus medius muscle, the remaining four subjects without axonal lesion signs on electromyography showed weakness in the gluteus medius muscle. The 0/10 subjects had axonal lesion signs in the rectus femoris muscle. Paravertebral electromyography disclosed ipsilateral to the paresis axonal damaging in 9/10 subjects at the level of the L5 vertebra, in cranial extension up to the L2 vertebra and in caudal direction down to the S2 vertebra (Fig. 4). On the contralateral side to the paresis there were signs of axonal lesion paravertebral at the level of the S1 vertebra and in cranial direction up to the L2 vertebra (Fig. 5). Additional electrophysiological data are shown in Tables 1 and 2.
Table 2.
Data of the 10 patients with a nerve compression injury to the L5 nerve root
| Age (range) (years) | Female : Male | Duration of the paresis (weeks) | MRC foot extensors (medium range) | Weakness of hip abduction (patients) | Atrophy of the ipsilateral gluteus maximus muscle (patients) | Nerve conduction studies normal : N.suralis/mot. Peroneus (patients) | Axonal lesion signs in the ipsilateral tibialis anterior muscle/peroneus longus muscle (patients) | Axonal lesion signs in the ipsilateral gluteus medius muscle (patients) | Axonal lesion signs in the rectus femoris muscle/contralateral tibialis anterior muscle (patients) | Straight leg raise test positive |
|---|---|---|---|---|---|---|---|---|---|---|
| 59.8 (38–81) | 6:4 | 7.1 (2–20) | 4/5 (4/5) | 10/10 | 0/9a | 9/9a (N. peroneus) 10/10 (N. suralis) | 10/10 | 6/10 | 0/10 | 10/10 |
MRC Medical Research Council
aND in one patient
Fig. 4.
Distribution of pathological spontaneous activity on the ipsilateral side in 10 patients with a L5 nerve root lesion in cranial and caudal extension. The filled bars correspond to axonal lesion signs in the sacral segments, the hatched bars to axonals lesion signs in the lumbar segments of each patient. Duration of the paresis in weeks (w)
Fig. 5.
Distribution of pathological spontaneous activity on the contralateral side in 10 patients with a L5 nerve root lesion in cranial and caudal extension. The filled bars correspond to axonal lesion signs in the sacral segments, the hatched bars to axonals lesion signs in the lumbar segments of each patient. Duration of the paresis in weeks (w)
Discussion
The analysis has been conducted to determine the distribution of spontaneous activity on electromyographic examination in medial paraspinal muscles in patients with a monoradicular nerve root lesion of the L5 or S1 nerve root. In dissection studies it had been shown that the iliocostalis and the longissimus muscles receive polysegmental innervation, but the most medial multifidus muscle fibers at one lumbar level are innervated from only one nerve root level [1, 2, 5, 8]. Recently electrophysiologic studies performed in paraplegic patients found pathological spontaneous activity and axonal lesions two segments below the paraplegic level as well as voluntary motor unit action potentials below and adjacent to the lesion [6, 14, 22]. Wu reported a subject with a monoradicular lesion of L3 nerve root revealing positive sharp waves on electromyography down to the L5 medial paraspinal muscles [22]. Lalive described axonal lesion on electromyography in the medial paraspinal muscles on adjacent levels in monoradicular lumbar nerve root lesion [14]. Our analysis addresses the cranial extension of the innervation respectively denervation in monoradicular L5 or S1 nerve root lesion. To be sure to examine only at the level of one single segment, we adapted the technique of Haig and did not change the direction of the needle in a cranial or caudal direction [9, 11, 12].
In case of an L5- and S1-nerve root lesion, pathological spontaneous activity could be detected on the ipsilateral side down to the S2-segment. These findings can be explained either by a polyradicular innervation of the medial paraspinal muscles as Wu [22] and Lalive [14] suspected or by the extension of the multifidus muscle itself coursing from the spinous process of L5 to the transverse process of the S1 vertebra at the L5-S1 interspace, so that the spontaneous activity at the lower segments could originate from the multifidus musle innervated by a nerve root situated cranially [12]. To our surprise electromyography revealed spontaneous activity in L5 nerve root lesion subjects up to three segments cranial to the L5 vertebra, e.g. to L2 vertebra. This widespread distribution of denervation is thought to be related to the lesion of the L5-nerve root as there is an accumulation of spontaneous activity around the L5-nerve root in the whole group and a decrease with the distance. Additionally, we ruled out a general distribution of spontaneous activity by examining the following segment as far as there was no further spontaneous activity. The paraspinal cranial extension of spontaneous activity of the subjects presenting an S1-nerve root lesion compromised only two segments. According to Haig, the S1 nerve root does not substantially innervate the paraspinal muscles [5]. In our analysis the denervation potentials of the medial paraspinal muscle in an S1 nerve root lesion reached down to the S2 segment. As the size of the S2 spinous process is very small, the localisation is difficult, and as the sacral vertebra become smaller, there might be an overlapping zone between the S1 nerve root and the S2 nerve root.
We cannot exclude reinnervation in the patients without pathological spontaneous activity in the medial paraspinal muscles, especially in the cases with a longer duration of the paresis. In patients with an L5-nerve root lesion, the extension of the pathological spontaneous activity was equally distributed between subjects with a shorter and patients with a longer duration of paresis. But in patients with an S1-nerve root lesion, those patients with a longer duration of paresis disclosed no pathological spontaneous activity in the medial paraspinal muscles.
The inconsistency between the anatomic studies and our electrophysiological findings in L5 and S1 nerve root lesion probably reflects the limitations of postmortem investigations of the peripheral nervous system. Anatomically, small nerve branches and anastomoses can be missed. The difference to the electrophysiological studies of Haig, studying patients with various diseases might originate from the group studied in this analysis complaining a short duration of the weakness, presenting an obvious paresis and disclosing spontaneous activity in the distal muscles and a nerve root compression on imaging studies [7]. The declining frequency of spontaneous activity with distance from the damaged nerve root argues against a normal distribution of pathological spontaneous activity ranging from 14.5 to 41.5% in recent studies examining a normal collective without back pain [3, 18]. The extension of spontaneous activity to the contralateral side to the nerve root damage might originate from a cross-over innervation of the contralateral medial paraspinal muscles. Another explanation of widespread pathological spontaneous activity in a monoradicular nerve root lesion might originate from an intersegmental reflex pathway. A lumbar nerve root compression caused by a disk herniation is followed by a contraction of the paraspinal muscles. According to the studies in cats this contraction could be the result of an intersegmental reflex leading to this muscle contraction [13]. So this hypothesis of ischemic damage of muscle fibers and following spontaneous activity on electromyographic examination by longstanding muscle contraction can be discussed. The loss of lordosis in older patients might be a result of repeated lumbar nerve injuries with a widespread damage of the paraspinal multisegmental innervated muscles, leading to weakness and fatty transformation of the dorsal muscles. The anterior part of the vertebra is more stressed and, in case of osteoporosis, results in wedge-shaped vertebra.
In summary the distribution of spontaneous activity on electrophysiological studies in monoradicular nerve root L5 and S1 reveals a widespread innervation of the medial paraspinal muscles. Our results demonstrate that the examination of the lumbar paraspinal muscles cannot identify a specific level of lumbar radiculopathy. The widespread innervation respectively denervation must be taken into account in the valuation of electromyography of the paraspinal muscles in the consideration of differential diagnosis.
References
- 1.Bogduk N, Twomey L. Clinical anatomy of the lumbar spine and sacrum. 3rd edn. New York: Churchill Livingstone; 1997. [Google Scholar]
- 2.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383–397. [PMC free article] [PubMed] [Google Scholar]
- 3.Date ES, Mar EY, Bugola MR, Teraoka JK. The prevalence of lumbar paraspinal spontaneous activity in asymptomatic subjects. Muscle Nerve. 1996;19:350–354. doi: 10.1002/(SICI)1097-4598(199603)19:3<350::AID-MUS11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Dillingham TR, Lauder TD, Andary M, Kumar S, Pezzin LE, Stephens RT, Shannon S. Identifying lumbosacral radiculopathies: an optimal electromyographic screen. Am J Phys Med Rehabil. 2000;79:496–503. doi: 10.1097/00002060-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Geiringer S. Anatomic localisation for needle electromyography. 2nd edn. Philadelphia: PA:Hanleyn & Belfus; 1999. [Google Scholar]
- 6.Gough JG, Koepke GH. Electromyographic determination of motor root levels in erector spinae muscles. Arch Phys Med Rehabil. 1966;47:9–11. [PubMed] [Google Scholar]
- 7.Haig AJ. Clinical experience with paraspinal mapping. I: neurophysiology of the paraspinal muscles in various spinal disorders. Arch Phys Med Rehabil. 1997;78:1177–1184. doi: 10.1016/S0003-9993(97)90328-2. [DOI] [PubMed] [Google Scholar]
- 8.Haig AJ. Clinical experience with paraspinal mapping. II: a simplified technique that eliminates three-fourths of needle insertions. Arch Phys Med Rehabil. 1997;78:1185–1190. doi: 10.1016/S0003-9993(97)90329-4. [DOI] [PubMed] [Google Scholar]
- 9.Haig AJ, LeBreck DB, Powley SG. Paraspinal mapping. Quantified needle electromyography of the paraspinal muscles in persons without low back pain. Spine. 1995;20:715–721. doi: 10.1097/00007632-199503150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Haig AJ, Levine JW, Ruan C, Yamakawa K. Describing paraspinal EMG findings: inadequacy of the single 0–4+ score. Am J Phys Med Rehabil. 2000;79:133–137. doi: 10.1097/00002060-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Haig AJ, Moffroid M, Henry S, Haugh L, Pope M. A technique for needle localization in paraspinal muscles with cadaveric confirmation. Muscle Nerve. 1991;14:521–526. doi: 10.1002/mus.880140606. [DOI] [PubMed] [Google Scholar]
- 12.Haig AJ, Talley C, Grobler LJ, LeBreck DB. Paraspinal mapping: quantified needle electromyography in lumbar radiculopathy. Muscle Nerve. 1993;16:477–484. doi: 10.1002/mus.880160508. [DOI] [PubMed] [Google Scholar]
- 13.Kang YM, Choi WS, Pickar JG. Electrophysiologic evidence for an intersegmental reflex pathway between lumbar paraspinal tissues. Spine. 2002;27:E56–E63. doi: 10.1097/00007632-200202010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lalive PH, Truffert A, Magistris MR. Lombosacral radiculopathy (L3–S1) and specificity of multifidus EMG. Neurophysiol Clin. 2004;34:41–47. doi: 10.1016/j.neucli.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Lauder TD, Dillingham TR, Huston CW, Chang AS, Belandres PV. Lumbosacral radiculopathy screen. Optimizing the number of muscles studies. Am J Phys Med Rehabil. 1994;73:394–402. doi: 10.1097/00002060-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 16.MacIntosh JE, Valencia S, Bogduk N, Monro RR. The morphology of the human multifidus. Clin Biom. 1986;1:196–204. doi: 10.1016/0268-0033(86)90146-4. [DOI] [PubMed] [Google Scholar]
- 17.Nardin RA, Patel MR, Gudas TF, Rutkove SB, Raynor EM. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22:151–155. doi: 10.1002/(SICI)1097-4598(199902)22:2<151::AID-MUS2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Nardin RA, Raynor EM, Rutkove SB. Fibrillations in lumbosacral paraspinal muscles of normal subjects. Muscle Nerve. 1998;21:1347–1349. doi: 10.1002/(SICI)1097-4598(199810)21:10<1347::AID-MUS20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Olmarker K, Rydevik B. Pathophysiology of sciatica. Orthop Clin North Am. 1991;22:223–234. [PubMed] [Google Scholar]
- 20.Spengler DM, Ouellette EA, Battie M, Zeh J. Elective discectomy for herniation of a lumbar disc. Additional experience with an objective method. J Bone Joint Surg Am. 1990;72:230–237. [PubMed] [Google Scholar]
- 21.Tullberg T, Svanborg E, Isaccsson J, Grane P. A preoperative and postoperative study of the accuracy and value of electrodiagnosis in patients with lumbosacral disc herniation. Spine. 1993;18:837–842. doi: 10.1097/00007632-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wu PB, Kingery WS, Frazier ML, Date ES. An electrophysiological demonstration of polysegmental innervation in the lumbar medial paraspinal muscles. Muscle Nerve. 1997;20:113–115. doi: 10.1002/(SICI)1097-4598(199701)20:1<113::AID-MUS18>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]